- LPS
lipopolysaccharide
Obesity is one of the major current public health problems because of its increasing prevalence and association with important chronic disorders(Reference James1). These include type-2 diabetes mellitus, atherosclerosis, CVD, non-alcoholic fatty liver disease and cancer. Obesity is the result of a long-term positive imbalance between energy intake and expenditure, which is regulated by multiple pathways involving metabolites, hormones and neuropeptides. High-fat diet-induced obesity and metabolic disorders are also associated with a state of chronic low-grade inflammation and increased susceptibility to infection, due to malfunction of the immune system. Obese individuals have increased macrophage infiltration in the adipose tissue along with the production of inflammatory adipokines, cytokines and associated immune factors. Inflammatory immune mediators (e.g. C-reactive protein, TNFα, IL-6 and monocyte chemotactic protein 1) and some adipokines (e.g. leptin) are usually elevated in obese mice and human subjects, whereas the production of the anti-inflammatory and insulin-sensitizing adipokine adiponectin is reduced(Reference Zeyda and Stulnig2). In fact, chronic activation of the innate immune system is regarded as a risk factor for the development of obesity and associated disorders; this activation might partly depend on the immunomodulatory effects exerted by dietary compounds in the gut and beyond(Reference Zeyda and Stulnig2).
The human intestinal tract is populated by a vast number of bacterial species that reach concentrations ranging from 107 to 1012 cells/g intestinal content, from the small intestine to the colon. This microbial community develops with its host throughout life by establishing mutualistic symbiotic relationships which favour their co-existence(Reference Xu, Mahowald and Ley3). The collective genome (microbiome) of the gut microbiota contains at least 100 times as many genes as the human genome, providing additional features and contributing to human physiological diversity(Reference Gill, Pop and Deboy4, Reference Turnbaugh, Ley and Hamady5). In particular, the gut microbiota has been considered to be a possible causative factor of metabolic conditions as well as a therapeutic target in recent years(Reference Turnbaugh, Ley and Hamady5). Herein, the proposed modes of action of the gut microbiota in obesity and associated-metabolic disorders and the effects of interventions with the probiotic, prebiotics and synbiotics are reviewed.
Obesity, weight loss and gut microbiota composition
Obesity is associated with phylum and group-specific changes in the microbiota, and with reduced bacterial diversity(Reference Turnbaugh, Hamady and Yatsunenko6, Reference Waldram, Holmes and Wang7). Increases in the relative abundance of Firmicutes and reductions in Bacteroidetes have been associated with obesity by comparisons between the distal gut microbiota of genetically obese ob/ob mice (leptin deficient) and their lean (ob/+ or +/+) littermates using DNA sequencing techniques(Reference Ley, Bäckhed and Turnbaugh8). A higher proportion of Archaea was also found on the caecal microbiota of these genetically obese mice in comparison with their lean littermates(Reference Samuel, Hansen and Manchester9). Diet-induced obesity in mice has also been associated with an increased proportion of Eubacterium dolichum, belonging to the Firmicutes division(Reference Turnbaugh, Bäckhed and Fulton10). Compared to lean rats, obese Zucker rats (fa/fa) showed reduced Bifidobacterium counts quantified by fluorescence in situ hybridization and increased abundance of Halomonas and Sphingomonas, detected by PCR and denaturing gradient gel electrophoresis(Reference Waldram, Holmes and Wang7). Obesity induced by a high-fat diet was also associated with lower Bifidobacterium numbers in caecal content in mice(Reference Cani, Amar and Iglesias11).
Similar alterations in the relative proportions of Firmicutes and Bacteroidetes in faeces have been associated with human obesity(Reference Ley, Turnbaugh and Klein12). In addition, obese human adults submitted to a hypoenergic diet (either low carbohydrate- or low-fat diet) showed significant increases in the faecal proportions of Bacteroidetes parallel to weight loss over a 1-year-long intervention(Reference Ley, Turnbaugh and Klein12). Furthermore, a lower proportion of Bacteroidetes and a higher proportion of Actinobacteria have been associated with obesity by comparisons between the faecal microbiota of obese and lean twin human subjects(Reference Turnbaugh, Hamady and Yatsunenko6). A larger-scale intervention trial has recently demonstrated that both an energy-restricted diet and increased physical activity induce changes in the gut microbiota structure of obese adolescents, correlated with weight loss and BMI Z-score reductions(Reference Nadal, Santacruz and Marcos13, Reference Santacruz, Marcos and Wärnberg14). Clostridium histolyticum, Clostridium lituseburense and Eubacterium rectale–Clostridium coccoides proportions dropped significantly, while those of the Bacteroides–Prevotella group increased after the intervention in those adolescents that experienced significant weight reduction (8·1% of their body weight) as determined by fluorescence in situ hybridization(Reference Nadal, Santacruz and Marcos13). When the microbiota was analysed by quantitative real-time PCR, increased Bacteroides fragilis and Lactobacillus group numbers and reduced C. coccoides and Bifidobacterium longum group numbers were detected in those adolescents that experienced important weight loss after the intervention(Reference Santacruz, Marcos and Wärnberg14). Moreover, the effectiveness of lifestyle intervention on body-weight loss seems to be influenced by the composition of the individual's microbiota(Reference Santacruz, Marcos and Wärnberg14). Alterations in the faecal microbiota composition also seem to precede overweight in children, early in life. Children maintaining normal weight showed an increased number of Bifidobacterium, whereas children becoming overweight showed an increased number of Staphylococcus aureus in faeces during infancy(Reference Kalliomäki, Collado and Salminen15).
Perturbations in the composition of gut microbiota associated with genetic or diet-induced obesity seem to be reversible by oral transfer of the gut microbiota from lean or obese mice to a germ-free recipient(Reference Turnbaugh, Bäckhed and Fulton10, Reference Turnbaugh, Ley and Mahowald16) or by the administration of prebiotic substrates to animal models at least over short-term periods(Reference Cani, Neyrinck and Fava17). Studies on the evolution of mammals and their gut microbes by DNA sequencing also indicate that the diet is a fundamental promoter of change in gut bacterial diversity(Reference Ley, Lozupone and Hamady18). Altogether, this evidence supports the hypothesis that the modulation of gut microbiota via dietary intervention is a potential strategy to help manage obesity and metabolic-associated disorders(Reference Turnbaugh, Bäckhed and Fulton10, Reference Turnbaugh, Ley and Mahowald16, Reference Cani, Neyrinck and Fava17), although actual proof is still limited.
Role of the gut microbiota in nutrient metabolism and energy storage
The intestinal microbiota develops an important biochemical activity within the human body by providing additional metabolic functions(Reference Gill, Pop and Deboy4) and regulating the diverse aspects of cellular differentiation and gene expression via host–microbe interactions(Reference Hooper, Midtvedt and Gordon19). In fact, comparisons between germ-free mice and mice colonized by the conventional distal gut microbiota showed that the microbiota, as a whole, increases the host's ability to extract energy from the diet and store this energy in adipocytes, contributing to body-weight gain(Reference Bäckhed, Ding and Wang20). The intestinal microbiota provides enzymes involved in the utilization of non-digestible carbohydrates and host-derived glycoconjugates, deconjugation and dehydroxylation of bile acids, cholesterol reduction and biosynthesis of vitamins (K and B group), isoprenoids and amino acids (e.g. lysine and threonine)(Reference Gill, Pop and Deboy4, Reference Hooper, Midtvedt and Gordon19). In particular, the ability of the commensal microbiota to utilize complex dietary polysaccharides which would otherwise be inaccessible to human subjects and to generate SCFA seems to contribute to the ability of the host to harvest energy from the diet(Reference Bäckhed, Ding and Wang20). This may represent 10% daily energy supply in omnivores and up to 70% in herbivores(Reference Flint, Bayer and Rincon21). Specific components of the commensal microbiota also regulate serum lipids and cholesterol by taking part in bile-acid recycling and metabolism. Bacterial enzymes mainly catalyse the deconjugation and dehydroxylation of bile acids, which alter the solubilization and absorption of dietary lipids throughout the intestine(Reference Ridlon, Kang and Hylemon22). Faecal commensal bacteria also reduce cholesterol to coprostanol and, thus, increase its excretion in faeces(Reference Norin23).
In addition, the commensal microbiota and its metabolites regulate the expression of genes involved in the processing and absorption of dietary carbohydrates and complex lipids in the host, favouring fat storage(Reference Bäckhed, Ding and Wang20, Reference Hooper, Wong and Thelin24). The expression of a monosaccharide transporter (Na+/glucose co-transporter) has been induced in Bifidobacterium thetaiotaomicron mono-colonized mice, leading to increased absorption of dietary monosaccharides and SCFA and, thereby, promoting de novo synthesis of lipids in the liver(Reference Hooper, Wong and Thelin24). In fact, the colonization of germ-free mice by the conventional microbiota leads to increased liver expression of key enzymes (acetyl-CoA carboxylase and fatty acid synthase) involved in de novo fatty acid biosynthetic pathways and the transcriptional factors (carbohydrate response element-binding protein and sterol regulatory element-binding protein-1) involved in hepatocyte lipogenic responses to insulin and glucose(Reference Bäckhed, Ding and Wang20). Furthermore, microbial colonization reduces the levels of circulating fasting-induced adipose factor in the gut, skeletal muscle and liver levels of phosphorylated AMP-activated protein kinase, which jointly contribute to reducing fat oxidation and enhancing fat storage(Reference Bäckhed, Manchester and Semenkovich25).
Role of the gut microbiota in neurohormonal function
The gut microbiota could also interact with the production and function of hormones and neuropeptides synthesized by the nervous system and enteroendocrine cells of the gastrointestinal tract mucosa and peripheral organs (adipose tissue, pancreas and liver), which are critical to the regulation of energy balance.
Colonization of the germ-free intestine of mice by conventional microbiota stimulates adipokine leptin synthesis, with a proportional increase in body fat and insulin resistance(Reference Bäckhed, Ding and Wang20). Although leptin is the dominant long-term signal informing the brain of energy stores and inhibiting food intake, leptin deficiency is not a common cause of obesity but leptin resistance is(Reference Matarese and La Cava26). Obese subjects usually have increased serum leptin levels associated with increased hunger and reduced energy expenditure. Increased leptin levels could also induce the production of pro-inflammatory T-helper 1-type cytokines and contribute to the inflammatory tone associated with obesity(Reference Matarese and La Cava26). SCFA, which are mainly produced by the gut microbiota, act as ligands for G protein-coupled receptors, such as Gpr41, expressed in the intestine, colon and adipocytes, which upon activation stimulate the expression of peptide hormones (e.g. leptin and peptide tyrosine–tyrosine) involved in appetite and energy metabolism(Reference Samuel, Shaito and Motoike27). In particular, Gpr41-deficient mice show a reduced expression of peptide tyrosine–tyrosine, which modulates gut motility and reduced harvest of energy from the diet, in a microbiota-dependent manner. Autoantibodies against key appetite-regulating neuropeptides and peptide hormones (e.g. alpha-melanocyte-stimulating hormone, neuropeptide Y, agouti-related protein, ghrelin and leptin) have also been detected in the sera of human subjects and rats(Reference Fetissov, Hamze-Sinno and Coëffier28). The sequence homology found between these neuropeptides and proteins from some members of the intestinal microbiota would suggest that the microbiota could influence their production and, therefore, eating behaviour. Mice infected with Helicobacter pylori showed delayed gastric emptying, increased visceral perception and abnormal feeding patterns(Reference Bercik, Verdú and Foster29). Feeding behaviour remained altered for up to 2 months post-infection, possibly due to altered gastric mechanosensitivity, increased postprandial cholecystokinin release inducing satiety and increased TNFα expression in the central nervous system(Reference Verdu30). However, the administration of Lactobacillus strains after H. pylori eradication normalized the feeding behaviour(Reference Verdu, Bercik and Huang31).
Interactions between the gut microbiota composition and stress-related hormones, which affect energy balance, have also been identified. Stress at late stages during pregnancy, parallel to elevated cortisol plasma levels, was found to lead to reductions in faecal Bifidobacterium counts in infant monkeys(Reference Bailey, Lubach and Coe32). Stress induced in male rat pups by maternal separation early in life also led to increased plasma corticosterone and the systemic immune response with alterations in the faecal microbiota compared to the control group(Reference O'Mahony, Marchesi and Scully33). In germ-free mice, higher plasma adrenocorticotropic hormone and elevated corticosterone were detected in response to restraint stress as compared to conventional mice(Reference Sudo, Chida and Aiba34). However, the excessive hypothalamic–pituitary–adrenal stress response in germ-free mice was reversed by inoculation with a Bifidobacterium infantis strain. Glucocorticoids are well known for their critical role in metabolism and, in particular, alterations in tissue-specific cortisol levels influencing lipogenic and gluconeogenetic pathways in fat and liver, associated with obesity and the development of insulin-resistance(Reference Simonyte, Rask and Näslund35).
Role of the gut microbiota in immune function
Obesity induced by high-fat diets and the associated metabolic disorders are characterized by a state of low-grade inflammation which has been related to alterations in the gut microbiota composition and increased plasma lipopolysaccharide (LPS) levels(Reference Cani, Amar and Iglesias11, Reference Cani and Delzenne36). Mice fed a high-fat diet exhibited a significant increase in plasma LPS, which was termed ‘metabolic endotoxemia’, associated with changes in the gut microbiota (reductions in Bifidobacterium and E. rectale/C. coccoides). A mouse model chronically infused with a dose of LPS to reach the same plasma LPS levels as those measured in the high-fat-diet-fed mice also mimicked the phenotype of high-fat-fed mice. This was characterized by fasting hyperglycaemia, obesity, steatosis, adipose tissue macrophages infiltration, hepatic insulin resistance and hyperinsulinemia. Furthermore, mice knocked out in CD14, a key molecule in Toll-like receptor 4 signalling, were completely resistant to the development of inflammation induced by both high-fat feeding and chronic LPS administration in the visceral and subcutaneous adipose depots, the liver and the muscle(Reference Cani and Delzenne36). In contrast, the inhibition of the gut microbiota by antibiotic administration (norfloxacin and ampicillin) in two different mouse models of insulin resistance resulted in reduced serum LPS levels, low-grade inflammation, obesity and type-2 diabetes(Reference Kalliomäki, Collado and Salminen15). Altogether, these findings demonstrate the link between the gut microbiota, LPS and certain metabolic disorders. In human subjects, increased LPS plasma levels have also been associated with an elevated BMI and high-fat feeding(Reference Lajunen, Vikatmaa and Bloigu37, Reference Erridge, Attina and Spickett38). These increased LPS concentrations were sufficient to activate the synthesis of inflammatory cytokines (e.g. TNFα) by monocytes in vitro. Therefore, metabolic endotoxemia has also been considered a possible factor contributing to the postprandial inflammatory state, which could favour certain chronic disorders, including type-2 diabetes and atherosclerosis in human subjects(Reference Erridge, Attina and Spickett38).
The colonization of the germ-free mouse intestine also regulates the expression of serum amyloid A proteins, which are mediators of inflammation and metabolism and whose serum levels are increased in subjects with obesity, chronic hyperglycaemia, insulin resistance and CVD(Reference Reigstad, Lundén and Felin39). The serum amyloid A3 protein expression was significantly augmented in adipose and colonic tissues by the presence of intestinal microbes, when comparing germ-free and conventionally raised mice. The authors propose that LPS, and potentially other products of gut bacteria, activate Toll-like receptors and mediate signalling through MyD88 and NF-κB to promote increased serum amyloid A3 and pro-inflammatory cytokine expression (e.g. TNFα), thereby exacerbating the chronic low-grade inflammation associated with obesity(Reference Reigstad, Lundén and Felin39).
Effects of probiotics and prebiotics on obesity and metabolic disorders in animals
A summary of trials evaluating different modes of action of classical probiotics (Lactobacillus and Bifidobacterium strains), prebiotics or a combination thereof synbiotics, on diverse biomarkers of body-weight balance, immunity and metabolism in conventional animals and animal models of obesity, diabetes and hyperlipidemia is shown in Table 1. For example, feeding rats with skim milk fermented by Lactobacillus gasseri SBT2055 led to reduction in adipocyte size and increased numbers of small adipocytes in white adipose tissue, also reducing serum leptin concentrations compared with control rats, suggesting that the probiotic plays a role in regulating adipose tissue growth(Reference Sato, Uzu and Yoshida40). Dietary supplementation of high fructose-induced diabetes and streptozotocin-induced diabetes in rats with a probiotic product (dahi) containing Lactobacillus acidophilus NCDC14 and Lactobacillus casei NCDC19 improved the biomarkers of glucose and lipid metabolism and delayed or suppressed glucose intolerance, hyperglycaemia, hyperinsulinemia, dyslipidemia and oxidative stress(Reference Yadav, Jain and Sinhá41, Reference Yadav, Jain and Sinha42). The administration of either Lactobacillus paracasei NCC2461 or Lactobacillus rhamnosus NCC4007 to germ-free mice colonized with human baby microbiota also decreased plasma concentrations of VLDL and LDL and stimulated glycolysis(Reference Martin, Wang and Sprenger43). Similarly, when the same murine model was administered galactosyl oligosaccharides combined with L. rhamnosus NCC4007 as a synbiotic, the levels of plasma lipoproteins, hepatic TAG and kidney lipids were reduced(Reference Martin, Sprenger and Yap44). It seems that the reduction in TAG in the liver was mainly due to the prebiotic, while the decrease in plasma lipoproteins was mainly due to the probiotic L. rhamnosus. This synbiotic also induced a remarkable stimulus to both growth and activity of bifidobacteria and, in particular, of B. longum (Reference Martin, Sprenger and Yap44).
Table 1. Effects of probiotics, prebiotics and synbiotics on biomarkers of body weight, immunity and metabolism in animals
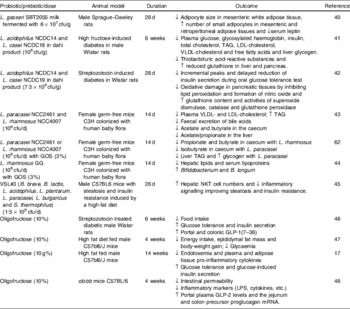
cfu, colony-forming units; GOS, galactosyl-oligosaccharides; LPS, lipopolysaccharide; NKT, natural killer T cells; ↑, increase; ↓, decrease.
The oral administration of the probiotic product VSL#3 to wild-type male C57BL6 mice fed a high-fat diet significantly improved their insulin resistance, hepatic natural killer T cell depletion and hepatic steatosis induced by the high-fat diet. This effect was natural killer T cell dependent, resulting from the attenuation of the TNFα and IκB kinase inflammatory signalling and leading to improved sensitivity in insulin signalling(Reference Ma, Hua and Li45).
Inulin-type prebiotics have also been demonstrated to modulate lipid and glucose metabolism in different animal models. Oligofructose decreases food intake, fat mass development and hepatic steatosis in normal and obese rats and mice; moreover, it exerts an anti-diabetic effect in streptozotocin-treated rats and high-fat-fed mice(Reference Cani, Daubioul and Reusens46, Reference Delmée, Cani and Gual47). The positive effects of oligofructose on diverse metabolic parameters are partly explained by its ability to regulate the expression of anorexigenic peptides, such as GLP-1 that promotes satiety, as well as other gastrointestinal peptides (such as peptide tyrosine-tyrosine and ghrelin), which could jointly be involved in controlling food intake as detected in rats(Reference Cani, Daubioul and Reusens46). Moreover, the administration of oligofructose to high-fat-fed mice increased the intestinal Bifidobacterium numbers and normalized the endotoxemia and inflammatory tone associated with the high-fat diet(Reference Cani, Neyrinck and Fava17). Furthermore, the administration of oligofructose to genetically obese mice (ob/ob) induced specific changes in the gut microbiota, characterized by increases in Lactobacillus, Bifidobacterium and C. coccoides–E. rectale groups, which led to reductions in intestinal permeability and an improvement in tight-junction integrity and inflammatory markers (plasma LPS and cytokines)(Reference Cani, Possemiers and Van de Wiele48). These effects were associated with increases in portal plasma GLP-2 levels and its precursor (the proglucagon mRNA), in the jejunum and colon.
Effects of probiotics and prebiotics on obesity and metabolic disorders in human subjects
A summary of human clinical trials that have evaluated different effects of probiotic, prebiotic and synbiotic intake on biomarkers of lipid and glucose metabolism, blood pressure and body weight is shown in Table 2. Supplementation of hypercholesterolemic patients with the probiotic bacteria Lactobacillus plantarum 299v significantly lowered serum concentrations of LDL cholesterol and fibrinogen(Reference Bukowska, Pieczul-Mróz and Jastrzebska49). A functional food product containing the same strain, L. plantarum 299v, also decreased different biomarkers of CVD risk in heavy smokers(Reference Bukowska, Pieczul-Mróz and Jastrzebska49), (Table 2). Monocytes isolated from the subjects treated with L. plantarum 299v also showed significantly reduced adhesion to native and stimulated human umbilical vein endothelial cells, suggesting that the probiotic product could reduce CVD risk(Reference Bukowska, Pieczul-Mróz and Jastrzebska49). A yoghurt supplemented with L. acidophilus 145, B. longum 913 and oligofructose increased HDL cholesterol concentrations and decreased the ratio of LDL:HDL cholesterol in comparison with control yoghurt in women(Reference Kiessling, Schneider and Jahreis50). However, the administration of other probiotic strains did not exert significant effects on serum lipids, cholesterol or lipoproteins(Reference Simons, Amansec and Conway51, Reference Greany, Bonorden and Hamilton-Reeves52). The effects of probiotic supplementation together with dietary counselling exerted positive effects on glucose metabolism in normoglycaemic pregnant women(Reference Laitinen, Poussa and Isolauri53). Blood glucose concentrations were the lowest in the diet/probiotic group during pregnancy and over the 12 months' postpartum period. Glucose tolerance was also better in the diet/probiotic group compared with the control/placebo group during the last trimester of pregnancy and over the 12-month postpartum period(Reference Laitinen, Poussa and Isolauri53). In human subjects, inulin-type fructans have also generally been found effective on normalization of metabolic disorder biomarkers, although the results have not been as consistent as those reported in animals(Reference Reimer and Russell54). Supplementation of inulin to subjects under a moderately high-carbohydrate, low-fat diet led to decreased hepatic lipogenesis and plasma TAG concentrations, suggesting an effect on the reduction of atherosclerosis risk factors(Reference Letexier, Diraison and Beylot55). Oligofructose intake also led to slightly significant effects on postprandial insulin response, but not on lipid metabolism in individuals with mild hypercholesterolemia(Reference Giacco, Clemente and Luongo56). An infant formula containing galactosyl-oligosaccharides and long chain fructo-oligosaccharides in a ratio of 9:1 did not exert significant effects on total cholesterol and LDL cholesterol in infants compared with those receiving a control infant formula(Reference Alliet, Scholtens and Raes57). Daily consumption of oligofructose decreased the basal hepatic glucose production in healthy subjects, without any effect on insulin-stimulated glucose metabolism(Reference Luo, Rizkalla and Alamowitch58). However, this prebiotic had no effect on glucose and lipid metabolism in type-2 diabetics(Reference Luo, Van Yperselle and Rizkalla59). In a pilot study with 10 human subjects, oligofructose treatment also increased satiety following breakfast and dinner, reduced hunger and prospective food consumption following dinner(Reference Cani, Joly and Horsmans60). A long-term study (12 months) including 100 subjects revealed that those who received the prebiotic supplement had a smaller increase in BMI, BMI Z-score and total fat mass, compared with the control group(Reference Abrams, Griffin and Hawthorne61).
Table 2. Effects of probiotics, prebiotics and synbiotics on biomarkers of body weight regulation and metabolic disorders in human subjects
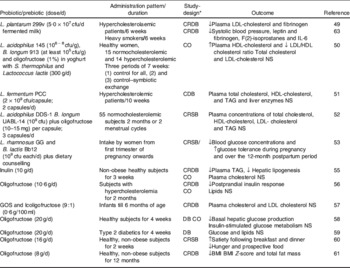
* C, placebo-controlled; R, randomized; DB, double-blind; SB, single-blind trial; CO, cross-over.
Conclusions
A number of ecological studies have uncovered the association between the composition of the gut microbiota and body weight and the prominent role played by the diet in these interactions. Mechanistic studies have also revealed that the gut microbiota may perform specific functions in the metabolic, neurohormonal and immune dysfunction associated with obesity. In this scenario, the use of dietary strategies targeting the gut ecosystem emerges as an additional tool to control metabolic disorders. A small number of trials have demonstrated that the administration of probiotics, prebiotics and their combination (synbiotics) exert positive effects in vivo, which are often more remarkable in animals than in human subjects. Nevertheless, the findings indicate that advances in this field could be of value to improve intervention strategies to manage obesity and its associated metabolic disorders.
Acknowledgements
This work was supported by grants AGL2008-01440/ALI and Consolider Fun-C-Food CSD2007-00063 from the Spanish Ministry of Science and Innovation and AP 124/09 from Consejería de Sanidad (Valencia, Spain). The scholarships to A. S. from CONACYT (Mexico) and to P. G. from CONICET (Argentina) are fully acknowledged. This review was drafted by Y. S. and discussed and approved by all authors. Authors declare no conflict of interest.




