Several types of intermittent fasting have emerged as an alternative to energetic restriction and to a prolonged period of fasting because this diet strategy is relatively easy to maintain(Reference Barnosky, Hoddy and Unterman1) and there is some evidence from studies in animals and humans that it might improve overall health(Reference Mattson, Allison and Fontana2) and body composition(Reference Tinsley and La Bounty3), protects against cardiometabolic risk factors(Reference Melkani and Panda4,Reference Ahmet, Wan and Matsson5) and extends life span(Reference Goodrick, Ingram and Reynolds6). A specific type of intermittent fasting is time-restricted feeding, in which the daily eating period is limited to 12 h or less per d. Studies in humans have shown that time-restricted feeding is associated with weight loss(Reference Marianna, Iolanda and Andrea7), fat mass decrease(Reference Moro, Tinsley and Bianco8), metabolic disease risk reduction(Reference Melkani and Panda4) and improvement in glycaemic response(Reference Hutchinson, Regmi and Manoogian9).
Several physiological mechanisms might explain how intermittent fasting can affect metabolic pathways(Reference De Cabo and Mattson10). Generally, after 12 h of food deprivation, the hepatocytes glycogen stores are depleted and the liver shifts from glucose storage to gluconeogenesis and produces ketone bodies from fatty acids, such as β-hydroxybutyrate and acetoacetate, to satisfy the energy requirements of brain cells and to preserve muscle mass and muscle function(Reference Mattson, Allison and Fontana2,Reference Anton, Moehl and Donahoo11,Reference Di Francesco, Di Germanio and Bernier12) . Thus, individuals engaging in intermittent fasting might maintain or even enhance their physical function(Reference Anton, Lee and Donahoo13–Reference Tinsley, Moore and Graybeal15). Although the relatively short duration of fasting in time-restricted fasting may not lead to ketosis, it can trigger other processes such as autophagy, where damaged macromolecules and organelles can be cleared to preserve normal cell function(Reference Antunes, Erustes and Costa16). Moreover, intermittent fasting may have an impact on the circadian rhythm that regulates metabolism, energetics and sleep–wake cycles(Reference Patterson and Sears17,Reference Froy and Miskin18) .
Yet, evidence about the effects of intermittent fasting in free-living populations is scarce. Specifically, the impact of time-restricted fasting on physical function in older adults is unknown. In this population, time-restricted fasting happens unintentionally as prolonged nightly fasting and may pose individuals at higher risk of malnutrition. In spite of the fact that the need of energy intake is substantially reduced with age, macronutrient needs in older adults are similar or even greater than in younger adults in order to prevent sarcopenia and frailty(Reference Cruz-Jentoft and Sayer19). We hypothesised that prolonged nightly fasting could be related to functional impairment in older adults. Therefore, the aim of this study was to assess prolonged nightly fasting in association with performance-based lower-extremity function in a large population of community-dwelling older adults.
Materials and methods
Study design and participants
We performed a cross-sectional analysis of data obtained from the Seniors-ENRICA-II (Study on Nutrition and Cardiovascular Risk in Spain) cohort, among 3273 community-dwelling individuals aged 64 years or older residing in the city of Madrid and four surrounding towns: Alcalá de Henares, Alcorcón, Getafe and Torrejón de Ardoz. This cohort followed the same design as the Seniors-ENRICA-I(Reference Rodríguez-Artalejo, Graciani and Guallar-Castillón20). The study participants were selected by random sampling sex- and district-stratified among all individuals with a national healthcare card between 2015 and 2017. Given that all people residing in Spain are entitled to free healthcare, the list of card holders closely approximates the entire resident population of Madrid. Information was collected by trained staff in three stages: (1) a computer-assisted telephone interview on socio-demographic data, lifestyle, health status and morbidity; (2) a first home visit to perform a physical examination and obtain biological samples and (3) a second home visit, conducted 7 d after the first one, to take a diet history and obtain other questionnaire data. Participants were suggested to have a proxy to accompany them for the home visits to help them respond to the diet history and to help them feel comfortable and safe. At the end of the first home visit, an accelerometer was located on the wrist of participants and was returned during the second visit, 7 d apart(Reference Cabanas-Sánchez, Esteban-Cornejo and Migueles21). Study participants gave written informed consent. The study was approved by the Clinical Research Ethics Committee of ‘La Paz’ University Hospital in Madrid (Spain).
Study variables
Diet
Trained interviewers obtained information on food consumption through a validated computerised dietary history, which was developed from the one used in the EPIC-Spain cohort study(Reference Guallar-Castillón, Sagardui-Villamor and Balboa-Castillo22). The diet history included 880 foods along with 127 sets of photographs to help estimate the serving size. This instrument collects food consumption by occasions of intake and accommodates habitual diet information to a 24-h format, by asking participants to indicate the time in which they usually had their meals, including snacks. Fasting time was defined as the window (in h) between the last food ingested before going to sleep and the first food consumed upon getting up in the morning. Participants were classified in three categories: those reporting a fasting time of ≤9 h/d, those with fasting time between 10 and 11 h/d and those with fasting time ≥12 h/d, which can be considered prolonged nightly fasting(Reference Di Francesco, Di Germanio and Bernier12).
Nutrient intakes were estimated using standard food composition tables for the Spanish population(Reference Moreiras, Carbajal and Cabrera23). The Mediterranean Diet Adherence Screener score was also estimated for all participants in order to define the overall diet quality(Reference Schröder, Fitó and Estruch24). This score ranged from 0 to 14, and higher scores indicated greater adherence and thus higher diet quality. The validity of the diet history was evaluated by comparing results from this instrument with seven 24-h recalls over a 1-year period among a subsample of participants; the observed correlations ranged between 0·27 and 0·71 across food groups and nutrients, which are in line with those for most instruments assessing self-reported diet in population studies(Reference Yuan, Spiegelman and Rimm25).
Physical function
We assessed lower-extremity function with the Short Physical Performance Battery (SPPB), which includes three timed tasks: gait speed, standing balance and ability to rise from a chair(Reference Guralnik, Ferrucci and Pieper26,Reference Guralnik, Ferrucci and Simonsick27) . Gait speed was calculated as the time that a participant walked at usual pace across 2·44 m, timed from a standing position. The standing balance test evaluated the time that participants could maintain three hierarchical tandem positions: side-by-side, semitandem and tandem positions. The ability to rise from a chair was assessed by the time required to stand up and sit down from a chair five times consecutively as fast as possible and with their arms folded across their chest. Each test was scored from 0 to 4, and the total SPPB score was the sum of these three components (range 0–12); a higher score indicates better physical performance. We used the standard cut-off of ≤9 to define impaired lower-extremity function (ILEF). In addition, balance impairment, difficulty to raise from a chair and slow gait were defined as a score of ≤3 in each scale(Reference Guralnik, Ferrucci and Pieper26).
Other variables
We obtained information on age, sex, education (primary, secondary or university level), smoking status (never, former or current smoker), sedentary behaviour (h/week spent watching television), alcohol intake (g/d), sleep duration (h/d) and energy intake (kJ/d). Physical activity (metabolic equivalent-h/week) was assessed with the ActiGraph GT9X (ActiGraphInc) accelerometer, which was asked to be worn for seven consecutive days(Reference Cabanas-Sánchez, Esteban-Cornejo and Migueles21). Weight and height were measured under standardised conditions, using electronic scales and portable extendable stadiometers. BMI was calculated as weight (kg) divided by height squared (m2) and classified as <25, 25–29·9 or ≥30 kg/m2. In addition, the following physician-diagnosed diseases were self-reported: musculoskeletal disease, CVD, diabetes, cancer, chronic lung disease and depression requiring treatment.
Statistical analyses
From the 3273 participants in the cohort, we selected the 1732 participants with information on timing of food consumption. Then, we excluded one participant with implausibly energy intake and another one without information on educational level. Additionally, we excluded 454 individuals without measurement of SPPB and fifty without accelerometry. Thus, the analyses were conducted with 1226 individuals. There is no available power calculation for these analyses since data were obtained from a population study with multiple a priori hypothesis based on lifestyles and healthy ageing; the analyses of this manuscript were performed only among the available sample with information on habitual fasting.
Participants’ characteristics were summarised as means and standard deviations for continuous variables and as percentages for categorical variables. Differences in characteristics across participants in the different fasting categories were tested by ANOVA for quantitative variables and by the χ 2 test for categorical variables. Logistic regression was used to estimate the OR and the 95 % CI for the association between different fasting categories and ILEF.
Several logistic models were built with sequential adjustment for potential confounders: the first one included sex, age and energy intake; the second one was additionally adjusted for educational level, smoking status, sedentary behaviour, alcohol intake (g/d), BMI, morbidity, sleep duration, protein intake and Mediterranean Diet Adherence Screener score and the third model was also adjusted for physical activity since this variable could also be considered a potential mediator in the studied association. Additionally, we assessed the linear dose–response relationship (P trend) by modelling fasting time as a continuous variable. To test non-linear risk trends, we used three knot-restricted cubic splines for fasting time and the risk of ILEF. Test for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. The association between prolonged nightly fasting and each SPPB component was also evaluated.
We replicated the analyses for the associations by strata of sex, BMI (<30, ≥30 kg/m2), sleep time (above and below the median), depression (no, yes), energy intake (above and below the median), total protein intake (above and below the median) and diet quality (above and below the median) to assess the robustness of the results. The possible modifying effect of these variables on the studied association was assessed with the likelihood-ratio test. Finally, we examined the joint effect of fasting categories and physical activity on ILEF, by considering physical activity in tertiles and using as reference category the simultaneous condition of a fasting time of ≤9 h/d and being in the highest tertile of physical activity. Last, in a sensitivity analysis, we examined the impact of depression in the studied association by excluding participant with a diagnosis of this disease. Statistical significance was set at two-tailed P < 0·05. Analyses were conducted using Stata (version 15.1; Stata Corp.)
Results
Characteristics of study participants according to the three fasting time categories are presented in Table 1. Those with prolonged nightly fasting (≥12 h) were significantly older, more often women, never smokers, more likely to have a diagnosis of depression and reported more hours of sleep, compared with those in the shortest fasting time category. In addition, total energy, protein and fat intake were lower in those with prolonged nightly fasting, although adherence to the diet quality index was similar across groups.
Table 1. Baseline characteristics of the study participants (n 1226) by categories of fasting time
(Mean values and standard deviations; numbers and percentages)

MEDAS, Mediterranean Diet Adherence Screener.
* ANOVA test was used for quantitative variables and the χ 2 test for categorical variables.
† Osteo-arthritis, arthritis and hip fracture.
‡ IHD, stroke and heart failure.
In Table 2, a significant association between a longer fasting time and ILEF was found; in age-, sex- and energy intake-adjusted models, the OR for 10–11 and ≥12 h/d of fasting were 2·06 (95 % CI 1·44, 2·94) and 2·56 (95 % CI 1·76, 3·73), respectively, using ≤9 h as the reference category (P trend < 0·001). After additional adjustment for other potential confounders including physical activity, we obtained similar results (OR 2·27 (95 % CI 1·56, 3·33); 2·70 (95 % CI 1·80, 4·04); P trend < 0·001). Fasting time also showed a significant association with balance impairment (OR for longest v. shortest fasting time: 2·48; 95 % CI 1·51, 4·08; P trend = 0·001) and difficulty to rise from a chair (OR 1·47; 95 % CI 1·05, 2·06; P trend = 0·01). Slow gait speed was not associated with fasting.
Table 2. Association between fasting time categories and impaired lower-extremity function (ILEF), balance impairment, difficulty to rise from a chair and slow gait
(Odds ratios and 95 % confidence intervals, n 1226)
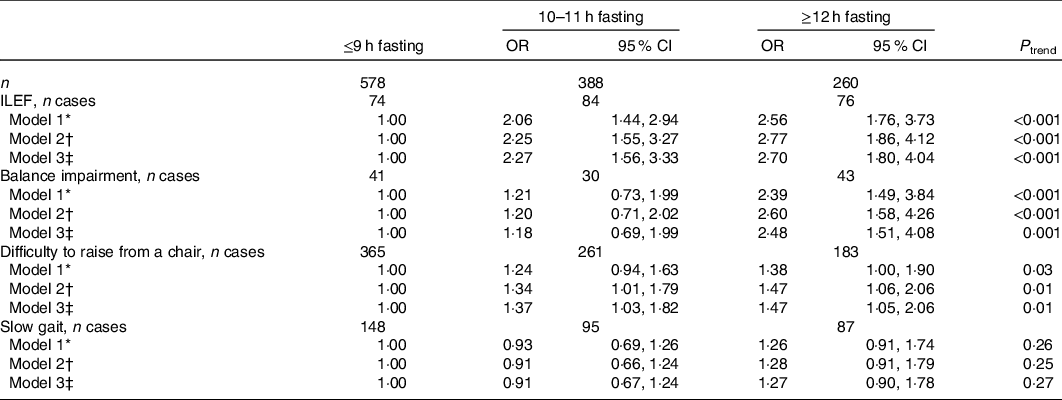
* Model 1: OR (95 % CI) adjusted for sex, age and energy intake (quintiles of kJ/d).
† Model 2: OR (95 % CI) additionally adjusted for educational level (≤primary, secondary or university), smoking status (never, former, current smoker), sedentary behaviour (tertiles of h/week watching television), alcohol intake (quintiles of g/d), BMI (<25, 25–29·9, ≥30 kg/m2), morbidity (musculoskeletal disease, CVD, cancer, diabetes, chronic lung disease and depression), sleep duration (tertiles of h/d), protein intake (quintiles of g/d) and for Mediterranean Diet Adherence Screener score (tertiles).
‡ Model 3: OR (95 % CI) additionally adjusted for physical activity (tertiles of MET-h/week).
The dose–response association between the fasting time and ILEF is assessed in Fig. 1; the test for non-linearity was not significant, except for gait speed (P ≤ 0·001). The association between different fasting categories and ILEF was assessed by strata of sex, BMI, sleep, depression, energy intake, total protein intake and diet quality and is shown in Table 3. No significant differences were identified across the strata (P interaction > 0·05 in all cases). In addition, when fasting time and physical activity were considered simultaneously, ≥12 h fasting had double risk of ILEF than those with <9 h of fasting (OR 1·50, 95 % CI 0·62, 3·60 v. OR 1) among those with high levels of physical activity. The risk associated with ≥12 h fasting among those with the lowest levels of physical activity was three times higher than among those with ≤9 h fasting with the same low level of physical activity (OR 4·60, 95 % CI 1·42, 14·84 v. OR 1·55, 95 % CI 0·49, 4·88) (P interaction < 0·001) (Fig. 2). Last, sensitivity analyses by excluding participants with depression yielded similar associations than in the main tables (online Supplementary Table S1).

Fig. 1. Multivariable-adjusted spline curves for the relation between fasting time and the risk of impaired lower-extremity function. Adjusted for sex, age, educational level (≤primary, secondary or university), smoking status (never, former, current smoker), sedentary behaviour (h/week watching television), alcohol intake (quintiles of g/d), BMI (<25, 25–29·9, ≥30 kg/m2), morbidity (musculoskeletal disease, CVD, cancer, diabetes, chronic lung disease and depression), sleep duration (tertiles of h/d), energy intake (quintiles of kJ/d), protein intake (quintiles of g/d), Mediterranean Diet Adherence Screener score (tertiles), and physical activity (tertiles of MET-h/week).
Table 3. Association between fasting time categories and impaired lower-extremity function, by specific subgroups of older adults*
(Odds ratios and 95 % confidence intervals, n 1226)
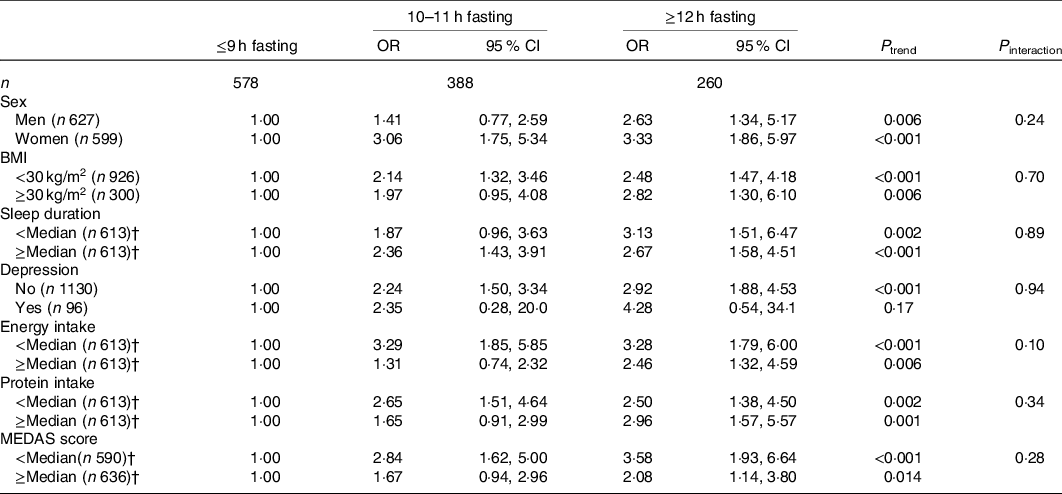
MEDAS, Mediterranean Diet Adherence Screener.
* From logistic regression models adjusted for sex, age, educational level (≤primary, secondary or university), smoking status (never, former, current smoker), sedentary behaviour (h/week watching television), alcohol intake (quintiles of g/d), BMI (<25, 25–29·9, ≥30 kg/m2), morbidity (musculoskeletal disease, CVD, cancer, diabetes, chronic lung disease and depression), sleep duration (tertiles of h/d), energy intake (quintiles of kJ/d), protein intake (quintiles of g/d), MEDAS score (tertiles) and physical activity (tertiles of MET-h/week) except for the stratification variable.
† Median sleep duration: 7 h; median energy intake: 7669 kJ; median protein intake: 90·3 g; median MEDAS score: 7.

Fig. 2. Odds ratios for the joint association of fasting time and physical activity categories with impaired lower-extremity function. Adjusted for sex, age, educational level (≤primary, secondary or university), smoking status (never, former, current smoker), sedentary behaviour (h/week watching television), alcohol intake (quintiles of g/d), BMI (<25, 25–29·9, ≥30 kg/m2), morbidity (musculoskeletal disease, CVD, cancer, diabetes, chronic lung disease and depression), sleep duration (tertiles of h/d), energy intake (quintiles of kJ/d), protein intake (quintiles of g/d) and Mediterranean Diet Adherence Screener score (tertiles). Cut-off points to define levels of physical activity were: ≤57·5 (low), 57·6–82·0 (intermediate) and ≥82·1 MET-h/week (high). Reference category included participants with ≤9 h/d of fasting time and with high physical activity level. ![]() , High physical activity;
, High physical activity; ![]() , intermediate physical activity;
, intermediate physical activity; ![]() , low physical activity.
, low physical activity.
Discussion
In this cross-sectional study of community-dwelling older adults, a fasting time of more than 12 h/d was associated with a higher risk of ILEF, balance impairment and difficulty to rise from a chair. These associations were robust across different strata, including sex, BMI, having depression, energy intake, total protein intake and diet quality. In addition, these results held on different levels of physical activity, which suggests that despite the beneficial effect of physical activity on function, fasting time has an independent association with this endpoint in older adults. Notwithstanding this, high physical activity could compensate for part of the excess frequency of ILEF associated with longer fasting.
Most of the evidence on the effects of intermittent fasting has been obtained in animal studies(Reference Melkani and Panda4–Reference Goodrick, Ingram and Reynolds6). Human studies have been restricted to younger adults in experimental designs with extreme intermittent fasting and a small number of participants(Reference Marianna, Iolanda and Andrea7–Reference Hutchinson, Regmi and Manoogian9). To our knowledge, this is the first study assessing fasting in association with ILEF. Our findings of an increased risk of balance impairment in participants on prolonged nightly fasting are in line with previous publications in older adults who practiced Ramadan fasting(Reference Laatar, Borji and Baccouch28,Reference Laatar, Baccouch and Borji29) . A study among fifteen older men showed that fasting approximately 17 h/d for 4 weeks had a detrimental effect on postural balance performance and in simple reaction time(Reference Laatar, Borji and Baccouch28). Another study compared the effects of Ramadan fasting on postural control between a group of twelve participants with a history of at least two spontaneous and unexpected falls during the previous year and a group of twelve non-fallers. Ramadan fasting had similar adverse effects on postural control in both groups that could lead to balance dysfunction and mobility limitation in older adults(Reference Laatar, Baccouch and Borji29). Despite Ramadan fasting is a common variety of time-restricted fasting, it is of note that fasting time is approximately 12 h/d from dawn to sunset(Reference Patterson and Sears17), which contrasts with prolonged nightly fasting in our study and with usual time-restricted diets.
In our population, fasting time was not associated with the risk of slow gait. The tests included in the SPPB reflect different abilities. Balance and difficulty to raise from a chair reflect diminished muscle mass and energy deficit, and slow walking speed integrates these aspects with neurological control(Reference Guralnik, Ferrucci and Pieper26). We can hypothesise that prolonged nightly fasting may have a detrimental effect on some aspects related to physical function, such as muscle mass and strength but not in others related to neurological function. Of note, in a small non-randomised clinical trial among ten overweight older adults who were asked to fast for 16 h/d during 4 weeks, slow gait, grip strength and quality of life were improved, although this may be driven by the weight loss that all participants experienced, which was the main target of the intervention(Reference Anton, Lee and Donahoo13). This study lacked of a control group, so causal effects were not demonstrated. In another recent clinical trial(Reference Martens, Rossman and Mazzo30), a maintained 14 h/d of fasting for 6 weeks without weight loss did not influence lean mass, bone density or nutrient intake. In our results, stratification for BMI level showed a similar association of prolonged nightly fasting with ILEF; however, since this was a cross-sectional study, we could not assess whether weight loss mediated the study association.
It is well known that the best approach to counteract physical function impairment is regular practice of physical activity(Reference Simonsick, Guralnik and Volpato31–Reference Yorston, Kolt and Rosenkranz33). In our study, although participants with a higher level of physical activity had a lower risk of ILEF than their less active counterparts, the augmented risk of ILEF associated with longer fasting was present at all physical activity levels. Adequate dietary protein intake is also strongly related to physical function. Proteins have been associated with maintenance of physical function, muscle mass and bone mass density(Reference Coelho-Júnior, Milano-Teixeira and Rodrigues34–Reference Rizzoli, Biver and Bonjour38) and have been shown to prevent frailty in older adults(Reference Otsuka, Tange and Tomida39). A previous study found that a higher intake of dietary animal protein in combination with physical activity was associated with higher skeletal muscle mass and a lower likelihood of functional limitation development(Reference Bradlee, Mustafa and Singer40). In our study, participants on prolonged nightly fasting had lower intake of dietary protein and energy in comparison with participants with fasting time <9 h/d; however, all participants in the three fasting groups had a protein intake above the current RDA, of 0·8 g protein/kg per d(41,Reference Estrada-DeLeón, Struijk and Caballero42) . Despite participants on prolonged nightly fasting were meeting their adequate intake of proteins, prolonged fasting might reduce the intake of other relevant nutrients for older adults resulting in reduced function.
The involuntary loss of appetite that leads to reduced food intake is a common problem in older adults, usually known as anorexia of ageing(Reference Martone, Onder and Vetrano43). Several factors for anorexia of ageing have been described(Reference Landi, Calvani and Tosato44), including hearing and vision impairment, specific medical conditions such as gastrointestinal diseases, poly-medication, social factors including social isolation and depression. In our study, participants on prolonged fasting presented more depression than those who did not fast ≥12 h. A recent study in community-dwelling older adults found a higher prevalence of depression in participants with poor appetite, in comparison with those with normal appetite. Also, they found an association between poor appetite and lower skeletal muscle mass, lower grip strength and low muscle mass(Reference İlhan, Bahat and Erdoğan45). However, in our sensitivity analysis among participants without depression, we still found a detrimental association between prolonged nightly fasting and physical function; therefore, this association was not totally explained by the effect of depression on eating patterns. Whether other causes for the anorexia of ageing may explain prolonged nightly fasting among our study participants and are ultimate responsible of the association found will require additional research.
A variety of intermittent fasting regimens such as time-restricted fasting, periodic fasting, complete alternate-day fasting, modified fasting regimens and religious fasting have been associated with health benefits, including improvements in glucose regulation, blood pressure and heart rate(Reference Mattson, Allison and Fontana2,Reference Anton, Moehl and Donahoo11,Reference Di Francesco, Di Germanio and Bernier12,Reference Antunes, Erustes and Costa16–Reference Froy and Miskin18) . There are also evidence of a beneficial effect of these regimes on stress resistance pathways, including increased expression of antioxidant defences, DNA repair, protein quality control, mitochondrial biogenesis and autophagy and down-regulation of inflammation(Reference De Cabo and Mattson10). However, intermittent fasting has been mostly studied as a practical approach to restrict energy intake for weight loss(Reference Anton, Lee and Donahoo13). Thus, the extrapolation of the health benefits of common intermittent fasting to the general population is difficult. In addition, since prolonged nightly fasting is unintentional, assumption of similar effects of intermittent fasting and prolonged nightly fasting is un-appropriated.
One of the strengths of this study was the assessment of fasting time through a validated diet history that collected habitual diet information in a 24-h format. Another strength was the adjustment for many potential confounders, including sleep duration, energy intake, protein intake, co-morbidities and physical activity. Additionally, the use of an accelerometer was a more reliable way to assess physical activity than self-reported questionnaires, since it provided objective measures on 24-h activity cycle.
On the other hand, the main limitation of this study was the cross-sectional design, which does not allow for causal inference. The possibility for reverse causation in the interpretation of the results is plausible; for example, it is possible that prolonged nightly fasting only reflects a situation in which participants have already developed certain degree of disability that force them to stay longer time in bed, needing to wait for external help to get up and eat. Another limitation of this study is that weight changes associated with fasting could not have been assessed because of the study design; thus, whether weight loss is a mediator in the association between prolonged nightly fasting and ILEF is uncertain. Moreover, as in all population studies, some residual and unmeasured confounding cannot be ruled out, despite the associations between the main variables studied were adjusted for an important number of potential confounders. Last, since the cross-sectional design in this study, OR obtained should not be interpreted as proxies of relative risk for incidence of ILEF.
In conclusion, fasting time ≥12 h/d was associated with a higher frequency of ILEF, balance impairment and difficulty to rise from a chair in older adults, independent of physical activity level. These results suggest a detrimental effect of prolonged nightly fasting in older adults. Further longitudinal studies of fasting in relation to ILEF would be valuable to understand if this is a causal association.
Acknowledgements
This work was supported by Consejo Nacional de Ciencia y Tecnología de México (CONACyT 493867), FIS grants 16/609, 16/1512 and 19/319 (Instituto de Salud Carlos III, State Secretary of R+D+I, and FEDER/FSE); the ‘Cátedra UAM Epidemiología y control del riesgo cardiovascular’ (no. 820024) and the Joint Programming Initiative - A Healthy Diet for a Healthy Life (SALAMANDER project; State Secretary of R+D+I PCIN-2016-145). Mercedes Sotos Prieto holds a Ramón y Cajal contract from the Ministry of Science, Innovation and Universities and FEDER/FSE.
The funding agencies had no role in study design, data analysis, interpretation of results, manuscript preparation or in the decision to submit this manuscript for publication.
D. B. E., E. A. S. and E. L. G. designed the research; D. B. E. and F. F. C. performed the statistical analyses; D. B. E., E. A. S. and E. L. G. drafted the manuscript; E. L. G. supervised the conduct of research and had primary responsibility for final content; all authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520005218








