A substantial number of studies on the prevalence of dementia in old age have been published over the past years. Exponentially increasing prevalence rates between 65 and 85 years are a clearcut finding of these studies (Reference Jorm, Korten and HendersonJorm et al, 1987). However, it still remains unclear whether this will continue in the oldest of the elderly. Actual reported prevalence rates in these studies differ widely. In an effort to increase comparability, the use of psychiatric classification systems for case definition has become standard in epidemiological studies. However, the prevalence of dementia diagnosis by applying different classification systems remains poorly understood. This paper reports and compares prevalence rates of dementia according to DSM—III—R (American Psychiatric Association, 1987) and ICD—10 (World Health Organization, 1992).
METHOD
Sample
A total of 1500 community-dwelling individuals aged 75 years and over living in the community of Leipzig-South were identified by systematic random sampling from an age-ordered list provided by the official registry office for each of the seven subdistricts. In order to avoid high sample attrition owing to death and substantial movement out of the study area, as well as to facilitate public support for the study, we sampled in sequence for each of the sub-districts just before starting field work. In case of a move, a search for a new address was conducted; and in case of death, the deceased individual's heir (mainly close relatives) was identified by the official registry office staff. Institutionalised individuals were included in the study by proportion (n=192), because in the study area residential homes were overrepresented and nursing homes were underrepresented. The number was based on the situation in Leipzig in 1997, where 39.9 individuals aged 75+ years per 1000 inhabitants were cared for in residential homes and 88.4 individuals aged 75+ years per 1000 inhabitants were cared for in nursing homes. Sixty residential home residents and 132 nursing home residents were identified by systematic random sampling from an age-ordered list provided by the four institutions in the study area. The baseline study (Leipzig Longitudinal Study of the Aged, LEILA75+) was conducted between January 1997 and June 1998. For details on contact procedure, see elsewhere (Reference Riedel-Heller, Schork and MatschingerRiedel-Heller et al, 2000).
Instruments
A fully structured face to face interview was administered to the study subjects by trained female physicians and psychologists at a home visit. Cognitive function was assessed by the Structured Interview for Diagnosis of Dementia of Alzheimer Type, Multiinfarct Dementia and Dementia of other Aetiology according to ICD—10 and DSM—III—R (SIDAM; Zaudig et al, Reference Zaudig, Mittelhammer and Hiller1991a ,Reference Zaudig, Mittelhammer and Hiller b ). The SIDAM comprises a test performance part, a section for clinical judgement and third party information to determine psychosocial impairment. The SIDAM test performance part consists of a range of cognitive tests that constitute a short neuropsychological battery with 55 questions, including all 30 items of the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975). The SIDAM gives detailed instructions about the application of the items. All individuals interviewed face to face were asked to name an informant. If an individual interviewed face to face scored below 24 on the MMSE (as incorporated in the SIDAM test performance part) or reported impairment of the activities of daily living that was not caused by physical or sensory deficits, a comprehensive informant interview was conducted. Otherwise only short informant interviews were applied. Deaf study participants were offered a large print version of the interview. Blind study participants received test versions without tasks involving eyesight and the final scores were projected. In the case of sensory impairment, comprehensive informant interviews were conducted. The SIDAM diagnostic algorithms were available to derive ICD—10 and DSM—III—R diagnoses of dementia. Consensus conferences were held on each potential case to discuss the psychosocial impairment. The interview contained further structured enquiries on socio-demographic and other health aspects.
If relatives of study subjects refused participation on behalf of the elderly person cared for or the study participant died between sampling and planned examination, we offered the option of a fully structured proxy interview. Instead of cognitive testing, the Clinical Dementia Rating Scale (CDR) was used for assessment of cognitive function (Reference Hughes, Berg and DanzigerHughes et al, 1982). A very good agreement between the SIDAM-derived DSM—III—R diagnosis for moderate and severe dementia had been shown in a sample of n=180 (κ=0.83) (Reference Riedel-Heller, Schork and MatschingerRiedel-Heller et al, 2000). The prevalence results presented are based on those individuals interviewed face to face. By including the respondents by proxy, prevalence rates derived from face to face interviews increased insignificantly by 1% for DSM—III—R and 1.4% for ICD—10.
Test—retest reliability was assessed in a sample of 30 subjects aged 75-99 years living in the community and in institutions. It was carried out by two raters within a 1-week interval. Test—retest reliability was found to be excellent for SIDAM DSM—III—R diagnoses of dementia (κ=1.00) and high for the severity level of dementia (κ=0.94). Similar results were found for SIDAM ICD—10 diagnoses (κ=1.00) and the severity level of dementia (κ=0.92).
The validity of dementia diagnosis was investigated using two strategies. The first was to find out whether study participants were treated during the past 5 years by checking the records of the two psychiatric hospitals in the study area. Records of 34 participants in the field study were identified. Although 32 were diagnosed with dementia in the field study, only 31 suffered from dementia as diagnosed by the clinicians. The discordant case suffered from depression. However, agreement between clinical and field diagnosis was found to be good (κ=0.78). The second strategy involved a subsample of the study participants (n=74) being thoroughly investigated clinically: physical and neurological status, cognitive testing, electroencephalogram, brain imaging and blood test. The agreement was found to be very good (κ=0.85).
Analysis
Frequency of dementia is described in terms of percentage prevalence. Confidence intervals were calculated based on binomial distribution. Owing to multiple comparisons using the χ2 test, the level of α error was adjusted to 0.01.
RESULTS
A total of 1265 individuals (74.8%) were interviewed face to face. Information on 113 (6.7%) study subjects who were shielded by their relatives was gathered solely by proxy interview. Only 57 (3.4%) individuals died, 15 (0.9%) were not traceable and 242 (14.2%) refused participation. Individuals interviewed face to face did not differ significantly from the rest of the sample with regard to age (χ2=1.2591, d.f.=2, P=0.533), gender (χ2=0.391, d.f.=1, P=0.532) and marital status (χ2=5.267, d.f.=3, P=0.170).
Of the study participants interviewed face to face, 17.4% (95% CI=15.9-19.5) suffered from dementia according to DSM—III—R. This includes mild, moderate and severe cases. Age— and gender-specific prevalence rates are summarised in Table 1. Up to age 89 years the prevalence rates for women and men do not differ significantly. Almost half of the dementia cases (42.7%) have mild dementia and over a quarter have moderate (29.1%) and severe (28.2%) dementia. According to the severity rating, there is no statistical difference between the age groups (χ2=6.1584, d.f.=8, P=0.629).
Table 1 Age— and gender-specific prevalence rates according to DSM—III—R

| Age (years) | Women (n=964) | Men (n=301) | Total (n=1265) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dementia cases/ | Prevalence | 95% CI | Dementia cases/ | Prevalence | 95% CI | Dementia cases/ | Prevalence | 95% CI | |
| n | (%) | n | (%) | n | (%) | ||||
| 75-79 | 17/375 | 4.5 | 2.4-6.7 | 9/144 | 6.3 | 2.3-10.2 | 26/519 | 5.0 | 3.1-6.9 |
| 80-84 | 44/264 | 16.7 | 12.1-21.2 | 12/86 | 14.0 | 6.5-21.4 | 56/350 | 16.0 | 12.1-19.9 |
| 85-89 | 68/229 | 29.7 | 23.7-35.7 | 11/48 | 22.9 | 10.6-35.3 | 79/277 | 28.5 | 23.2-33.9 |
| 90-94 | 41/76 | 53.9 | 42.5-65.4 | 6/22 | 27.3 | 7.1-47.5 | 47/98 | 47.9 | 37.9-58.0 |
| 95+ | 12/20 | 60.0 | 36.5-83.5 | -/1 | - | - | 12/21 | 57.1 | 34.0-80.2 |
| 75+ | 182/964 | 18.9 | 16.4-21.3 | 38/301 | 12.6 | 8.8-16.4 | 220/1265 | 17.4 | 15.3-19.5 |
| 85+ | 121/325 | 37.2 | 31.9-42.5 | 17/71 | 23.9 | 13.7-34.1 | 138/396 | 34.8 | 30.1-39.6 |
Dementia according to ICD—10 was diagnosed in 12.4% (95% CI=10.6-14.2) of the study participants interviewed face to face. Age— and gender-specific prevalence rates are shown in Table 2. Prevalence rates for men and women do not differ significantly; 19.7% of the study participants suffering ICD—10 dementia have mild dementia, 40.8% have moderate dementia and 39.5% have severe dementia. Regarding the severity rating, there were no statistical differences found in the age groups (χ2=6.6317, d.f.=8, P=0.577).
Table 2 Age— and gender-specific prevalence rates according to ICD-10

| Age (years) | Women (n=964) | Men (n=301) | Total (n=1265) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dementia cases/ | Prevalence | 95% Cl | Dementia cases/ | Prevalence | 95% Cl | Dementia cases/ | Prevalence | 95% Cl | |
| n | (%) | n | (%) | n | (%) | ||||
| 75-79 | 12/375 | 3.2 | 1.4-5.0 | 6/144 | 4.2 | 0.8-7.5 | 18/519 | 3.5 | 1.9-5.1 |
| 80-84 | 30/264 | 11.4 | 7.5-15.2 | 7/86 | 8.1 | 2.2-14.0 | 37/350 | 10.6 | 7.3-13.8 |
| 85-89 | 49/229 | 21.4 | 16.1-26.8 | 8/48 | 16.7 | 5.7-27.6 | 57/277 | 20.6 | 15.8-25.4 |
| 90-94 | 33/76 | 43.3 | 32.0-54.8 | 4/22 | 18.2 | 0.7-35.7 | 37/98 | 37.8 | 28.0-47.5 |
| 95+ | 8/20 | 40.0 | 16.5-63.5 | -/1 | - | - | 8/21 | 38.1 | 15.4-60.7 |
| 75+ | 132/964 | 13.7 | 11.5-15.9 | 25/301 | 8.3 | 5.2-11.4 | 157/1265 | 12.4 | 10.6-14.2 |
| 85+ | 90/325 | 27.7 | 22.8-32.6 | 12/71 | 16.9 | 8.0-25.8 | 102/396 | 25.7 | 21.4-30.1 |
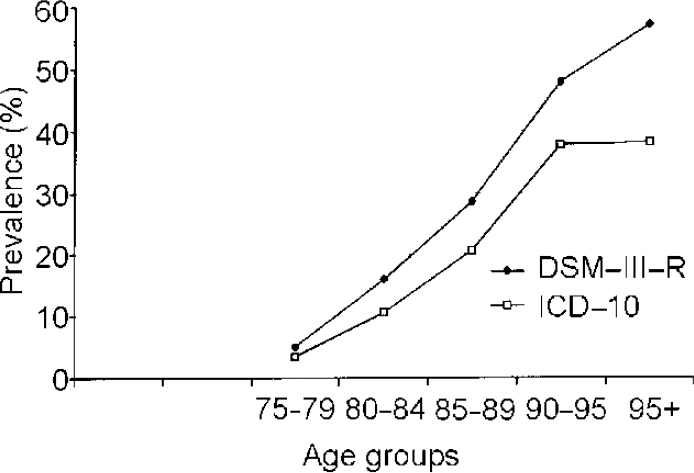
Figure 1 compares age-specific prevalence rates of dementia according to DSM—III—R and ICD—10. The comparison of DSM—III—R and ICD—10 rates indicates that dementia prevalence increases with age regardless of the classificatory system used. Beyond age 95 years the curve levels off. This trend is observed in both DSM—III—R and ICD—10 rates but it is more obvious using ICD—10. Also, dementia prevalence rates according to ICD—10 are lower than dementia according to DSM—III—R in all the investigated age groups. The largest differences appear in the eldest age groups.

Fig. 1 Age-specific prevalence rates of dementia according to DSM—III—R and ICD—10.
Finally, detailed analysis of dementia prevalence according to DSM—III—R and ICD—10 in the mild, moderate and severe severity stages shows that DSM—III—R and ICD—10 differences are based solely on different judgements of mild dementia cases (see Table 3). Using DSM—III—R, individuals are included in the mild dementia category who do not get a dementia diagnosis according to ICD—10.
Table 3 Age-specific prevalence rates for mild, moderate and severe dementia according to DSM-III-R and ICD-10

| Age (years) | DSM-III-R dementia | ICD-10 dementia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mild | moderate | severe | mild | moderate | severe | |||||||
| Cases | % | Cases | % | Cases | % | Cases | % | Cases | % | Cases | % | ||
| 75-79 | 519 | 13 | 2.5 | 7 | 1.3 | 6 | 1.2 | 5 | 1.0 | 7 | 1.3 | 6 | 1.2 |
| 80-84 | 350 | 27 | 7.7 | 12 | 3.4 | 17 | 4.9 | 8 | 2.3 | 12 | 3.4 | 17 | 4.9 |
| 85-89 | 277 | 33 | 11.9 | 25 | 9.0 | 21 | 7.6 | 11 | 4.0 | 25 | 9.0 | 21 | 7.6 |
| 90-94 | 98 | 17 | 17.3 | 14 | 14.3 | 16 | 16.3 | 7 | 7.1 | 14 | 14.3 | 16 | 16.3 |
| 95+ | 21 | 4 | 19.0 | 6 | 28.6 | 2 | 9.5 | - | - | 6 | 28.6 | 2 | 9.5 |
| 75+ | 1265 | 94 | 7.4 | 64 | 5.1 | 62 | 4.9 | 31 | 2.5 | 64 | 5.1 | 62 | 4.9 |
| 85+ | 396 | 54 | 13.6 | 45 | 11.4 | 39 | 9.8 | 18 | 4.5 | 45 | 11.4 | 39 | 9.8 |
DISCUSSION
Dementia according to DSM—III—R
A substantial number of recent field studies are based on the revised edition of the third Diagnostic and Statistical Manual of Mental Disorders (DSM—III—R). Diagnosis of dementia according to DSM—III—R requires (A) impairment in short— and long-term memory, (B) impairment in abstract thinking or judgement or impairment of higher cortical functions or personality changes and (C) evidence that the cognitive disturbance resulting from criteria (A) and (B) significantly interferes with work, usual social activities or relationship with others. Symptoms do not occur exclusively during the course of delirium (D) and there is either (E1) evidence of a specific organic factor judged to be aetiologically related to the disturbance, or (E2) in the absence of such evidence, an aetiological factor can be presumed if the disturbance cannot be accounted for by any non-organic mental disorder (American Psychiatric Association, 1987).
Figure 2 shows age-specific prevalence rates of recent field studies considering more than three age groups and applying DSM—III—R for case definition compared with the LEILA75+ results (Reference Rocca, Bonaiuto and LippiRocca et al, 1990; Reference Letenneur, Jacqmin and CommengesLetenneur et al, 1993; Reference Roelands, Wostyn and DomRoelands et al, 1994; Reference Lobo, Saz and MarcosLobo et al, 1995; Reference De Ronchi, Fratiglioni and RucciDe Ronchi et al, 1998; Reference Ott, Breteler and van HarskampOtt et al, 1995). Up to age 89 years the LEILA75+ results correspond with the results of the studies reviewed. Beyond 90 years the LEILA75+ prevalence rates exceed the rates found in these studies. A similar pattern emerges when our results are compared with the EURODEM pooled findings of 12 studies (age 75-79 years, 5.7%; 80-84 years, 13.0%; 85-89 years, 21.6%; 90-94 years, 32.2%) (Reference Hofman, Rocca and BrayneHofman et al, 1991). However, mild dementia cases were only partially included in the EURODEM studies.
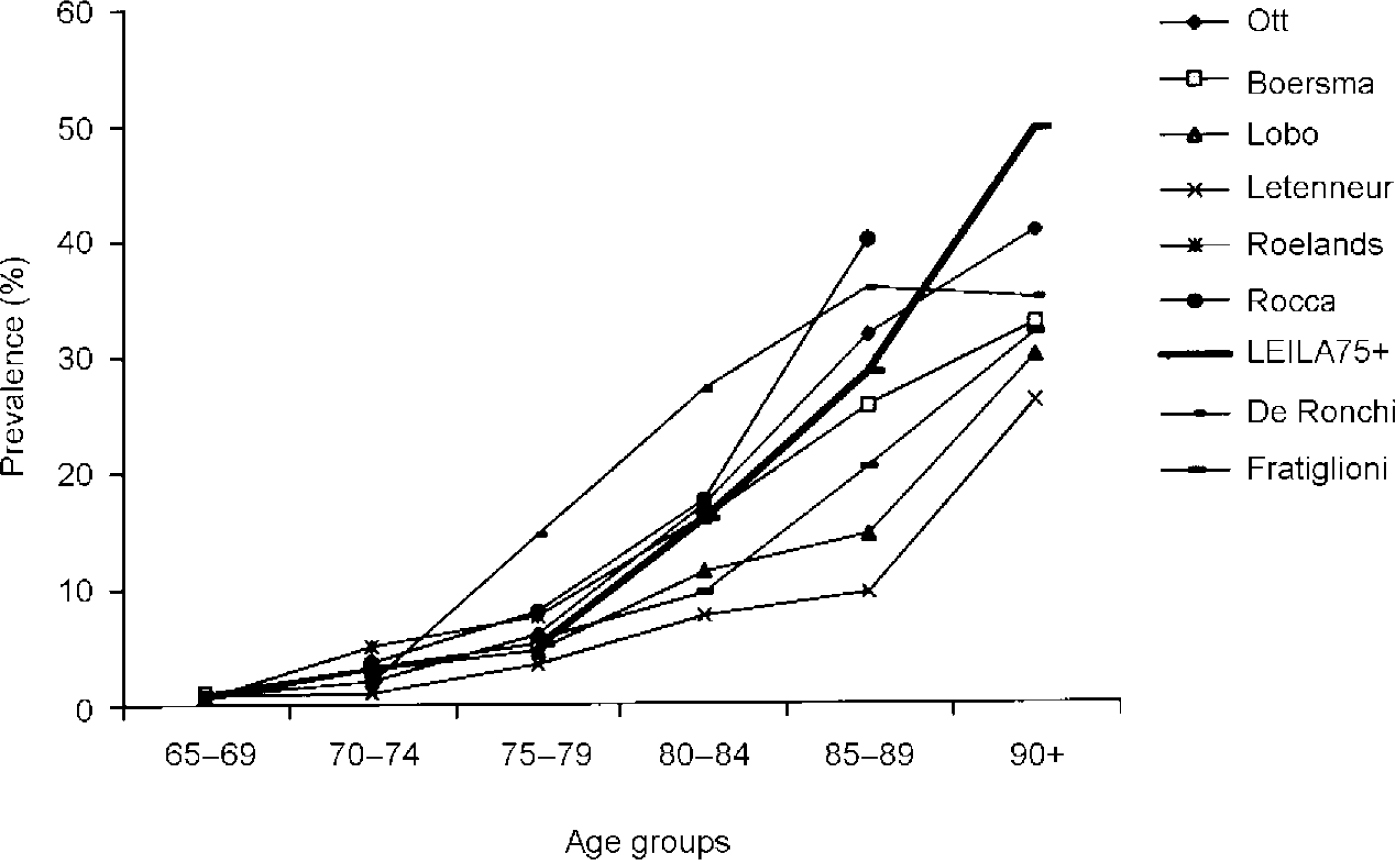
Fig. 2 Age-specific prevalence rates of dementia according to DSM—III—R of recent field studies compared with the LEILA75+ results (age groups 90-94 and 95+ years were collapsed).
Figure 2 illustrates that results differ, especially in the eldest age groups. This points to difficulties in applying criteria for case definition in this population segment, where cognitive deficits often are accompanied by physical and sensorial impairment. This makes it difficult to tease apart psychosocial impairment owing to cognitive deficits or other reasons. The SIDAM includes 14 questions enquiring about impairment of activities of daily living owing to cognitive deficits. These questions have to be answered by caregivers. Therefore, it is not surprising that our results correspond much better with the results of the Munich study of the oldest of the elderly using SIDAM as the method of case identification (Reference Fichter, Meller and SchröppelFichter et al, 1995). For individuals aged 90+ years the Munich study reported SIDAM DSM—III—R rates of 40.2%. Clinical diagnoses also were determined, yielding an even higher rate (57.3%).
Dementia according to ICD—10
Compared with DSM—III—R, ICD—10 criteria for dementia appear to be more restrictive. The ICD—10 requires (G11) memory decline and (G12) a decline in other cognitive abilities sufficient to impair personal activities of daily living. The awareness of the environment has to be preserved (G2). A decline in emotional control or motivation or a change in social behaviour has to be established (G3). The criterion G1 should have been present for at least six months (World Health Organization, 1994).
Therefore, it is not surprising that our results show a lower prevalence according to ICD—10 compared with DSM—III—R. The DSM—III—R includes cases that are not considered to have dementia according to ICD—10. Our data show that this is owing to the ICD—10 criterion that requires a decline in emotional control or motivation or a change in social behaviour, which is not an obligatory criterion in DSM—III—R. There are individuals who are mildly cognitively impaired but do not show these changes, or these disturbances might not be recognised or reported by caregivers, probably owing to negative age stereotypes. However, this constellation is found mainly in the eldest age groups, where the largest differences in the prevalence rates on comparing both classification systems were found.
Comparison of ICD—10 and DSM—III—R dementia rates
To our knowledge, only two studies have reported ICD—10 and DSM—III—R rates. They confirm lower rates by applying ICD—10 criteria. The Munich study of the oldest of the elderly (age groups 85-89 years and 90+ years) yielded prevalence rates according to SIDAM ICD—10 of 13.6% (95% CI=9.3-17.9) and 24.0% (95% CI=14.3-33.7), respectively (Reference Fichter, Meller and SchröppelFichter et al, 1995). Despite using the same method for case identification, the rates are lower than those reported in our study. The Australian study reported even lower prevalence rates by applying ICD—10 criteria strictly (age 75-79 years, 1.2%; age 80-84 years, 5.2%; 85+ years, 10.3%) (Reference Henderson, Jorm and MackinnonHenderson et al, 1994). However, the authors explicitly mention that their results are not representative owing to a lack of informant information on a substantial number of participants. The authors concluded that ICD—10 criteria proved to be demanding to apply in community surveys because they are more dependent on reliable information from informants. This corresponds to our experience and refers especially to the judgement of decline of emotional control or motivation or a change in social behaviour in the oldest of the elderly.
Dementia prevalence in the oldest of the elderly
Despite the mentioned difficulties in applying case definitions in the oldest of the elderly, our results suggest that prevalence rates of dementia do not increase exponentially beyond age 90 years, rather they level off. Few recent studies show how prevalence rates perform in individuals aged 90 years and over. Our results are in line with what was found by Heeren et al (Reference Heeren, Lagaay and Hijmans1991), Reischies et al (Reference Reischies, Geiselmann and Gessner1997) and by a meta-analysis conducted by Ritchie & Kildea (Reference Ritchie and Kildea1995). However, the majority of studies revealed contrasting results, suggesting further increasing prevalence rates in the oldest of the elderly (Reference Graves, Larson and EdlandGraves et al, 1996; Reference von Strauss, Viitanen and De Ronchivon Strauss et al, 1999; Reference Ebly, Parhad and HoganEbly et al, 1994; Reference Fichter, Schröppel and MellerFichter et al, 1996; Reference Blansjaar, Thomassen and Van SchaickBlansjaar et al, 2000).
We argue that the performance of prevalence of dementia diagnosis in the oldest of the elderly will remain unclear as long as substantial methodological difficulties exist in determining dementia in the oldest of the elderly. However, epidemiology of dementia in the oldest of the elderly is of great interest because there are practical consequences pertaining to the planning of health services and conceptual consequences regarding the question of whether dementia is an inevitable result of ageing or a disorder occurring within a specific age range (ageing— v. age-related).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Dementia is a common condition of old age that challenges the medical and social system.
-
• Discrepancies in prevalence rates of dementia according to different case definitions may result in inadequate assessment of resources required for health and social care.
-
• Revisions of psychiatric classification systems should strive to reduce these discrepancies.
LIMITATIONS
-
• Although extensive effort was made to ensure participation, non-response bias cannot be excluded completely.
-
• The survey was carried out in a large city, reflecting the situation in an urban setting.
-
• Dementia diagnosis was determined under field study conditions.






eLetters
No eLetters have been published for this article.