Introduction
There is a growing body of literature on cervicogenic headache and an increasing acceptance that headache can originate from the cervical spine. Diagnostic criteria have been established by a variety of expert groups 1 - Reference Sjaastad, Fredriksen and Pafeffenrath 7 all of which agree that these headaches originate in the neck or occipital region and implicate cervical paraspinal tissues. Prevalence estimates range from 0.4% to 2.5%Reference Sjaastad and Fredriksen 8 , Reference Nilsson 9 in the general population, and up to 15% to 20% 4 , Reference Nilsson 9 of patients with chronic daily headache. Cervicogenic headache affects four females for one male and peaks at a mean age of 42. Although its etiology remains a source of controversy, 4 , Reference Merskey and Bogduk 6 , Reference Fredriksen, Fougner and Tangerud 10 , Reference Merskey and Bogduk 11 structures implicated in the genesis of cervicogenic headache have their sensory input at the level of C1-C2-C3, and converge within the spinal nucleus of the trigeminal nerve to form the trigeminal-cervical complex, which is a common final pathway for many types of headache.Reference Bovim, Berg and Dale 12 - Reference Hack 14
Despite the significant impact of chronic daily cervicogenic headache, a relative paucity of studies has been published examining the use of pharmacotherapy for its relief. In our clinical experience chronic cervicogenic headache seems unresponsive to common headache medication.
Peripheral nerve injury, as may be occurring in cervicogenic headache, can lead to persistent neuropathic pain in which allodynia, hyperalgesia, hyperesthesia, hypoesthesia or dysesthesia may develop. Experiments have demonstrated a marked up regulation of the alpha-2-delta calcium channel subunit after peripheral nerve injuryReference Martelletti, Di Sabato and Granata 13 , Reference Luo, Chaplan and Higuera 15 - Reference Dooley, Donavan, Meder and Whetzel 18 and an improvement of tactile allodynia secondary to neuropathic injury when these calcium channel subunits are targetedReference Wallin, Cui, Yakhnitsa, Schechtmann, Meyerson and Lenderoth 19 , Reference Field, McCleary, Hughes and Singh 20 for treatment. Systematic and pragmatic reviews have lamented the poor quality of the literature and the fact that most treatments lack a compelling evidence base.Reference Bogduk 21 Given that pregabalin, a potent ligand for the alpha-2-delta calcium channel subunit that exhibits analgesic properties,Reference Ben-Menachem 22 we posited that it could be effective in reducing the frequency of chronic daily unilateral cervicogenic headache, caused by a C2 radiculopathy.
Methods
Study Design
The overall study design was a single center, double -blind, randomized, placebo-controlled, parallel-group study. The criteria of the Cervicogenic Headache International Study Group (7) were employed to diagnose patients. Clinical signs of C2 radiculopathy on the painful side only were obligatory.
Patients were randomized to placebo or to a pregabalin regimen (1:1) consisting of a flexible schedule with escalation based on patient’s individual response and tolerability (30 days). The full study duration was 16 weeks: 4 weeks for the baseline period and 12 weeks of active treatment.
The study was conducted in compliance with good clinical practice guidelines and in accordance with the regulations of the declaration of Helsinki and approved by an institutional review board. Study subjects provided informed consent prior to enrollment.
Subjects
Sixty subjects, 18-70 years of age, were screened with the objective of randomizing 40 patients suffering chronic unilateral cervicogenic headache, and willing to participate in a placebo-controlled study for approximately 12 weeks, using pregabalin as the active drug. Inclusion criteria stipulated that patients suffer headache for more than three months, 15 or more headache days per four week period, with each day consisting of four or more hours of continuous headache of moderate to severe intensity measured with a numerical rating scale (NRS) defined as moderate (4-7/10 on NRS) to severe (8-10/10 on NRS) in both groups at screening, refractory to standard treatment such as neuromodulators, muscle relaxants, tricyclics, non steroidal anti inflammatory drugs since onset, with fifteen or more neck pain days per four week period, with each day consisting of four or more hours of continuous neck pain. Patients were further required to have an abnormal neck examination with clinical signs of neuropathic involvement (i.e., hyperesthesia, hypoesthesia, dysesthesia, allodynia or hyperalgesia on the painful side of the head and/or neck, in the C2 and C3 territory). Patients were excluded if they presented bilateral headaches, migraine, cluster headaches, hemicrania continua, or chronic paroxysmal hemicranias. Patients with a secondary headache in relation to any intracranial pathology, patients with secondary cervical pain in relation to tumors, neoplasm or infection were also excluded.
Also excluded from the study were all patients scheduled for somatic nerve blocks during their participation in the trial and patients who had undergone C2-C3 rhyzotomy by thermocoagulation of the medial sensory root, or facet joint, in the past six months. Patients were also excluded if they had undergone somatic nerve blocs of the neck in the past four weeks with a local anesthetic, or in the past six months with a local anesthetic and cortisone. Patients with fibromyalgia or those who had abused illicit drugs or alcohol within the last year and patients on a continuous opiate regimen for chronic pain or using episodic rescue medication for more than 15 days/month were excluded. Other exclusion criteria were a serious psychiatric condition, females who were pregnant or nursing and patients not using reliable contraception.
Treatment
Study medications consisted of pregabalin 75 mg and 150 mg, capsules and matching placebo capsules, all identical in appearance in order to preserve study blinding. After a −28 day baseline period, patients were randomly assigned to their treatment regimens (pregabalin/placebo in a 1:1 ratio), according to a computer-generated randomization list. Following randomization, an escalation phase consisted of three consecutive ten-day periods during which the dose was escalated. Dosage could be increased in four steps (150 mg, 225 mg, 300 g, 450 mg) but only decreased once (by only one step) during the escalation phase.
During the first 30 days on study treatment (escalation phase), two phone contacts were made, producing the three ten-day treatment periods. During each telephone contact the patients were instructed to return to the clinic for dose adjustment depending on individual tolerability and efficacy on the study medication and/or study medication replacement. If the patient had difficulty tolerating the escalated dose (i.e., 225 mg/day), then the patient could return to 150 mg/day, or choose to remain at 225 mg/day. Patients who elected to escalate their dose were instructed to take 300 mg/day for seven days, and then 450 mg/day (one 150 mg capsule in the morning and two at night) for the following three days. Patients were contacted by the research assistant by telephone and were given a final opportunity to adjust their dose. During the maintenance phase, doses were fixed at 150 mg/day, 225 mg/day, 300 mg/day, or 450 mg/day without possibility of dose adjustment.
Patients were not allowed to use preventive medication for their head and neck pain within the 28 days prior to randomization. Rescue medication such as non steroidal anti-inflammatory drugs or combination analgesics were not allowed more than 12 days per month. Non-headache medications had to be at a stable dose for at least one month prior and during the conduct of the trial.
Outcome measures
The primary efficacy variable was change from baseline in frequency of chronic cervicogenic headache per each 28-day period during the maintenance phase. Change from baseline in intensity of cervicogenic headache during the maintenance phase was included as a secondary efficacy variable.
Health outcome measures were: headache diaries for frequency and intensity (NRS 1-10/10) of cervicogenic headache, Headache Impact Test (HIT-6). At baseline a mean total HIT-6 score of more than 60 indicates severe headache impact Each HIT-6 question scored as never (6) rarely (8) sometimes (10) very often (11) or always (13) will add to a total score of 36-78.Reference Sauro, Rose and Becker 23 The Hospital Anxiety and Depression scale (HAD) is a structured auto questionnaire of 14 items, designed to identify the presence of anxiety and/or depression.Reference Zigmond and Snaith 24 With a score of less than 7 there is absence of psychopathology With a score of more than 8 specificity and sensitivity is high, with a maximum possible score of 21. The higher the score, the more severe the pathology. The EQ-5D Visual analogue scale, is a self classified health state profile measuring quality of health in % ( 0% being the worst and 100% being the best health state profile).
Tolerability and safety
Tolerability and safety measures were recorded in the Case Report Form for adverse events or serious adverse events. Adverse events were collected for 30 days following the final study visit. Physical examination, urine pregnancy tests (for all females of childbearing potential) and vital signs were monitored throughout the study. All adverse events occurring during the study were classified on the basis of Medical Dictionary for Regulatory Activities (MedDRA) terminology.
Statistics
For the purpose of statistical analyses, subjects were classified into one of the following groups: the modified intent-to-treat (mITT) population, the completers population, and the safety population.
The modified intent-to-treat (mITT) population consisted of all patients who received at least one dose of pregabalin or placebo and had at least one efficacy evaluation following a minimum of 14 days (owing to natural variations in cervicogenic headache frequency from week to week) of study medication during the maintenance phase of the study. All efficacy analyses (primary and secondary) were based on this population. The patients were analyzed according to the randomization assignment (pregabalin or placebo), regardless of actual treatment received.
Completers were patients who took study drug consistent with all study procedures and who had taken the study medication for the full 12 weeks. Confirmatory efficacy analyses were performed on this population.
Safety analysis included all patients assigned a subject number and who received at least one dose of study medication. The patients were analyzed according to the randomization assignment (pregabalin or placebo) regardless of actual treatment and dosage received.
Handling of Missing Data
During the maintenance phase, patients had to have a minimum of 14 efficacy measurements (i.e., days) for each 28-day period for efficacy analysis. If data was unavailable from the final 28-day period, then data from the patient’s final 28 days on protocol was used (assuming at least 14 days of data was available). Patients with no on-treatment data for a parameter were excluded from the analysis of that parameter.
Primary Efficacy Analysis
The primary efficacy endpoint was the change from baseline in number of cervicogenic headache days per each 28-day period. A headache day was defined as a day (00:00 to 23:59) with four or more continuous hours of headache per patient diary. Comparison between treatment groups was conducted using an analysis of covariance adjusting for the baseline frequency of headache.Reference Oakes and Feldman 25 - Reference Stephen 27
Study Success Criteria for Efficacy
A conclusion of superiority of pregabalin relative to placebo was reached if the group differences in (baseline adjusted) mean changes from baseline to each 28-day period in frequency of cervicogenic headache for pregabalin were found to be statistically significantly decreased when compared to placebo.
Non Parametric Analysis
Other supplemental non-parametric analysis was performed. Tests of normality for the primary endpoint were performed. Assessments included histograms and QQ-plots as well as test checks for normality with the Shapiro-Wilk test and the Kolmogorov-Smirnov test. If the normality assumptions were not tenable, corresponding non-parametric methods were implemented.
Secondary Efficacy Endpoint Analysis
The secondary efficacy endpoints were the change from baseline in intensity of cervicogenic headache during the maintenance phase. The mean intensity for each period (screening, escalation, maintenance) was determined, using the numerical rating scale (NRS). Formal statistical testing was based on the results from a linear mixed model for repeated measures. This mixed model expressed the (mean) headache intensity as a linear function of treatment, time, treatment-by-time interaction, screening phase (baseline) headache intensity, and a random patient effect. The difference between treatment groups was expressed by the treatment-by-time interaction.
The frequency of headache was based on the results from a linear mixed model. The HIT-6 for the impact of headache on patients’ lives was determined with a linear mixed model for repeated measures as above. The HAD scale for depression and anxiety, with an analysis of covariance adjusting for the baseline HAD score. The EQ-5D, was assessed with the health states converted into a weighted health state index by applying scores from the EQ-5D preference weights elicited from general United States population samples in order to give an EQ-5D index score to be analyzed with a t-test, as well as for the visual analog scale (VAS)-based health state score. The presence of over dispersion was checked and accounted for the patient’s Global Impression of Change (PGIC), with treatment groups compared.
Results
Populations and demographics
Pregabalin and placebo groups were similar. Forty one patients were randomized, 34 were part of the mITT population (18 pregabalin, 16 placebo), Table 1. Sixty seven percent of patients were females and 33% were males. Ninety four percent of patients were Caucasian, and 6% were Hispanic. The mean age of patients in the placebo group was 45 years old and in the pregabalin group the subjects mean age was 58 years-of-age, Table 2. No patients suffered migraine but two patients had a family history of headache. More placebo patients than pregabalin patients discontinued the study for lack of efficacy. Neurologic and musculoskeletal abnormalities at baseline were not different between the two groups. The maximum dose of pregabalin for efficacy and tolerability was a 450 mg/day regimen, Table 3.
Table 1 Patient disposition
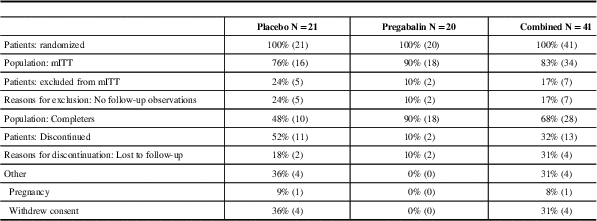
mITT=modified intent-to-treat
N=the number of subjects analysed.
Table 2 Patient Demographics and Baseline Characteristics - mITT Population
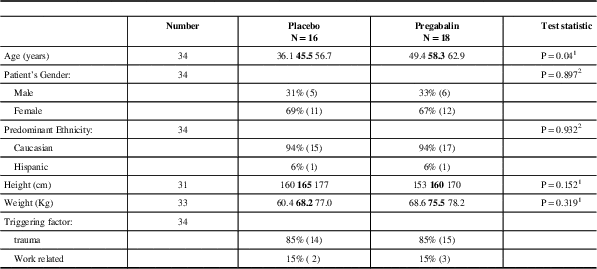
1 =Wilcoxon Test
2 =Pearson Test
N=the number of subjects analysed.
Table 3 Pregabalin doses achieved after escalation - m-ITT population
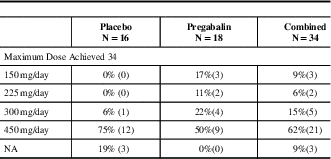
N=the number of subjects analysed.
Primary efficacy endpoints
Both groups had on average 26 headache-days per 28 day period at screening. This number dropped to 16 for the pregabalin group by the end of the study while remaining stable for the placebo group, Table 4. There was, however, no statistical difference between groups for the first 28-day period (p=0.058), while the pregabalin group had significantly less headache-days during the second period (p=0.013)), compared to the placebo group.
Table 4 Frequency of Headache-Days/month - mITT Population

N=the number of subjects analysed. Test used: t-test
Secondary end points
The treatment-by-time for the intensity of cervicogenic headache between the two groups showed a statistical difference,Table 5. During the baseline phase, the mean pain intensity was of moderate intensity (4-7/10 on the NRS). During the maintenance phase, for the pregabalin group, mean pain intensity changed from moderate pain to mild pain (1-3/10 on NRS) of short duration mostly upon awakening, compared to the placebo group that continued with a moderate intensity (4-7/10 on NRS) of pain throughout the day.
Table 5 Intensity of headache - mITT Population
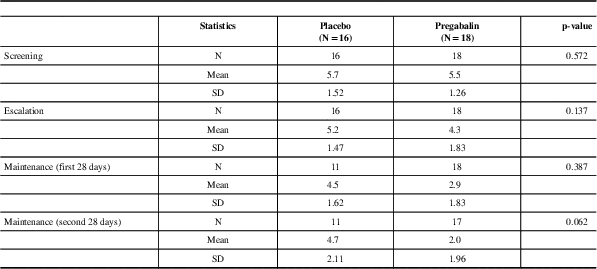
N=the number of subjects analysed. Test used: t-test
NRS scale of 10 (1-3/10: mild) (4-7/10: moderate) (8-10/10: severe); SD=standard deviation
We assessed change from baseline in Headache-Related disability. The HAD scores and the HIT-6 scores are seen in Table 6 and the EQ-5D VAS self-classified health state profiles are presented in Table 7.
Table 6 Change from baseline in headache-related disability: Pregabalin versus Placebo. mITT Population

HAD=Hospital Anxiety and Depression scale; HIT-6=Headache Impact Test
* VAS (visual analog scale): 0%=worst value, 100%=better value
Table 7 Patient Global Impression of Change (PGIC), - mITT Population
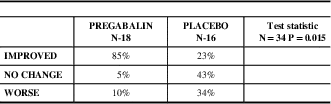
For the global impression of change there was a significant difference between the two treatment groups (p=0.015). Patients on pregabalin said they improved more (85% improved ) than the placebo group (23% on placebo). Safety analysis included all patients assigned a subject number and who received at least one dose of study medication. There were no serious adverse events, 5% of patients on placebo and pregabalin had severe adverse events, 15% on placebo and 35% on pregabalin suffered moderate adverse events and 35% of patients on pregabalin had mild adverse events, Table 8 and 9.
Table 8 Summary of Adverse Events - Safety Population
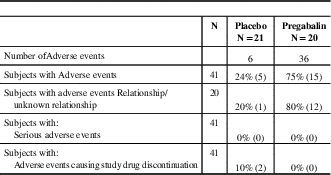
N=the number of subjects analysed.
Table 9 Summary of Adverse Events by System Organ Class - Safety Population

Most frequent adverse events
N=the number of subjects analysed.
Discussion
Headaches, frequently occipital or hemicranial, may be associated with disorders of the cervical spine.Reference Edmeads and Soyka 28 The term cervicogenic refers to the suspected origin of the pain. The obligatory symptoms of cervicogenic headache are: a unilateral pain constantly on the same side, aggravated by particular movements of the cervical spine or external pressure applied to the ipsilateral neck or occipital region. It is experienced as a continuous unilateral cervical and hemicranial pain. Occipital nerve, facets, and nerve roots may respond to blockade with local anesthetics (refer to reference 7 for details)
The pain is dull, tearing, boring, and burning in quality. An important diagnostic feature of cervicogenic headache is a transient pain-free interval after anesthetic blockade of the C2 roots or the greater occipital nerve on the symptomatic side.
Cervicogenic headache is sometimes preceded by cervical whiplash injury as those subjects who suffer acute neck pain after whiplash have a three times greater risk of developing chronic neck pain. According to the Oslo whiplash study, cervicogenic headache was diagnosed in 8% of whiplash patients after 6 weeks.Reference Pollmann, Keidel and Pfaffenrath 29 In post-traumatic headache, symptoms were identical to cervicogenic headache in many instances but unilaterality of pain occurred in 24% of patients.Reference Magnusson 30 The pain of cervicogenic headache appears to be transmitted via thin unmyelinated C-fibers.Reference Groenbaek 31 Pain impulses from the occipital region of the head and neck enter the spinal cord via the C2-C5 dorsal rami,Reference Kerr 32 , Reference Bogduk 33 the C2 root probably plays a central role in pain transmission. Intrathecal nerve roots are more vulnerable to injury than peripheral nerves due to their lesser connective tissue covering and their greater vascularity,Reference Rydevic, Brown and Lundborg 34 and an injured nerve root and dorsal root ganglion may be a cause of chronic pain secondary to neck sprain.Reference Taylor, Twomey and Kakula 35
In this study, pregabalin improved the frequency of headache days by ten days per month in the second month; which is remarkable, considering the long-term potentiation and wind-up in these patients. Headache intensity reduced from moderate to mild compared to placebo. Patents on pregabalin who were depressed or anxious improved; possibly because of pain reduction, improved sleep, increased hope of improvement or because of the anxiolytic properties of pregabalin. For many patients on pregabalin, the headache impact test improved, reducing their score to less than 60. Results measured with the Patients Global Impression of Change were significantly in favor of active pregabalin treatment. Seventy five percent of patients on placebo escalated to the maximum dose of 450 mg. and 52% of patients on placebo discontinued because of lack of efficacy or withdrew consent.
The most common adverse events were: somnolence, nausea, and peripheral edema, even though patients in the active group maintained their participation because of the benefit they obtained. The occasional patient with ankle edema suspected he was on the active drug.
Patient selection in this study was very difficult for four reasons: No patients with migraine were included and family history of migraine was scrutinized. Previous use of pregabalin was not permitted; pregabalin is a frequently prescribed drug. Patients refused to participate because of a 1:1 risk of receiving a placebo and many patients were desperate and in need of an active treatment for their chronic daily cervicogenic headache.
Conclusion
Forty one patients were recruited. In this study both the placebo and pregabalin groups had, on average, 26 headache days per month at baseline. The difference between placebo and pregabalin was statistically significant only for the second half of their maintenance period, reducing the number of headache days per month by ten days. In the first half of the maintenance phase a reduction of seven days per month also showed a trend of improvement. There are no significant studies on the use of medication for cervicogenic headache. In our clinical experience, chronic cervicogenic headache seems unresponsive to common headache medication. Patients with chronic daily cervicogenic headache are severely afflicted but a change from baseline in the headache-related disability occurred in the pregabalin group. Their involvement in a double blind clinical study was very difficult. Although this was a small study, positive results suggest that large multi-site trials are warranted.
Acknowledgements and Funding
This study was funded by Pfizer Inc., Québec, Canada, through an unrestricted research grant. Biostatistics: Integrated Research, Dollard-Des-Ormeaux (Québec) Canada.











