Article contents
Ferro-ferri-katophorite, a new clinoamphibole from the silicocarbonatite dykes in Sierra de Maz, La Rioja, Argentina
Published online by Cambridge University Press: 19 January 2023
Abstract
Ferro-ferri-katophorite (IMA2016–008), ideally Na(NaCa)(Fe2+4Fe3+)(Si7Al)O22(OH)2, was found as xenocrysts up to 3 cm long and replacement rims around aegirine–augite in silicocarbonatite dykes cropping out in the Sierra de Maz, La Rioja province, NW Argentina. Ferro-ferri-katophorite is black and has vitreous lustre and a pale green streak. The new mineral is brittle, with perfect {110} cleavage and has a Mohs hardness of 6. The measured density is 3.32(1) g/cm3. In plane-polarised light it is strongly pleochroic, X = light greenish brown, Y = dark greyish brown and Z = dark greyish olive green. Absorption (very strong) is Z > Y > X. The orientation is: Z ∥ b, and X forms a small angle with [001]. Ferro-ferri-katophorite is biaxial (–), with α = 1.688(3), β = 1.697(3), γ = 1.698(3) and 2V(calc) = 36.7°. It is monoclinic, space group C2/m, a = 9.8270(7), b = 18.0300(8), c = 5.316(4) Å, β = 104.626(4)°, V = 911.4(6) Å3 and Z = 2. The strongest five lines in the powder X-ray diffraction pattern [d in Å (I)(hkl)] are: 8.416(100)(110), 3.135(50)(310), 2.815(26)(330), 2.720(18)(151) and 1.4422(15)($\bar{6}$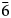 61). The chemical composition is SiO2 43.08, TiO2 2.76, ZrO2 0.15, Al2O3 8.76, V2O3 0.07, Fe2O3 9.28, FeO 13.85, MnO 0.43, MgO 6.88, CaO 6.58, ZnO 0.06, Na2O 5.55, K2O 1.18, Cl 0.01, H2O calc 1.36, total 99.95 wt.%. The formula unit (confirmed by single-crystal structural analysis) is (Na0.74K0.23)Σ0.97(Ca1.08Na0.91Mn0.01)Σ2.00(Fe2+1.78Mg1.57Fe3+1.07Ti4+0.32Al0.19Mn2+0.04Zr0.01V3+0.01Zn0.01)Σ5.00(Si6.61Al1.39)Σ8.00O22(OH1.59O0.61)Σ2.00. Aluminium is strongly ordered at the T(1) site. Ferro-ferri-katophorite is the 9th species carrying the katophorite root name and is related to katophorite by the Fe2+ + Fe3+ → Mg2+ + Al3+ substitution. Type material was deposited at the Museo de Mineralogía “Stelzner”, Universidad Nacional de Córdoba, Argentina, under catalogue number MS003341.
61). The chemical composition is SiO2 43.08, TiO2 2.76, ZrO2 0.15, Al2O3 8.76, V2O3 0.07, Fe2O3 9.28, FeO 13.85, MnO 0.43, MgO 6.88, CaO 6.58, ZnO 0.06, Na2O 5.55, K2O 1.18, Cl 0.01, H2O calc 1.36, total 99.95 wt.%. The formula unit (confirmed by single-crystal structural analysis) is (Na0.74K0.23)Σ0.97(Ca1.08Na0.91Mn0.01)Σ2.00(Fe2+1.78Mg1.57Fe3+1.07Ti4+0.32Al0.19Mn2+0.04Zr0.01V3+0.01Zn0.01)Σ5.00(Si6.61Al1.39)Σ8.00O22(OH1.59O0.61)Σ2.00. Aluminium is strongly ordered at the T(1) site. Ferro-ferri-katophorite is the 9th species carrying the katophorite root name and is related to katophorite by the Fe2+ + Fe3+ → Mg2+ + Al3+ substitution. Type material was deposited at the Museo de Mineralogía “Stelzner”, Universidad Nacional de Córdoba, Argentina, under catalogue number MS003341.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2023. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Giancarlo Della Ventura
References
- 2
- Cited by


