Introduction
Although schizophrenia commonly presents in late adolescent or early adulthood, a small proportion of patients first present with symptoms later in life (Howard et al. Reference Howard2000). An international consensus defined very late-onset schizophrenia-like psychosis (VLOSLP) as an onset of psychotic symptoms at the age of 60 years or above, which cannot be attributed to a primary affective disorder or structural brain abnormalities (Howard et al. Reference Howard2000). VLOSLP typically presents with multimodal hallucinations, partition and paranoid delusions (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015) in the absence of formal thought disorder and negative symptoms seen in early-onset schizophrenia (EOS). A two-stage model of onset has been proposed, characterised by initial suspicion, irritability and ideas of reference, followed by florid hallucinations and delusions (Zarit & Zarit, Reference Zarit and Zarit2012); however, disease onset remains challenging to pinpoint.
The aetiology of VLOSLP is unclear. VLOSLP differs significantly from EOS in clinical presentation, and unlike EOS, occurs more commonly in women and is less associated with family history or childhood trauma (Reeves & Brister, Reference Reeves and Brister2008). The presentation of VLOSLP also diverges from that of late-onset schizophrenia (LOS), defined as a primary onset of psychotic symptoms at the age of 40 years or above, being associated with a stronger female predominance, brain abnormalities on imaging and overall neuropsychological decline. Compared to VLOSLP, LOS has also been observed to be more commonly associated with positive family history and presenting with negative symptoms (Palmer et al. Reference Palmer, McClure and Jeste2001).
As VLOSLP often shares clinical features seen in neurodegenerative illnesses, such as cognitive impairment (Palmer et al. Reference Palmer, McClure and Jeste2001), late-life presentation, and behavioural and psychological symptoms (Savva et al. Reference Savva, Zaccai, Matthews, Davidson, McKeith and Brayne2009), it has been suggested as a prodromal phase of neurodegenerative dementias (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009; Sin Fai Lam et al. Reference Sin Fai Lam, Reeves, Stewart and Howard2016; Van Assche et al. Reference Van Assche, Morrens, Luyten, Van de Ven and Vandenbulcke2017). Patients with VLOSLP are subject to greater risk of a dementia than aged-matched controls (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009), but the condition does not always predict cognitive or functional decline (Sin Fai Lam et al. Reference Sin Fai Lam, Reeves, Stewart and Howard2016). Where impairment does arise, patients with VLOSLP often demonstrate non-progressive impairment similar to static encephalopathy in EOS (Van Assche et al. Reference Van Assche, Morrens, Luyten, Van de Ven and Vandenbulcke2017). The condition is also more prevalent in ethnic minority groups, which may implicate psychosocial stressors as a risk factor (Mitter et al. Reference Mitter, Reeves, Romero-Rubiales, Bell, Stewart and Howard2005). Nevertheless, the current consensus is that all cases satisfying diagnostic criteria for schizophrenia, regardless of onset age, fall under the same illness category (Howard et al. Reference Howard2000).
VLOSLP patients experience substantially incapacitating symptoms and represent a highly vulnerable group. Nevertheless, few systematic reviews have been conducted on the topic, focusing on clinical presentation, neuropsychology and neurobiology (Van Assche et al. Reference Van Assche, Morrens, Luyten, Van de Ven and Vandenbulcke2017; Suen et al. Reference Suen, Wong, Hui, Chan, Lee, Chang and Chen2019) in LOS and VLOSLP, all of which jointly evaluate outcomes of both conditions. There is debate around whether LOS is driven by the neurodevelopmental origins of EOS or the suggested neurodegenerative processes of VLOSLP (Palmer et al. Reference Palmer, McClure and Jeste2001), and the clinical difference between both conditions remains unclear despite international consensus establishing separate cut-offs for onset age for both conditions (Howard et al. Reference Howard2000). Consequently, this review focuses on examining only VLOSLP in relation to neurodegenerative disease and cognition.
The objectives of this review are therefore to review cognitive profiles and longitudinal course of cognitive function in patients with VLOSLP and to assess the quality of current evidence.
Methods
A systematic literature search was conducted based on Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (Moher et al. Reference Moher, Liberati, Tetzlaff, Altman and Group2009) on 5 February 2021 covering PubMed, PsycINFO and Web of Science, using a free text search strategy based on synonyms for ‘geriatrics’, ‘epidemiology’, ‘psychosis’ and ‘dementia’. As a consensus on the criteria for VLOSLP was only introduced in 2000 (Howard, Reference Howard2000), literature predating this uses a range of nomenclature to classify psychosis of first onset after the age of 60 years. Conditions with concepts overlapping with VLOSLP including paraphrenia, affective psychoses and very late-onset delusional disorder (VLODD) were therefore included in search terms for comprehensiveness. Additional studies were identified through searching reference lists of included sources. Only peer-reviewed original research available in English and as full texts were withheld for review. Full search terms are described in Supplementary Table 1.
Included studies investigated cognitive functioning, or dementia diagnosis, in patients with a primary onset of psychotic symptoms aged 60 years or above (Howard, Reference Howard2000) on a cross-sectional or longitudinal basis. We excluded studies investigating patients with pre-VLOSLP history of neurological disease, studies which did not separately describe outcomes of LOS and VLOSLP patient groups, studies which did not specify the age of onset of psychotic symptoms and cross-sectional studies without a control group.
Two-stage screening was performed to determine article eligibility, first by title and abstract and then by full text. Two authors (VY and SFL) independently reviewed titles and abstracts yielded from the systematic search to select full texts for review, and differences were resolved through discussion and mutual agreement. Both authors then independently screened selected full texts against inclusion and exclusion criteria, compared and resolved differences to determine final eligibility. A flowchart of the screening process is displayed in Fig. 1.

Fig. 1. PRISMA flowchart of study selection process.
Study quality was assessed according to GRADE criteria (Ryan & Hill, Reference Ryan and Hill2016). Due to heterogeneity in study design and outcome measures used, data were synthesised in a narrative structure. Information on study design, participant age and setting, outcome measures and findings was extracted and summarised in Tables 1 and 2. Duration of follow-up, defined as the length of time after which participants were re-assessed for dementia or cognitive ability, was also noted for longitudinal studies.
Table 1. Summary of results of cross-sectional studies
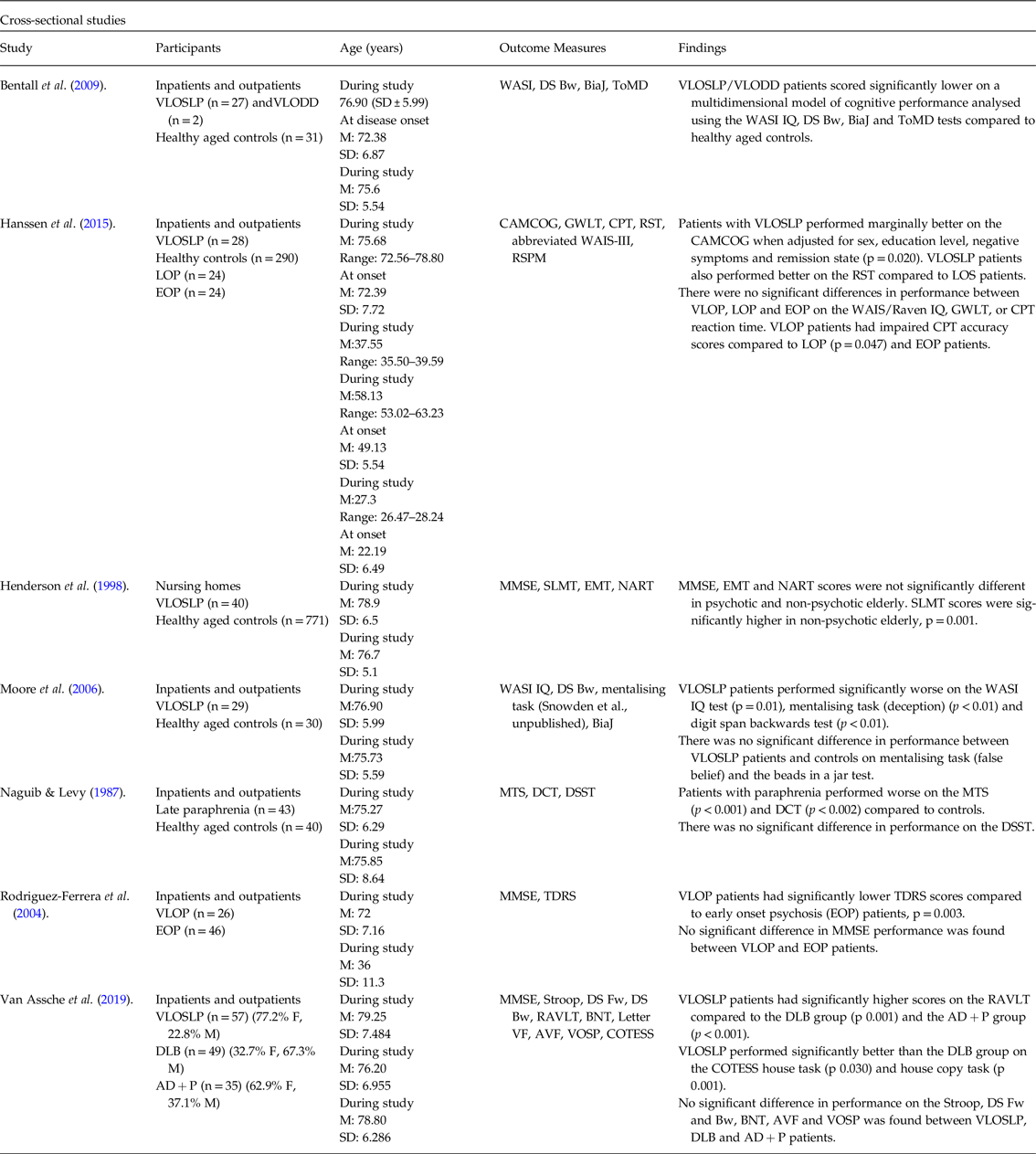
VLOSLP, very late-onset schizophrenia-like psychosis; LOS, late-onset schizophrenia; VLODD, very late-onset delusional disorder; WASI, Wechsler Abbreviated Scale of Intelligence; DS Bw, Digit Span Backward; BiaJ, Beads in a Jar; ToMD, Theory of Mind Deception; CAMCOG, Cambridge Cognitive Assessment Battery; GWLT, Groningen Word Learning Test; CPT, Continuous Performance Test; RST, Response Shifting Task; WAIS-R, Wechsler Adult Intelligence Scale-Revised; LOP, late-onset psychosis; EOP, early-onset psychosis; RSPM, (Raven Standard) Progressive Matrices; MMSE, Mini Mental State Examination; SLMT, Symbol Letter Modalities Test; EMT, Episodic Memory Test; NART, National Adult Reading Test; MTS, Mental Test Score; DCT, Digit Copying Test; DSST, Digit Symbol Substitution Test; VLOP, very late-onset psychosis; TDRS, Tardive Dyskinesia Rating Scale; Stroop I, Stroop Colour Word Interference Task Card I; Stroop IF, Stroop Colour Word Interference Task Interference Factor; DS Fw, Digit Span Forward; DLB, dementia with Lewy bodies; AD + P, Alzheimer’s type dementia with psychosis; RAVLT, Rey Auditory Verbal Learning Test; BNT, Boston Naming Test; AVF, Animal Verbal Fluency; VOSP, Visual Object and Space Perception Battery; COTESS, Cognitieve Testbatterij voor Senioren (Cognitive Test for Seniors).
An expanded version of this table is included in online-only material as Supplementary Table 3.
Table 2. Summary of results of longitudinal Studies.
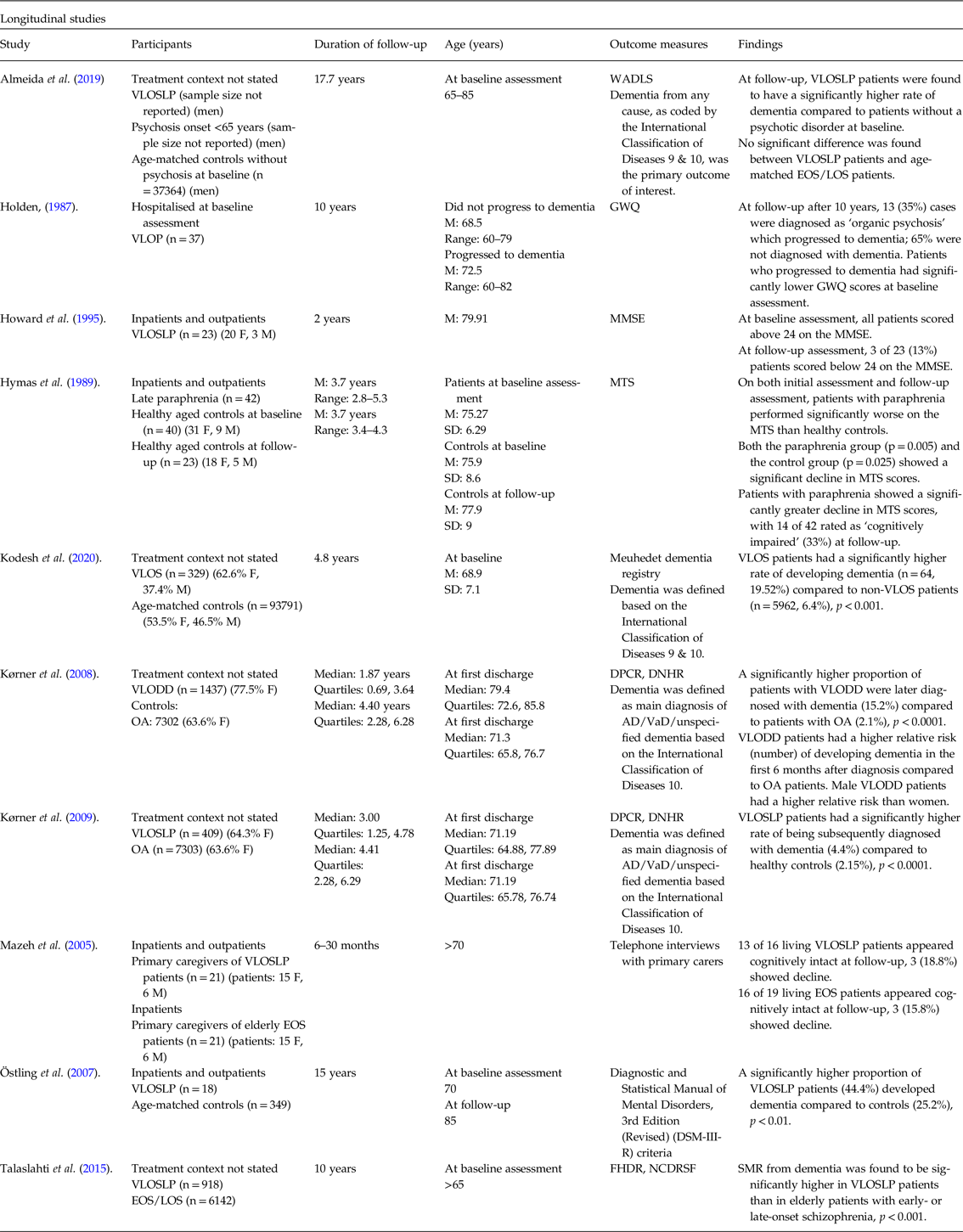
WADLS, Western Australia Data Linkage System; VLOP, very late-onset psychosis; GWQ, Gresham Ward Questionnaire; VLOS, Very Late-Onset Schizophrenia; VLODD, Very Late-Onset Delusional Disorder; OA, Osteoarthritis; DPCR, Danish Psychiatric Central Register; DNHR, Danish National Hospital Register; AD, Alzheimer’s Disease; VaD, vascular dementia; FDHR, Finnish Hospital Discharge Register; NCDRSF, National Causes of Death Register of Statistics Finland; SMR, Standard Mortality Ratio; VLOSLP, very late-onset schizophrenia-like psychosis; LOS, late-onset schizophrenia; EOS, early-onset schizophrenia; MMSE, Mini Mental State Examination.
An expanded version of this table is included in online-only material as Supplementary Table 4.
Results
Summary of included studies
Study characteristics
Nine hundred and sixteen records (excluding duplicates) were identified through the initial search, and 41 records were selected through title and abstract screening. Seventeen studies were identified through full-text review, of which seven were cross-sectional and ten longitudinal. Of seven cross-sectional studies, four compared cognition in VLOSLP patients with that of healthy aged controls (Naguib & Levy, Reference Naguib and Levy1987; Henderson et al. Reference Henderson, Korten, Levings, Jorm, Christensen, Jacomb and Rodgers1998; Moore et al. Reference Moore, Blackwood, Corcoran, Rowse, Kinderman, Bentall and Howard2006; Bentall et al. Reference Bentall, Rowse, Shryane, Kinderman, Howard, Blackwood, Moore and Corcoran2009) and two compared VLOSLP patients to young patients with EOP (Rodriguez-Ferrera et al. Reference Rodriguez-Ferrera, Vassilas and Haque2004, Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015) using a range of assessment tools. One study (Van Assche et al. Reference Van Assche, Van Aubel, Van de Ven, Bouckaert, Luyten and Vandenbulcke2019) compared individual cognitive domains between patients with VLOSLP and patients with neurodegenerative disease, matched for age, years of formal education and general cognitive function assessed using the Mini Mental State Examination (MMSE). Participants came from settings including nursing homes, inpatient and outpatient populations.
Of ten longitudinal studies, six investigated rates of developing dementia over a follow-up period in VLOSLP patients through searching hospital records (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009; Almeida et al. Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2019; Kodesh et al. Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020) or assessing patients using diagnostic criteria (Holden, Reference Holden1987; Östling et al. Reference Östling, Pálsson and Skoog2007). Of these, two compared VLOSLP patients to age-matched controls without VLOSLP (Östling et al. Reference Östling, Pálsson and Skoog2007; Kodesh et al. Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020), two compared VLOSLP patients to osteoarthritis patients (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009), one compared VLOSLP patients to non-psychotic elderly (Almeida et al. Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2019) and one did not use a control group (Holden, Reference Holden1987). Three studies investigated the course of cognition over a follow-up period using cognitive assessment tools (Hymas et al. Reference Hymas, Naguib and Levy1989; Howard et al. Reference Howard, Dennehey, Lovestone, Birkett, Sham, Powell, Castle, Murray and Levy1995) or carer impressions (Mazeh et al. Reference Mazeh, Zemishlani, Aizenberg and Barak2005), and one study compared rates of mortality from dementia in VLOSLP patients with that of EOS/LOS patients (Talaslahti et al. Reference Talaslahti, Alanen, Hakko, Isohanni, Häkkinen and Leinonen2015).
Study quality
Of 60 outcomes, 45 were rated ‘very low’ in quality, 13 ‘low’, one ‘moderate’ and one ‘high’. As all included studies were observational in design, all outcomes were assigned a ‘low’ baseline rating and then downgraded or upgraded based on assessment criteria for risk of bias, inconsistency, indirectness, imprecision, publication bias, large magnitude of effect, dose–response phenomena or the potential of plausible confounding factors to reduce an observed effect. Only studies which were not downgraded for a quality concern were eligible for upgrading (Ryan & Hill, Reference Ryan and Hill2016). Full results of quality assessment are included in Supplementary Table 2.
Global cognitive profiles
Cognitive ability in patients with VLOSLP was impaired compared to healthy age-matched controls in three studies (Naguib & Levy, Reference Naguib and Levy1987; Moore et al. Reference Moore, Blackwood, Corcoran, Rowse, Kinderman, Bentall and Howard2006; Bentall et al. Reference Bentall, Rowse, Shryane, Kinderman, Howard, Blackwood, Moore and Corcoran2009). One study found impairment in attention, perceptual speed, motor speed and visual scanning among patients with VLOSLP when compared to age-matched controls (Henderson et al. Reference Henderson, Korten, Levings, Jorm, Christensen, Jacomb and Rodgers1998).
One study found that levels of cognitive impairment in VLOSLP patients were not significantly different from that of aged patients with EOS using the MMSE (Rodriguez-Ferrera et al. Reference Rodriguez-Ferrera, Vassilas and Haque2004). Another study observed better performance from VLOSLP patients on the Cambridge Cognitive Assessment Battery (CAMCOG) compared to LOS patients (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015).
Impairment in individual cognitive domains
Patients with VLOSLP had impaired reasoning and perceptual organisation ability compared to healthy controls (Östling et al. Reference Östling, Pálsson and Skoog2007), and impaired language ability compared to age-matched controls (Bentall et al. Reference Bentall, Rowse, Shryane, Kinderman, Howard, Blackwood, Moore and Corcoran2009; Harris et al. Reference Harris, Kotsopoulos and Yamin2014). Memory and recall were also impaired in VLOSLP patients compared to healthy age-matched controls (Naguib & Levy, Reference Naguib and Levy1987). However, VLOSLP patients performed significantly better on short-term auditory-verbal memory and learning tasks compared to Alzheimer’s dementia with psychosis and Lewy body dementia patients with similar MMSE scores (Van Assche et al. Reference Van Assche, Van Aubel, Van de Ven, Bouckaert, Luyten and Vandenbulcke2019).
VLOSLP patients had lower accuracy of sustained attention than EOP or LOP patients, although no difference in reaction time of sustained attention measured using the continuous performance test (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015). Attention and vigilance were impaired in patients with VLOSLP compared to healthy controls (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015). However, notably, VLOSLP patients showed significantly less accurate responses on a vigilance task compared with LOS (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015).
Two studies reported that patients with VLOSLP had significant deficits in working memory (Harris et al. Reference Harris, Kotsopoulos and Yamin2014; Mazeh et al. Reference Mazeh, Zemishlani, Aizenberg and Barak2005) compared to healthy controls.
One study, comparing patients with VLOSLP to those with EOS and LOS, found patients with VLOSLP performed better on response-switching tasks (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015). VLOSLP patients were also found to perform better on tasks assessing visuo-constructive skills compared to patients with Alzheimer’s dementia with psychosis and Lewy body dementia; however, no difference was found with visuospatial perception (Van Assche et al. Reference Van Assche, Van Aubel, Van de Ven, Bouckaert, Luyten and Vandenbulcke2019).
Longitudinal course of cognition
Six studies reported that VLOSLP groups exhibited a significantly greater decline in cognitive function compared to controls over follow-up periods ranging from 6 months to 17.7 years (Hymas et al. Reference Hymas, Naguib and Levy1989; Östling et al. Reference Östling, Pálsson and Skoog2007; Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009; Almeida et al. Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2019; Kodesh et al. Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020). In two studies, follow-up periods were significantly different between subjects and controls (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009).
The proportion of patients with VLOSLP developing dementia ranged from 4.4% over 3 years (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009) to 44.4% over 15 years (Östling et al. Reference Östling, Pálsson and Skoog2007). One study reported that hallucinations conferred additional risk; 60% of patients experiencing hallucinations developed dementia, compared with 30% of those with delusions alone (Östling et al. Reference Östling, Pálsson and Skoog2007).
When compared with age-matched patients with EOS, VLOSLP participants had a similar rate of dementia but showed greater stability in cognitive and everyday functioning scores (Mazeh et al. Reference Mazeh, Zemishlani, Aizenberg and Barak2005).
Two studies found that VLODD groups exhibited a higher rate of developing dementia than age-matched patients with osteoarthritis (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009); one noted that 15% of VLODD patients compared to 2% of controls developed dementia within 6 months of initial presentation (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008).
One study, in which 13 of 37 patients with paraphrenia were diagnosed with dementia, reported that a higher proportion of patients with lower cognitive scores at initial presentation subsequently developed dementia (Holden, Reference Holden1987).
Summary
VLOSLP patients were found to have impaired global cognition compared to age-matched non-psychotic controls, but no strong evidence was found for a difference between VLOSLP patients and aged EOS patients.
Compared to age-matched healthy controls, VLOSLP patients had impairments in reasoning, perception, language, memory and recall, and attention. Compared to age-matched patients with neurodegenerative disease, VLOSLP patients had less impairment of memory, learning and visuo-constructive skills. Compared to age-matched patients with EOS/LOS, VLOSLP patients had lower vigilance and accuracy of attention, but performed better on response-switching tasks.
VLOSLP patients generally exhibited a greater decline in cognitive function and higher rates of later developing dementia compared to age-matched controls, but no difference in rate of developing dementia was found between VLOSLP patients and aged EOS patients.
Discussion
Global cognitive function and individual cognitive domains
Review of studies reporting on cognitive function in VLOSLP was complicated by heterogeneity of both study populations and outcome measures. Cognitive impairment in patients with VLOSLP compared to healthy age-matched controls was observed only in three studies (Naguib & Levy, Reference Naguib and Levy1987; Moore et al. Reference Moore, Blackwood, Corcoran, Rowse, Kinderman, Bentall and Howard2006; Bentall et al. Reference Bentall, Rowse, Shryane, Kinderman, Howard, Blackwood, Moore and Corcoran2009). Evidence comparing VLOSLP with LOS patients was conflicting, with VLOSLP patients outperforming LOS patients on the CAMCOG in one study (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015) but performing similarly on the MMSE in another (Rodriguez-Ferrera et al. Reference Rodriguez-Ferrera, Vassilas and Haque2004). Small sample sizes in both studies may account for this inconsistency in findings.
No single cognitive domain was observed to be consistently affected in VLOSLP compared to controls. Executive dysfunction, although commonly observed deficits in all forms of schizophrenia (Orellana & Slachevsky, Reference Orellana and Slachevsky2013), did not appear to be consistently impaired. Deficits in attention were however noted among patients with VLSOLP when compared to both healthy controls and individuals with LOS and EOS (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015).
VLOSLP patients were found to have less impaired short-term audio-visual memory and learning and visuo-constructive skills compared to patients with psychotic Alzheimer’s dementia and Lewy body dementia with similar MMSE scores, suggesting possible sparing of these domains (Van Assche et al. Reference Van Assche, Van Aubel, Van de Ven, Bouckaert, Luyten and Vandenbulcke2019).
As both attentional and executive function impairments are important clinical features of neurodegenerative conditions, longitudinal assessment of this patient group for later dementia diagnosis could be helpful in defining and separating the clinical profiles of both conditions.
Dementia in patients with VLOSLP
VLOSLP patient groups exhibited significantly higher rates of dementia diagnosis than non-psychotic controls over follow-up periods ranging from 6 months to 17.7 years (Hymas et al. Reference Hymas, Naguib and Levy1989; Östling et al. Reference Östling, Pálsson and Skoog2007; Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009; Almeida et al. Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2019; Kodesh et al. Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020). Rates of dementia diagnosis ranged from 4.4% over 3 years (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009) to 44.4% over 15 years (Östling et al. Reference Östling, Pálsson and Skoog2007), which may be attributable to differences in study design and methods of dementia diagnosis including Gresham Ward Questionnaire (GWQ), International classification of diseases (ICD)-10 and DSM-III-R.
Cohort prevalence of dementia was found to increase with longer follow-up periods as expected. Only one study identified a clinical risk factor for progression to dementia, the presence of hallucinations (Östling et al. Reference Östling, Pálsson and Skoog2007). Another reported lower cognitive scores at presentation as a risk factor for later progression (Holden, Reference Holden1987), but it is unclear if this represented a risk factor for subsequent dementia, or indicate possible misdiagnosis of a prodromal phase of neurodegenerative disease. Kørner and colleagues’ striking observation of a marked difference in progression to dementia within 6 months of initial presentation between patients with VLODD may raise the possibility of an initial high-risk period for progression to dementia, which subsequently regresses to a rate more comparable with that of controls (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008). However, it is worth noting that rate of progression to dementia differed significantly between two studies conducted by the same group in two consecutive years even with near-identical study design and methods (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008, Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009). The study investigating VLODD observed a rate of 15.2% despite a shorter follow-up period of 1.87 years (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008), while the study invsetigating VLOSLP observed 4.4% over 3 years (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2009), contradicting earlier findings of hallucinations as a risk factor for progression (Östling et al. Reference Östling, Pálsson and Skoog2007). A possible explanation for this inconsistency could be common misdiagnosis of early-stage dementia for VLODD initially, contributing to a high rate of subsequent diagnosis.
Importantly, irrespective of differences in study design and outcome measures used, less than half of VLOSLP patients developed dementia in all studies, even when 25% of healthy matched controls did (Östling et al. Reference Östling, Pálsson and Skoog2007), indicating a significant patient proportion without cognitive decline and supporting a distinct or diverging pathophysiological pathway from that of neurodegenerative disease. Even where male VLOSLP patients had higher rates of developing dementia compared to non-psychotic controls, there was no significant difference compared to aged male EOS/LOS patients (Almeida et al. Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2019), suggesting a similar course of cognition in old age across all schizophrenias regardless of onset age.
One study directly compared patients with VLOSLP to those with psychotic Alzheimer’s dementia and Lewy body dementia (Van Assche et al. Reference Van Assche, Van Aubel, Van de Ven, Bouckaert, Luyten and Vandenbulcke2019). In this study, dementia patients were diagnosed using a combination of clinical features, biomarkers and imaging, and VLOSLP patients were deemed to show no evidence of neurologic or major affective disorder. Participants were matched for MMSE scores, with both groups having a mean score just above 24, implying a similar stage of functional decline. Although the process of selecting for MMSE-matched participants was not elaborated on, the absence of neurodegenerative signs characteristic of dementias in the VLOSLP group despite similar levels of cognitive decline could point towards diverging mechanisms in both groups.
Interpretation of findings
Study quality was generally poor. Using the GRADE system (Ryan & Hill, Reference Ryan and Hill2016), five studies were attributed lower ratings for quality due to imprecision involving the use of meta-analysis to derive significant results (Bentall et al. Reference Bentall, Rowse, Shryane, Kinderman, Howard, Blackwood, Moore and Corcoran2009) and wide confidence intervals (Naguib & Levy, Reference Naguib and Levy1987; Hymas et al. Reference Hymas, Naguib and Levy1989; Henderson et al. Reference Henderson, Korten, Levings, Jorm, Christensen, Jacomb and Rodgers1998; Mazeh et al. Reference Mazeh, Zemishlani, Aizenberg and Barak2005). Studies rated as lower quality due to risk of bias had a low response rate (Henderson et al. Reference Henderson, Korten, Levings, Jorm, Christensen, Jacomb and Rodgers1998; Östling et al. Reference Östling, Pálsson and Skoog2007), excluded VLOSLP patients with clinical dementia for cognitive assessment at follow-up (Hanssen et al. Reference Hanssen, van der Werf, Verkaaik, Arts, Myin-Germeys, van Os, Verhey and Kohler2015) or used sample selection methods which could have excluded more EOP patients with more severe illness (Rodriguez-Ferrera et al. Reference Rodriguez-Ferrera, Vassilas and Haque2004). Studies rated down for indirectness relative to our research question investigated mortality from dementia as a primary outcome (Talaslahti et al. Reference Talaslahti, Alanen, Hakko, Isohanni, Häkkinen and Leinonen2015) or sampled only male patients (Almeida et al. Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2019) despite known female predominance in VLOSLP patients (Howard, Reference Howard2000). One study was attributed lower ratings for inconsistency as different groups of patients were administered different cognitive tasks without explanation of the selection process (Van Assche et al. Reference Van Assche, Van Aubel, Van de Ven, Bouckaert, Luyten and Vandenbulcke2019). Two studies were rated highly for large sample sizes and risk ratios, implying a large magnitude of effect (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008; Kodesh et al. Reference Kodesh, Goldberg, Rotstein, Weinstein, Reichenberg, Sandin and Levine2020). One study rated highly for plausible confounding factors reducing observed effects, as VLODD patients exhibited higher rates of dementia despite shorter follow-up periods compared to controls (Kørner et al. Reference Kørner, Lopez, Lauritzen, Andersen and Kessing2008).
Included studies spanned 33 years and were subject to multiple changes in the concept of the disease and shifting diagnostic criteria. This lack of uniformity in selection criteria implies a lack of generalisability of individual study findings. A wide range of measures were used across all studies including clinical diagnosis, functional decline and cognitive test scores. No two studies employed the same primary outcome measure, and studies examining functional decline and clinical diagnosis of dementia notably did not standardise assessments. Of all studies reporting development of dementia, only one adopted a rating scale routinely used in clinical practice, the MMSE; this, or CAMCOG, was used as an outcome measure in only 4 of 17 included studies.
Three non-retrospective longitudinal studies had high dropout rates (23%–41%) (Hymas et al. Reference Hymas, Naguib and Levy1989; Mazeh et al. Reference Mazeh, Zemishlani, Aizenberg and Barak2005; Östling et al. Reference Östling, Pálsson and Skoog2007). More generally, studies failed to consistently describe disease duration, points at which patients’ ages were measured, or specify length of time in changing cognitive function. The heterogeneity of outcome measures used made it difficult to compare studies, especially as some studies utilised subjective measures such as collateral interviews (Mazeh et al. Reference Mazeh, Zemishlani, Aizenberg and Barak2005). Studies further exploring this relationship should aim to follow-up participants up for 10 years or longer; only four studies included in this review adopted follow-up periods greater than 5 years (Holden, Reference Holden1987; Östling et al. Reference Östling, Pálsson and Skoog2007; Talaslahti et al. Reference Talaslahti, Alanen, Hakko, Isohanni, Häkkinen and Leinonen2015; Almeida et al. Reference Almeida, Ford, Hankey, Yeap, Golledge and Flicker2019).
All studies were limited by an absence of neuropathological data, the presence of which might indicate if VLOSLP represents a risk factor for dementia, a prodromal phase or a different manifestation of shared pathophysiology. One pathological study has associated VLOSLP with ventricular enlargement, generalised atrophy and vascular brain damage (Antonova et al. Reference Antonova, Sharma, Morris and Kumari2004). A high incidence of Lewy body dementia and argyrophilic grain disease pathology, including deterioration of the frontal and temporal lobes have also been detected in VLOSLP patients (Cloud et al. Reference Cloud, Carew, Rothenberg, Malloy and Libon1996; Nagao et al. Reference Nagao, Yokota, Ikeda, Takeda, Ishizu, Kuroda, Sudo, Terada, Murayama and Uchitomi2014). As Lewy body dementia is also associated with symptoms such as delusions and hallucinations, research comparing VLOSLP with these conditions, perhaps with the aid of neuroimaging or neuropathological data, could be helpful in improving differential diagnosis and understanding pathophysiology.
Our systematic, replicable search strategy, which adopted a fixed framework to analyse study quality, represents the greatest strength of this review. We did not conduct meta-analysis, but study heterogeneity and small sample size precluded this. Nevertheless, the absence of meta-analysis does render the review prone to interpretation bias.
Current and future directions
This study systematically reviewed 17 studies investigating cognitive impairment in patients with VLOSLP, and the proportion of such patients who develop dementia. Notwithstanding the caveats to interpretation discussed above, our overall findings point towards separate pathophysiology between VLOSLP and neurodegenerative disease and suggest similar courses of cognition between EOS, LOS and VLOSLP in old age.
A major concern our findings raise is the diagnostic integrity of VLOSLP. Despite an international consensus on criteria, the current definition of VLOSLP still encompasses a very broad range of presentations, and this heterogeneity is reflected in the selection of patient samples in included studies. One study comparing neurodegenerative disease to VLOSLP sampled 57 patients with an onset of psychosis after the age of 60 years, of which presentations ranged from mild neurocognitive disorder with schizophrenia spectrum disorder to mild neurocognitive disorder with psychosis, pure schizophrenia, delusional disorder, brief psychotic disorder, adjustment disorder with psychosis and initial anxiety with persisting psychosis (Van Assche et al. Reference Van Assche, Van Aubel, Van de Ven, Bouckaert, Luyten and Vandenbulcke2019). This diversity in presentation calls into question the categorisation of these patients under the same diagnosis, and the validity of comparison to dementia patients in this capacity.
In most studies, VLOSLP patients were selected on the basis of showing no evidence of neurological disorder; however, it was not established if imaging or biomarker investigations were conducted during the diagnostic process. Furthermore, as EOS, LOS and VLOSLP are distinguished by cut-offs for onset age, diagnostic delays or missed diagnoses can additionally conflate one condition with another.
While trends in clinical presentation, such as lack of negative symptoms, have been identified, the current lack of specificity in diagnostic guidelines creates uncertainty around the selection of VLOSLP patient samples, compromising the validity of findings even in well-designed studies. More research is therefore required to characterise VLOSLP as a clinical syndrome to strengthen the diagnostic process and enhance the applicability of subsequent findings.
Longitudinal cohort studies comparing the progression of clinical presentation, cognition and pathology in VLOSLP patients to that of EOS/LOS patients over time are needed to better define VLOSLP as a clinical syndrome and establish more robust selection criteria. Following that, studies comparing VLOSLP to neurodegenerative disease using standardised assessment tools and neuropathological investigations could shed light on any shared or diverging pathophysiology.
As older age groups are the fastest growing demographic in the world, VLOSLP and neurodegenerative conditions may become increasingly prominent in the global healthcare landscape. Managing VLOSLP is challenging as patients typically lack insight (Folsom et al. Reference Folsom, Lebowitz, Lindamer, Palmer, Patterson and Jeste2006) and are difficult to engage with (Sin Fai Lam et al. Reference Sin Fai Lam, Reeves, Stewart and Howard2016) and often have morbidities and concurrent drug treatments increasing the risk of antipsychotic drug-related morbidity and mortality (Reeves et al. Reference Reeves, Eggleston, Cort, McLachlan, Brownings, Nair, Greaves, Smith, Dunn, Marsden, Kessler, Taylor, Bertrand and Howard2018). This difficulty in engagement, amplified by a level of cognitive impairment in this patient cohort, could necessitate more assertive engagement methods, as successfully demonstrated with Early Intervention in Psychosis services for EOS patients (Neale & Kinnair, Reference Neale and Kinnair2017). Longitudinal follow-up should also be considered in VLOSLP patients who have lost contact with services, using methods such as linkage to national mortality databases, in order to better establish morbidity and mortality outcomes in disengaged patients.
Acknowledgements
The authors acknowledge the constructive criticism offered by the peer reviewers; their input unquestionably helped produce a more robust article.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committee on human experimentation with the Helsinki Declaration of 1975, as revised in 2008.
Author contribution statement
All authors declare that they have met the ICMJE criteria for authorship.
VY was responsible for the acquisition of data, analysis and interpretation, drafting of article, critical revision and approval of version of manuscript to be published. SFL was responsible for the acquisition of data, critical revision and approval of the manuscript. JK was responsible for the critical revision and approval of the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ipm.2021.48





