Introduction
Venous thromboembolism (VTE) (deep vein thrombosis [DVT] and pulmonary embolism [PE]) is commonly diagnosed and treated in the emergency department (ED). The annual incidence of first time VTE events in the general population (excluding hospitalized patients) is approximately 1 per 1000.Reference Franchini and Mannucci 1 An increasing proportion of patients with a diagnosis of low-risk PE is now also being managed predominantly as outpatients. Traditionally, patients are anticoagulated through the administration of low-molecular-weight heparin (LMWH) as a bridging agent and vitamin K antagonists such as warfarin.Reference Guyatt, Akl and Crowther 2 - Reference Fox, Kahn and Langleben 4 However, full anticoagulation is not achieved immediately with warfarin, and there is great inter-patient variability in dosage requirements due to genetic polymorphisms.Reference Franchini and Mannucci 1 - Reference Nutescu 6 For these reasons, it often takes a minimum of 5 days for patients to reach two consecutive therapeutic international normalized ratios (INRs) of between 2.0 and 3.0, requiring daily ED visits in some settings.Reference Franchini and Mannucci 1 - Reference Nutescu 6 In two large multicentre clinical trials comparing a direct oral anticoagulant (DOAC) to traditional therapy, the median duration of LMWH (enoxaparin) treatment while bridging to a vitamin K antagonist was 8 days (interquartile range 6 to 11 days).Reference Bauersachs, Berkowitz and Brenner 7 , Reference Büller, Prins and Lensin 8 After discharge from the ED, warfarin therapy poses additional challenges to the patient such as continued laboratory monitoring, compliance, and drug interactions.Reference Franchini and Mannucci 1 , Reference Pollack 3 - Reference Rudd and Phillips 5
From a hospital perspective, each ED visit is costly, in terms of personnel and resources. Additionally, from a patient perspective, the ED visits are inconvenient and time-consuming; each visit can consume up to 3 to 4 hours of time, often for a minimum of 5 days.
There is substantial evidence from randomized clinical trials, meta-analyses, and clinical practice guidelines supporting the use of DOACs, also called novel oral anticoagulants (NOACs), in the treatment of VTE.Reference Guyatt, Akl and Crowther 2 , Reference Pollack 3 , Reference Bauersachs, Berkowitz and Brenner 7 - Reference Sardar, Chatterjee and Mukherjee 9 Rivaroxaban (Xarelto), a factor Xa inhibitor, was the first DOAC approved for VTE treatment in Canada, based on EINSTEIN-DVT, which showed non-inferiority of rivaroxaban to standard therapy.Reference Bauersachs, Berkowitz and Brenner 7 Because it does not require therapeutic monitoring (i.e., daily INR testing) and its therapeutic effect is immediate, patients diagnosed with VTE can be treated with rivaroxaban in the outpatient setting without the need to return to the ED daily.Reference Franchini and Mannucci 1 , Reference Rudd and Phillips 5 However, rivaroxaban, as with other DOACs, is more expensive than warfarin, is not yet on many hospital formularies in Canada for VTE treatment, and has limited coverage by third-party payers.
This study was designed in order to evaluate the historical standard of VTE management in two large Canadian EDs and to compare the costs between traditional and rivaroxaban anticoagulation in this population. It was hypothesized that the administration of a DOAC for VTE in the ED would be less expensive than traditional anticoagulation with warfarin and LMWH, from a hospital perspective.
Methods
The study consisted of two parts. The first part was a chart review to determine historical outcomes of VTE management where LMWH/warfarin were used. The second part modeled these historical outcomes against VTE outcomes derived from published DOAC clinical trials. A cost minimization analysis was then performed comparing these two modes of anticoagulation, both from a hospital and a patient perspective, in Canadian dollars.
The protocol for the study was approved by University of British Columbia, Providence Health Care Research Ethics Board (UBC-PHC REB #H13-01730), and Fraser Health Research Ethics Board (FHREB #2013-078).
Chart review
In the first part, cases for review were identified via the Providence Health Emergency Department Discharge Database using keywords of “DVT,” “PE,” and “VTE” in the Presenting Complaint Description, National Ambulatory Care Reporting System Description, Diagnosis Name from January 1, 2010 to December 31, 2012. This included data from a large academic inner city hospital with an ED census of 78,000 visits, and an acute care community hospital with a census of 25,000 visits, in Vancouver, British Columbia.
Patients were included if they presented for a minimum of one ED visit with a diagnosis of VTE. For the purposes of this study, all subsequent visits within 30 days of eligibility and discharges (therapeutic INR for 2 consecutive days) were included as it was viewed that it was a resource burden to the ED. Patients with two separate diagnoses of VTE greater than 30 days after two therapeutic INRs had been obtained were quantified as two separate events. During the initial screening, patients without radiological findings consistent with VTE diagnosis and/or documentation of a negative d-dimer (less than 500 mcg/L) were excluded. In addition, patients who did not undergo radiological investigations in the ED were excluded because a diagnosis of VTE could not be confirmed. Those who presented with isolated superficial thrombophlebitis or were misclassified in the discharge database were also excluded. Other exclusion criteria were admission to hospital, lack of British Columbia personal health number, treatment with a DOAC, ongoing monotherapy with warfarin or LMWH, inferior vena cava filter in situ prior to VTE diagnosis, and patients who left against medical advice prior to emergency staff assessment (Figure 1). High-risk populations where DOACs are not recommended were also excluded, such as active cancer (currently undergoing chemotherapy, radiotherapy, or surgery), pregnancy, and renal dysfunction (estimated glomerular filtration rate [eGFR] less than 30 mL/min). Last, patients who did not have an updated eGFR within 6 months of diagnosis were also excluded.

Figure 1 Case inclusion flow chart. Number of VTE cases identified via emergency discharge database and, after screening and exclusions, were included in the historical cohort.
Demographic data and measures of health care utilization were collected during the chart review. Diagnosis and location of DVT or PE, gender, age, risk factors for VTE (i.e., recent surgery/trauma, immobilization, recent flight, thrombophilic disorder) were extracted from patient electronic charts. In addition, the total number of ED visits, length of stay per visit, number and type of laboratory tests per visit (excluding ultrasounds and computerized tomography scans), and types of medication received were recorded. Length of ED visits were rounded off to the nearest hour, with a minimum of 1 hour. INR, hemoglobin, and eGFR were also collected if performed during ED visit(s). Patient outcomes (death or unscheduled readmissions within 7 months of index visit) were determined from linkage to the Vancouver Coastal Health Regional Emergency Department database. Application Secure Access software was used to access outpatient lab information to follow up and assess for changes in hemoglobin and INR values.
The electronic chart review and data collection were completed in adherence with standardized chart review methodology.Reference Kaji, Schriger and Green 10 Two reviewers (SL and KH) were trained in chart abstraction and performed the chart reviews. The research team met regularly during the study to resolve any discrepancies identified during patient screening and data collection in order to maintain consistency and standardization. Data were cumulatively collected using a standardized Microsoft Excel spreadsheet. One of the investigators (DH) performed a review of a random sample of 20 of the VTE cases to determine reliability. The κ-statistic was used to measure inter-rater agreement.
Cost minimization analyses
Therapy with rivaroxaban has been shown to be non-inferior and comparable to standard therapy with LMWH/warfarin for VTE treatment.Reference Bauersachs, Berkowitz and Brenner 7 , Reference Büller, Prins and Lensin 8 In other words, both VTE outcomes (re-occurrence, death) and bleeding (major and minor) have been found to be non-inferior to LMWH/warfarin. Therefore, a cost minimization analyses was completed from both hospital and patient perspectives, using direct costs. Health care utilization costs were determined from Canadian Institute of Health Information (CIHI), 11 Centre for Health Services and Policy Research (CHSPR), British Columbia Ministry of Health, 12 and Lower Mainland Pharmacy Services (LMPS) where applicable.
Hospital cost perspective
Direct fixed and variable costs for first and subsequent ED visits were taken into account. This included personnel (admitting clerk, registered nurse, physician, phlebotomist, etc.), supplies (syringes, needles, cups, etc.), laboratory tests, and medications. For traditional anticoagulation, cost analysis was based on the mean number of ED visits identified from the historical cohort. For rivaroxaban, it was assumed that patients would visit the ED only once for diagnosis and appropriate treatment and would not return for subsequent visits.
Patient cost perspective
Direct costs endured during emergency visits and after discharge were taken into account for the patient perspective cost analysis. This included parking, mileage, time off work, cost of medications, and dispensing fees. It was conservatively assumed that a patient had a minimum wage occupation ($10.25 in BC) and had no extended health coverage. This analysis assumed a 6-month treatment duration. Parking fees and mileage from the hospital, lab, and physician office were estimated via BC Ministry of Finance mileage entitlement and current guidelines for monitoring of warfarin therapy. 13 The duration of ED visits, which is the expected time away from work, was obtained via the chart review. It was assumed that the patient had biweekly INRs until four consecutive therapeutic INRs, and then monthly INRs and biweekly family physician visits for the first 2 months then phone reviews if on warfarin. Two family physician visits were assumed if the patient was on DOAC therapy.
Outcomes
The primary outcome was the cost difference between standard and DOAC therapy for VTE treatment in the ED, from a hospital perspective. A patient perspective cost analysis was conducted to address potential concerns that the direct patient costs would be a barrier to DOAC therapy. Secondary outcomes included the rate of therapeutic anticoagulation at ED discharge, mean number of ED visits, all-cause mortality, all-cause readmission to hospital, and major bleeding, defined as a hemoglobin drop greater than 20 g/L. Clinical outcomes were followed over 6 months from time of diagnosis with an additional 30-day follow-up after discontinuation of therapy. Duration of anticoagulation varies with location and cause (provoked or unprovoked) of VTE, and 6 months of anticoagulation were deemed appropriate for this study among the study investigators after consultation with hematologists and internists at the outpatient anticoagulation clinic.
Results
Chart review
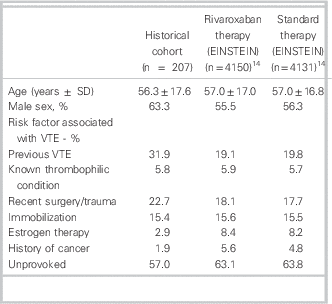
A total of 939 patient encounters were screened and after exclusions, 207 VTE cases were included (see Figure 1). Cohen’s kappa for inclusion in the study was found to be 0.83 (95% confidence interval = 0.78–0.88). Baseline characteristics are shown in Table 1. The mean age was 56.3 ± 17.6 yr, and 131 (63.3%) were men; 173 (83.6%) of the VTE cases were DVT, 12 (5.8%) were PE, and the remaining were a combination of both with or without superficial thrombophlebitis (Table 2); 130 (63.2%) VTE cases were discharged from the ED appropriately after achieving therapeutic anticoagulation, (INR 2.0–3.0) returning to the ED for a mean of 7.18 visits (range 1–21). The mean duration of visits was 1.65 hours (range 1–17). A total of 21 patients (10%) were readmitted to hospital, with 4 (1.9%) rehospitalizations related to VTE or anticoagulation complications. Major bleeding occurred in 8 (3.9%) patients, and 5 (2.4%) patients died during the predefined follow-up period of 7 months after diagnosis (Table 3).
Table 1 Baseline characteristics compared with pooled EINSTEIN studies
SD = standard deviation; VTE=venous thromboembolism.
Table 2 VTE diagnosis in historical cohort

Table 3 Clinical outcomes compared with EINSTEIN trials

Cost minimization analyses
Hospital perspective
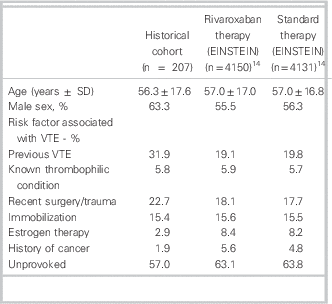
When traditional (warfarin/LMWH) and rivaroxaban anticoagulation were compared for the treatment of VTE in the ED, rivaroxaban was associated with a cost avoidance of $1,488.04 per VTE event. This was inclusive of costs incurred by the hospital during initial and subsequent visits (Table 4).
Table 4 Cost minimization breakdown and analysis: hospital perspective
Cost minimization analysis
= Total costs (traditional) – total costs (DOAC)
= ($247.50 + $1,435.31) – ($194.77 + $0.00)
= + $1,488.04/VTE event (in favour of DOAC)
CBC=complete blood count; DOAC=direct oral anticoagulant; INR=international normalized ratio; LMWH=low-molecular-weight heparin; VTE=venous thromboembolism.
Patient perspective
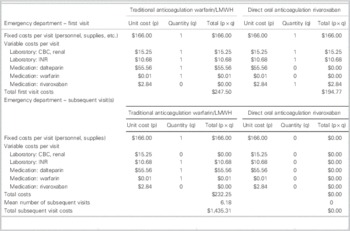
For a 6-month duration of therapy, the cost minimization analysis indicated that rivaroxaban would cost each patient an additional $204.10 to $349.04 (Tables 5 and 6). This was inclusive of costs during initial hospital ED visit(s) as well as outpatient therapy. Four visits to a family doctor for patients on traditional therapy were modeled on current guidelines for monitoring and therapyReference Guyatt, Akl and Crowther 2 ; two visits were estimated for those on rivaroxaban, that is, for re-prescribing and follow-up/counselling purposes.
Table 5 Breakdown of costs: patient perspective
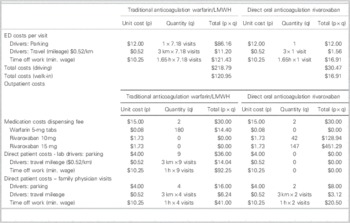
ED=emergency department; LMWH=low-molecular-weight heparin.
Table 6 Cost minimization analysis: patient perspective
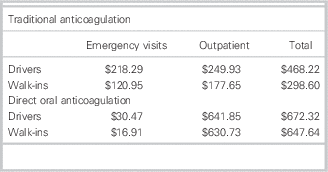
Cost minimization analysis
= Total costs (traditional) – total costs (DOAC)
Drivers: $468.22 – $672.32=– $204.10
Walk-ins: $298.60 – $647.64=– $349.04
DOAC=direct oral anticoagulant.
Discussion
In our historical VTE population treated with traditional anticoagulation, patient characteristics and clinical outcomes were similar to those seen in the control arms of the EINSTEIN trials (Tables 1 and 3).Reference Prins, Lensing and Bauersachs 14 However, only 63.2% of our cohort was appropriately anticoagulated with warfarin upon completion of their multiday ED course of therapy. This anticoagulation rate is considerably lower than that found in EINSTEIN-DVT, and -PE (80.8%, 83.2% respectively)Reference Bauersachs, Berkowitz and Brenner 7 , Reference Büller, Prins and Lensin 8 ; therefore, clinical trial results may be underestimating the benefit of rivaroxaban in a real world setting. The low therapeutic anticoagulation rate in our cohort could be attributed to that, in practice, practitioners may be more lenient regarding INRs—accepting bordering INR values of 1.9 to 3.1, rather than 2.0 to 3.0, as per clinical guidelines.Reference Guyatt, Akl and Crowther 2 Subtherapeutic anticoagulation might be avoided if rivaroxaban were used to manage this patient population. The VTE cases in the historical cohort returned to the ED for a mean of 7.18 visits, which is similar to what was seen in EINSTEIN-DVT (median 8 days).Reference Bauersachs, Berkowitz and Brenner 7
In the cost minimization analysis from the hospital perspective, rivaroxaban therapy was $1,488.04 less costly than warfarin/LMWH. This assumes no return visits for those patients prescribed rivaroxaban, which may be over-optimistic. In a sensitivity analysis, patients prescribed rivaroxaban may return up to eight times to the ED (for reassessment, for example) and still show a cost savings versus traditional therapy. In those settings that have outpatient anticoagulation clinics (or in pharmacy settings), the cost difference would be considerably less because those practice settings are less costly than the ED. However, rivaroxaban therapy would still cost less than traditional therapy. Very minor differences in costs exist between rivaroxaban and the other DOACs (dabigatran [Pradaxa] and apixaban [Eliquis]); therefore, these cost savings would be similar if other DOACs were used in the analysis.
Along with the direct hospital costs that were accounted for in this study, there are several indirect hospital costs that were not quantified. The use of a DOAC would reduce the number of overall ED visits, possibly reduce ED crowding, hypothetically improve clinician availability to treat higher acuity patients, and possibly improve clinician and patient satisfaction.
It could be argued that the use of a DOAC may misallocate health care costs to the patient by reducing hospital ED visits. Passing the costs to patients is a significant issue; this may result in noncompliance and poor outcomes, including more downstream visits and complications. Therefore, the implications of using a DOAC over traditional anticoagulation for VTE treatment from a patient perspective were considered. Indeed, the cost minimization analysis showed an increased cost of approximately $200 to $350 to the patient over a 6-month treatment period. The additional cost was dependent on patient mode of transportation to the ED and outpatient appointments. This analysis was based on very conservative cost assumptions: that the patient is making minimum wage ($10.25/hr) and does not have extended health coverage, including PharmaCare coverage. The transition point, in terms of patient wages, was found to be approximately $20.00/hr. Wages at or beyond this hourly rate would reverse the cost analysis in favour of rivaroxaban therapy. Indirect costs of being on traditional therapy that could not be quantified included patient satisfaction (dietary and drug restrictions due to interaction risks, labile INRs, etc.), infectious risks secondary to multiple hospital visits, and caregiver time off work, if applicable. Additionally, the indirect value that a patient may put on his or her time is variable and may ultimately alter therapeutic decision-making. Although using rivaroxaban clearly uses less health care costs from a hospital perspective compared with traditional treatment, the selection between the two modes of anticoagulation should be evaluated on a case-by-case basis.
Finally, there are currently no approved antidotes for the DOACs in Canada. Although in development, the lack of approved antidotal therapy may impact both patients and providers in their decision-making.
Limitations
This study is limited in that it is a comparison of historical data from a chart review against data from clinical trials. Real-world data will inherently have worse outcomes than outcomes derived from a clinical trial. We tried to mitigate this bias by assuming conservative outcomes and bleeding risks, and performing sensitivity analyses, where possible. In this study, bleeding risk was assumed to be equivalent with traditional versus DOAC therapy; this finding has been confirmed in the literature.Reference Beyer-Westendorf, Förster and Pannach 15 However, bleeding risk has not been firmly established with DOACs, in comparison to traditional anticoagulation, outside clinical trial settings. This may alter outcomes and, therefore, have cost implications that may alter the conclusions from this analysis.
In addition, this study is limited by the inherent biases of a chart review, including missing data and misclassification. Clinical outcomes such as rehospitalization and mortality may be incomplete if patient was out of the province or country. Rehospitalization data were not collected in EINSTEIN; therefore, a direct comparison could not be made. It is important to take note that both EINSTEIN trials included outpatients and inpatients, whereas our population consisted of outpatients only.
As with any economic analysis, there are limitations in the determination of outcomes and costs. This was a cost-minimization analysis; outcomes (both re-occurrence, mortality, and bleeding) were not included in the cost modelling because clinical trial data showed that rivaroxaban is non-inferior to traditional therapy, so outcomes were assumed to be equivalent. Furthermore, assumptions were made and several different sources were used in order to estimate various fixed and variable costs to complete the cost analyses. The cost analyses are specific to the practice at two hospitals in BC and other hospitals across Canada that have similar ED programs in place; they may not apply to outpatient anticoagulation clinics, which have different workflow and cost models.Reference Van Walraven, Jennings and Oake 16 In addition, costs are specific to the practice setting, city, and province of this study; although comparable to other jurisdictions, costs will obviously differ between settings.
Conclusions
In summary, rivaroxaban would realize a cost avoidance of $1,488.05 per VTE event versus traditional anticoagulation, with a resultant increase in costs to the patient of approximately $200 to $350. This analysis suggests that DOACs are a cost-effective and viable option for VTE treatment in the ED, for those patients appropriate for outpatient therapy. For those remaining on traditional anticoagulation, measures should be taken in order to minimize direct and indirect costs to the hospital and patient such as streamlining of ED visits, updating VTE treatment pathways, and/or specific anticoagulation clinics dedicated to bridging anticoagulation.
Acknowledgements: Presented at the Canadian Association of Emergency Physicians Annual General Meeting, Ottawa, Ontario, June 2014.
Special thanks to Kimberly Hilchie, UBC Pharmacy Student, for her assistance in data collection.
Competing interests: Dr. Harris has been supported by Bayer (consulting), Pfizer (consulting). No sponsorship or funding was provided for this research.