Introduction
Omicron BA.2, the dominant global variant of SARS-CoV-2 at the time of this study, has evolved from the original SARS-CoV-2 virus to be less virulent but much more transmissible, with an average R0 of 8.2 (World Health Organization, 2022). Lockdown policies were implemented to try to suppress viral community transmission and buy time for vaccination coverage (Ge et al., Reference Ge, Zhang, Wu, Ruktanonchai, Liu, Wang and Lai2022; Zhang, Zhang, & Chen, Reference Zhang, Zhang and Chen2022b). However, long-term lockdowns increase the risk of mental-health problems, especially among children with autism spectrum disorder (ASD) (Aknin et al., Reference Aknin, Andretti, Goldszmidt, Helliwell, Petherick, De Neve and Zaki2022; Butterworth, Schurer, Trinh, Vera-Toscano, & Wooden, Reference Butterworth, Schurer, Trinh, Vera-Toscano and Wooden2022). Identifying risk and protective factors for ASD clinical symptom exacerbation while under lockdown is essential to minimizing unintentional effects of any future lockdowns, but also for identifying important targets for therapeutic intervention.
Studies have reported an association between lockdowns and increased behavioral problems and psychiatric symptoms in children with ASD, including social impairment (Mutluer, Doenyas, & Aslan Genc, Reference Mutluer, Doenyas and Aslan Genc2020; Polónyiová et al., Reference Polónyiová, Belica, Celušáková, Janšáková, Kopčíková, Szapuová and Ostatníková2022; Vasa et al., Reference Vasa, Singh, Holingue, Kalb, Jang and Keefer2021). However, previous studies generally do not include pre-pandemic baseline data, and therefore are likely to contain recall bias (Adams, Zheng, Taylor, & Bishop, Reference Adams, Zheng, Taylor and Bishop2021; Vasa et al., Reference Vasa, Singh, Holingue, Kalb, Jang and Keefer2021). In addition, it was difficult to ascertain the extent of the lockdown from studies, which made it difficult to distinguish the effects of the lockdown from the effects of pandemic itself (Fong et al., Reference Fong, Cornish, Kirk, Ilias, Shaikh and Golden2021; Mutluer et al., Reference Mutluer, Doenyas and Aslan Genc2020; Polónyiová et al., Reference Polónyiová, Belica, Celušáková, Janšáková, Kopčíková, Szapuová and Ostatníková2022). The Omicron wave in China provided a unique opportunity to assess the effects of lockdowns on children with ASD in the setting of a low infection risk, low virulence virus, and strict restrictions (Burki, Reference Burki2022; Zhang et al., Reference Zhang, Zhang and Chen2022b). In Shanghai, where a citywide lockdown was implemented, only around 3% of residents were infected as of May 2022, and most were asymptomatic (Zhang et al., Reference Zhang, Zhang and Chen2022b). The regular follow-up plan of the Shanghai Autism Early Developmental Cohort (SAED-Cohort, Dai et al., Reference Dai, Liu, Zhang, Ren, Wang, Yu and Li2022) enabled us to compare the pre- and post-lockdown data of children with ASD in this current study.
While the pandemic was stressful, lockdowns created additional stresses for children and families. Being confined in relatively small spaces, not having access to the usual social activities and disruption to enjoyable activities and routines increased stress and mental health disorders. The long lockdowns led to chronic stress, especially when it was unclear how long the lockdown would continue for. Importantly, the response of ASD children to a lockdown can be modulated by parent/family-related factors (Aknin et al., Reference Aknin, Andretti, Goldszmidt, Helliwell, Petherick, De Neve and Zaki2022; Frigerio, Nettuno, & Nazzari, Reference Frigerio, Nettuno and Nazzari2023). It has been previously shown that parent/family-related factors, such as parental personality, resilience, emotional regulation, and family functioning, are associated with both parenting and symptom improvement in children with ASD (Bader & Barry, Reference Bader and Barry2014; Bekhet, Johnson, & Zauszniewski, Reference Bekhet, Johnson and Zauszniewski2012; Li et al., Reference Li, Wang, Wu, Wang, Huang and Li2017; Pruitt, Willis, Timmons, & Ekas, Reference Pruitt, Willis, Timmons and Ekas2016; Sekułowicz, Kwiatkowski, Manor-Binyamini, Boroń-Krupińska, & Cieślik, Reference Sekułowicz, Kwiatkowski, Manor-Binyamini, Boroń-Krupińska and Cieślik2022). During the initial COVID-19 waves, fewer maternal mood symptoms were associated with fewer emotional and behavioral problems in typically developing children during lockdowns (Frigerio et al., Reference Frigerio, Nettuno and Nazzari2023). However, the impact of parent/family modulation of the effects of pandemic lockdown on children with ASD remains unclear. The SAED cohort enabled us to investigate this modulatory effect.
The response of ASD children to lockdowns may also depend on their pre-pandemic symptoms and brain development. In healthy adolescents, pre-pandemic functional brain connectivity, in particular weaker connectivity within the frontal-parietal network, predicted increased anxiety during the COVID-19 pandemic (He et al., Reference He, Wei, Yang, Zhang, Cheng, Feng and Qiu2021). However, no neuroimaging evidence was available for children with ASD. In our study, we performed the first neuroimaging study of children with ASD to examine the pre-pandemic social brain, as defined by the neurosynth meta-analysis, to predict their response to a lockdown.
We hypothesized that the effects of a lockdown on core pediatric ASD symptoms are influenced by complex interactions between the duration of lockdown, the child's pre-pandemic behavioral symptoms, brain development, and parent/family-related factors. The study used the data collected within 6 months of the Omicron lockdown and the data from a follow-up assessment close to the end of the lockdown in the SAED-Cohort. We first evaluated the impact of the omicron lockdown on the child's clinical symptoms. We then explored the modulatory effects of parent/family-related factors. Lastly, we examined the contributions of pre-pandemic brain development to these associations.
Methods
Participant recruitment and assessment
The children with ASD who were involved in this study were already participants in the ASD registry cohort of SAED which contained integrative datasets that included neuroimaging, clinical assessments, and neuropsychology tests (Dai et al., Reference Dai, Liu, Zhang, Ren, Wang, Yu and Li2022; Ye, Reference Ye2022). The ASD registry cohort is a clinical cohort recruiting patients diagnosed with ASD in Xinhua Hospital, Shanghai, China, a tertiary hospital receiving patients from across the country. The diagnosis of ASD was established according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, then confirmed using the Autism Diagnostic Observation Schedule. ASD children in the SAED-Cohort had regular follow-ups, at 3–6 months, 1 year, and every 6 months thereafter. From 5 to 31 May 2022, we contacted the closest caregiver of each recruited ASD child who underwent a baseline assessment from 29 September 2021, to 28 March 2022 (Supplementary Fig. S1 ). The follow-up system was designed using the Fudan Toolbox of Neuropsychological tEst And Training (F-NEAT; fneat.medmine.com.cn) platform. We required that the questionnaire could be only submitted when all questions were answered. A total of 188 parents (of 188 ASD children) completed the follow-up program, which consisted of a virtual clinician interview and surveys regarding the omicron pandemic, family factors, and the child's behavioral problems.
Lockdown
In late February 2022, a wave of omicron infection started in Shanghai, China (Zhang et al., Reference Zhang, Zhang and Chen2022b). Due to the high transmissibility of the omicron variant, China implemented a dynamic zero-COVID policy with strict and comprehensive pandemic control strategies that included a lockdown of districts with outbreaks (Burki, Reference Burki2022; Zhang et al., Reference Zhang, Zhang and Chen2022b). We used an adjusted version of the Stringency Index (from 0 to 23, the strictest) created by the Oxford COVID-19 Government Response Tracker (OxCGRT) to quantify policy stringency in the residences of the children included in this study (Aknin et al., Reference Aknin, Andretti, Goldszmidt, Helliwell, Petherick, De Neve and Zaki2022; Hale et al., Reference Hale, Anania, de Mello, Angrist, Barnes, Boby and Zhang2022). The Stringency Index included the following areas: school closures, workplace closures, cancellation of public events, restrictions on gatherings, public transportation closures, stay at home requirements, restrictions on domestic travel, and international travel restrictions (Aknin et al., Reference Aknin, Andretti, Goldszmidt, Helliwell, Petherick, De Neve and Zaki2022). Cities under lockdown, primarily Shanghai, had high stringency scores of 19–21, while cities without lockdowns scored from 2 to 7. Restriction policies were generally stable during the lockdown as a part of the dynamic zero-COVID policy (Burki, Reference Burki2022). Whether to lockdown and the duration of lockdown were decided by the local government authorities. This information was self-reported by the caregivers of children with ASD and was collected with online survey. Children under lockdown were further categorized into shorter duration, strict lockdown group (<35 days) and longer duration, strict lockdown group (⩾35 days) by the median of their lockdown duration. In the current sample, 85 children were lockdown-free, 52 children were in the longer duration, strict lockdown group and 51 in the shorter duration, strict lockdown group.
Measurements
On enrollment, autistic children were assessed with social responsiveness scale (SRS), the strengths and difficulties questionnaire (SDQ), and the children's sleep habits questionnaire (CSHQ). Baseline structural neuroimaging was performed. The demographic information, such as sex, age, parental education, and parental income, was also collected at baseline. Data regarding pediatric outcomes as well as omicron-related stress and familial factors related with coping strategies were collected during the omicron pandemic.
Pediatric outcome measures
Clinical global impression (CGI) scale
The CGI-Severity (CGI-S) and CGI-Improvement (CGI-I) scales were used to evaluate the overall severity and degree of improvement of the child's symptoms compared to baseline (Zhang et al., Reference Zhang, Huang, Dai, Luo, Ji, Wang and Li2020). These ratings were made by developmental behavioral pediatricians during the virtual clinician interview.
Social responsiveness scale
ASD symptoms were evaluated using the SRS (Patel et al., Reference Patel, Masi, Dale, Whitehouse, Pokorski, Alvares and Guastella2018). The SRS provides a total score and five separate sub-scores: awareness, cognition, communication, motivation, and mannerisms. Higher SRS scores indicate increased ASD-related social impairment.
Strengths and difficulties questionnaire
The SDQ was used to identify coincident behavioral and emotional problems in the child (Croft, Stride, Maughan, & Rowe, Reference Croft, Stride, Maughan and Rowe2015; Lai et al., Reference Lai, Luk, Leung, Wong, Law and Ho2010). SDQ items are divided into five subscales: emotional symptoms, behavioral problems, hyperactivity, peer relationship problems, and prosocial behaviors. A higher score implies greater difficulty for all sub-scores except for prosocial behavior, where a higher score is better than a lower one.
Children's sleep habits questionnaire
The parent completed the CSHQ (Owens, Spirito, & McGuinn, Reference Owens, Spirito and McGuinn2000; Tan, Wang, Cheah, & Wang, Reference Tan, Wang, Cheah and Wang2018). The items of CSHQ were conceptually grouped into eight subscales reflecting the sleep habits and behaviors of young children at night and in the daytime (e.g. bedtime resistance and sleep duration). Total Sleep Disturbance score included all items of the eight subscales.
Survey on the omicron pandemic
A questionnaire was administered to caregivers regarding omicron infection in their neighborhood, stress related to the pandemic, and difficulties with ASD interventions (detailed in online Supplementary materials).
Family factor scales
Big five inventory (BFI)
Big five personality traits were measured with the BFI: dimensions of extraversion (e.g. talkative, energetic), agreeableness (e.g. merciful, warmhearted), conscientiousness (e.g. efficient, systematic), neuroticism (e.g. emotional, anxious), and openness (e.g. creative, artistic) (Zhang et al., Reference Zhang, Li, Li, Luo, Ye, Yin and John2022a). Higher scores indicated higher personality trends in that corresponding dimension.
10-item Connor–Davidson resilience scale (CD-RISC-10)
The self-administered abridged CD-RISC-10 scale reflects the participant's ability to tolerate experiences (e.g. illness, pressure, and failure) using the items such as ‘Able to adapt to change’ and ‘Coping with stress can strengthen me’ (Wang, Shi, Zhang, & Zhang, Reference Wang, Shi, Zhang and Zhang2010). The final score is the sum of the responses to each item, with higher scores indicating a higher resilience capacity.
Family adaptability and cohesion evaluation scale, second edition – Chinese version (FACESII-CV)
The FACESII-CV measures both family adaptability and cohesion (Li et al., Reference Li, Li, Wu, Cao, Zhang, Li and Kong2021). Adaptability refers to the ability of the family to adjust in response to problems arising during different stages of familial development using questions such as ‘when problems arise we compromise’ and ‘family members say what they want’, while cohesion refers to emotional connections between family members using questions such as ‘family members know each other's close friends’ and ‘our family does things together’. Higher scores correspond with greater adaptability and cohesion.
Emotion regulation questionnaire (ERQ)
The ERQ is a 10-item self-report questionnaire that is divided into two subscales: cognitive reappraisal including items such as ‘I control my emotions by changing the way I think about the situation I'm in’ and expressive suppression including items such as ‘When I am feeling negative emotions, I make sure not to express them’ (Tyra, Griffin, Fergus, & Ginty, Reference Tyra, Griffin, Fergus and Ginty2021). Higher subscale scores indicate higher use of emotion regulation strategies.
MRI acquisition and pre-processing
Image acquisition
Participants were scanned using a Siemens Verio 3.0-Tesla MRI scanner or Philips Inginia 3.0-Tesla MRI scanner, with a 32-channel head coil and four-channel neck coil. Earplugs, earphones, and extra foam padding were provided to the participants to reduce the impact of the sound of the scanner during the scan. A high-resolution anatomical T1-weighted magnetization-prepared rapid gradient echo image (Siemens: 192 sagittal slices; voxels = 1 × 1 × 1 mm3; repetition time [TR] = 2300 ms; echo time [TE] = 2.28 ms; inversion time = 1100 ms; flip angle = 8°, field of view = 192 × 192 × 192 mm3; Philips: 170 sagittal slices; voxels = 1 × 1 × 1 mm3; TR = 7900 ms; TE = 3.5 ms; inversion time = 1100 ms; flip angle = 7°, field of view = 250 × 193 × 170 mm3) was acquired. The scanner effect was controlled as the covariate during analysis.
Image pre-processing
T1-weighted images that passed visual inspection were processed using statistical parametric mapping SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/) with the computational anatomy toolbox CAT12, and incorporating the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) toolbox. The steps included segmentation, registration, normalization, and smoothing. Notably, the Template-O-Matic Toolbox was used to customize tissue probability maps (TPMs) for 6-year-olds (Zhao et al., Reference Zhao, Liao, Fonov, Wang, Men, Wang and He2019). These customized TPMs were used for the initial spatial registration and segmentation. A standard optimized method of iterative tissue segmentation and spatial normalization, using both linear (12-parameter affine) and non-linear transformations, was performed. So that the residuals in later analyses conformed more closely to a Gaussian distribution and to account for individual differences in brain anatomy, the modulated grey matter images in the MNI space were smoothed with an isotropic Gaussian kernel of 8 mm full-width at half maximum. The resulting voxel size was 1.5 × 1.5 × 1.5 mm3. The overall weighted image quality rating (IQR) score was employed and image rated as critical and unacceptable/failed would not pass the quality control. In this study, 185 of the 188 (98.4%) T1 structure imaging were rated as good (IQR score between 80 and 90) and the other three (1.6%) were rated as satisfactory (IQR between 70 and 80), the ratings that typical clinical data expected to get (https://neuro-jena.github.io/cat12-help/). The following neuroimaging analyses were performed on the social brain within the ‘social’ association test map/mask, extracted from the Neurosynth database (http://neurosynth.org).
Statistical analysis
Analysis of clinical symptoms
Enrolled children were categorized by their lockdown duration: no lockdown, shorter duration strict lockdown (less than 35 days), and longer duration strict lockdown (35 or more days). To compare the CGI-I scores among the three groups, we set the lockdown-free group as the control group, and used a linear regression, adjusted for sex, age, parental education, and parental income. This set of variables were adjusted in all the following analyses. The mixed-effects models were used for SRS, SDQ, and CSHQ scores, and their subscales, to determine whether time × group interactions were significant. We tested the fixed effects of time (0, baseline; 1, follow-up), group (no, shorter duration, and longer duration lockdown), and their interactions (time × group) by assuming customized random intercepts for each subject. The likelihood ratio test was used to compare the fits of the mixed-effect model assuming random slope and random intercept and the model assuming only random intercept for each subject, on scales with significant effect. Adjustment for multiple comparisons was performed at p = 0.05 using the false discovery rate (FDR) approach (Benjamin–Hochberg–adjusted p value). Analyses were performed with R version 4.0.4. The code is available at the following link: https://github.com/rt-asdf/Omicron_ASD_SAED_2022.
In the analysis of associations between a longer duration strict lockdown and CGI-I, we evaluated potential effect modification by family-related factors, including parental BFI, CD-RISC-10, FACESII-CV, and ERQ scores. We used a linear regression model to test whether there was a significant moderator × group (longer duration lockdown) interaction.
Analysis on the modulation effect of brain volumes
To investigate whether the child's social brain volume modified the relationship between lockdown status and CGI-I score, we assessed interactions between lockdown status and brain volume using voxel-wise linear regression models restricted within the social brain mask defined by NeuroSynth, adjusted for age, sex, IQR score, total intracranial volume (TIV), scanner, parental education, and parental income. We identified significant clusters using an FDR correction, with cluster sizes greater than 217 voxels (Luo et al., Reference Luo, Chen, Wang, Desrivières, Quinlan, Jia and Feng2019).
To investigate whether brain volume modulates the relationship between lockdown status and parent/family factors on CGI-I scores, we assessed voxel-wise interaction against lockdown status, parent/family factors, and brain volume. Age, sex, IQR score, TIV, scanner, parental education, and parental income were again included as covariates, and an FDR correction was used to control for family-wise error. Sensitivity analysis was also performed when we further adjusted for the baseline clinical social symptoms, the SRS total score and the SDQ prosocial score.
Results
Cohort characteristics
Of the 188 children diagnosed with ASD (82.4% male, mean age [s.d.] 5.2 [1.8] years), 103 (54.8%) experience strict lockdown, and 51 of them experienced the lockdown for less than 35 days while 52 were under lockdown for 35 or more days. Among these groups, baseline ASD symptoms and parental personality scores were comparable (Table 1). Compared with the lockdown-free group, parents in the lockdown-affected group had higher education levels and family incomes, which were adjusted for in later analyses.
Table 1. Baseline characteristics of the children diagnosed with ASD
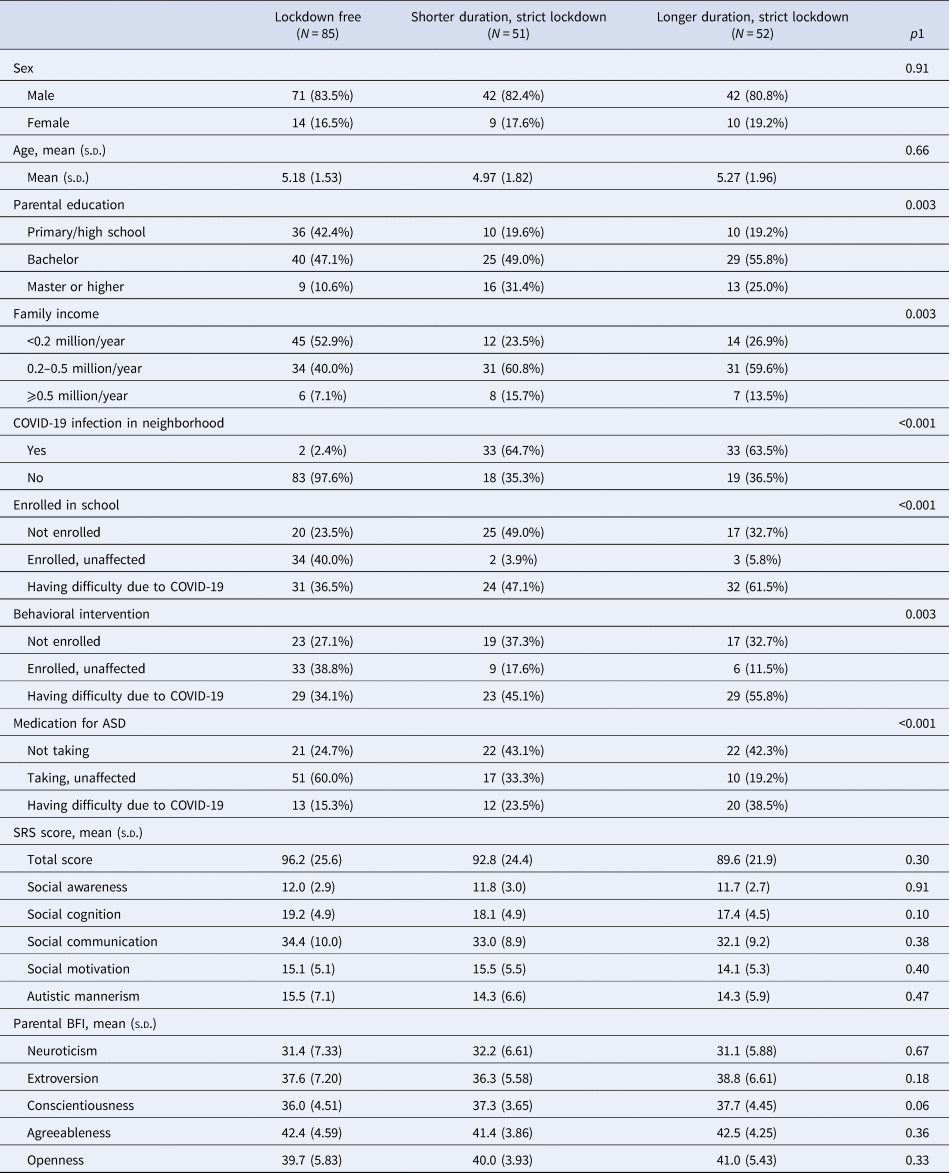
ASD, autism spectrum disorders; s.d., standard deviation; SRS, Social Responsiveness Scale; BFI, Big Five Inventory.
Continuous parameters were compared using analysis of variance.
1Categorical parameters were compared using likelihood ratio χ2 tests or Fisher's exact tests.
Longer duration lockdown was associated with less clinical improvement and worsening social cognition
Compared with lockdown-free children, children who were under strict lockdown had less clinical ASD improvement, as evidenced by higher CGI-I scores (β [95% CI], 0.36 [0.11–0.61], p = 0.005). No linear relationship was observed (r = 0.16, p = 0.11) between the length of lockdown and the CGI-I score across the entire lockdown group (online Supplementary Fig. S2). This slowed clinical improvement was particularly prominent in children under longer duration, strict lockdowns (⩾35 days; 0.49 [0.19–0.79], p = 0.001; Fig. 1a; Table 2). Reduced clinical improvement was mainly driven by worsening social cognition, as measured using the social cognition subscale of the SRS (2.62 [0.94–4.30], p = 0.002, FDR = 0.023 for the longer duration strict lockdown × time interaction; Fig. 1b; Table 3). The model containing the random slope did not improve the fit on social cognition (χ2 = 4.11, p = 0.128). No significant associations were observed between lockdown duration and SDQ and CSHQ (online Supplementary Table S1).

Figure 1. (a) CGI-I and (b) change in social cognition subscale of SRS in ASD children without lockdown, with shorter duration of strict lockdown, and longer duration of strict lockdown.
Table 2. Association between lockdown and CGI-I scores in children with ASD
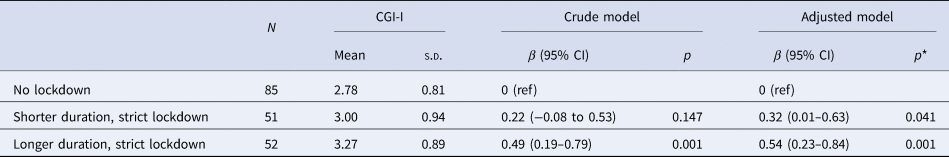
ASD, autism spectrum disorders; s.d., standard deviation; CGI, Clinical Global Impression-Improvement.
* Adjusted for sex, age, parental education, and parental income.
Table 3. Change in SRS score in ASD children from baseline to follow-up
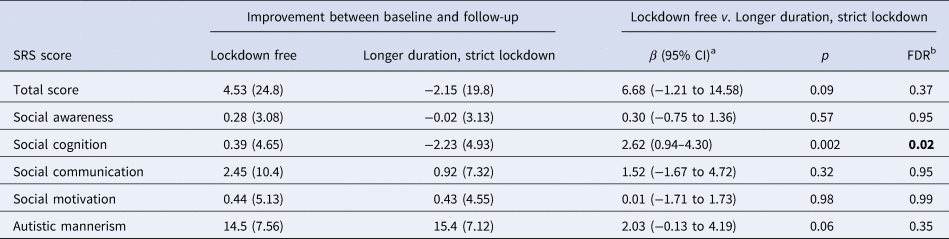
ASD, autism spectrum disorders; SRS, Social Responsiveness Scale; s.d., standard deviation; FDR, false discovery rate.
a Adjusted for sex, age, parental education, and parental income.
b The item that survived FDR correction is in bold
Lockdown-affected children were at a higher risk of infection and more likely to have difficulty reaching learning resources, continuing behavioral interventions, and accessing ASD-related medications (Table 1). After adjusting for these factors, longer duration of lockdowns was still associated with higher CGI-I scores (online Supplementary Table S2).
Parental personality impacted the association between prolonged lockdown and worse behavioral symptoms
A significant interaction between longer duration lockdown and parental agreeableness, measured with the BFI, was observed in the CGI-I (−0.11 [−0.17 to −0.05], p = 0.002, FDR = 0.009; online Supplementary Table S3). Specifically, among children who were under a longer duration strict lockdown, higher parental agreeableness scores were associated with better behavioral symptoms (−0.08 [−0.14 to −0.02], p = 0.004; Fig. 2), while no trend between parental agreeableness and CGI-I was observed in children who were not placed under lockdown (0.01 [−0.03 to 0.05], p = 0.489). No significant interactions were observed between longer duration lockdown and other family-related factors, including parental resilience, family adaptation, family cohesion, and parental emotion regulation (online Supplementary Table S3).
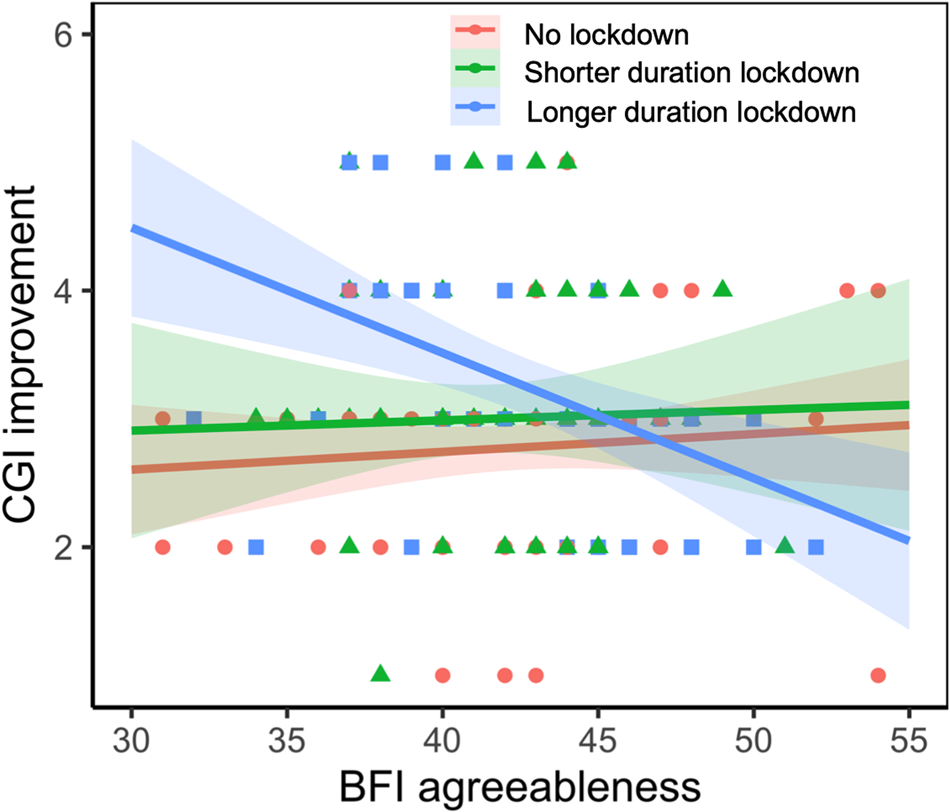
Figure 2. Parental agreeableness interacted with lockdown status on CGI improvement and a significant interaction between longer duration strict lockdown and parental agreeableness was observed on the CGI-I.
Parents who scored higher in agreeableness on the BFI reported higher resilience (measured with the CD-RISC), higher family adaptation and cohesion (measured with the FACSII-CV), higher cognitive reappraisal, lower expressive suppression (measured with the ERQ), and less stress related to COVID-19 (online Supplementary Table S4). Additional adjustments for these family-related variables did not alter the significant interactions between a longer duration lockdown and parental agreeableness (online Supplementary Table S5).
Relationships between brain volume, parental personality, and lockdown on behavioral symptoms
At baseline, no brain volumetric differences were observed between the three groups. No significant interactions between pre-pandemic brain volume and lockdown status were observed on CGI-I scores or SRS social cognition scores.
A significant interaction between the pre-pandemic grey matter volume, parental agreeableness, and longer duration strict lockdown on CGI-I scores was observed in brain regions across the temporal pole of the superior temporal gyrus and middle temple gyrus, the medial superior frontal gyrus, the superior temporal gyrus, the rectus gyrus, etc. (Fig. 3a, b). The larger the pre-pandemic brain volume, the stronger the protective effect of parental agreeableness on CGI-I score during a longer duration strict lockdown (Fig. 3c). This interaction effect remained significant after additionally adjusting for the baseline SRS total score (−0.18 [−0.26 to −0.11], p < 0.001) and the SDQ prosocial score (−0.19 [−0.27 to −0.11], p < 0.001).
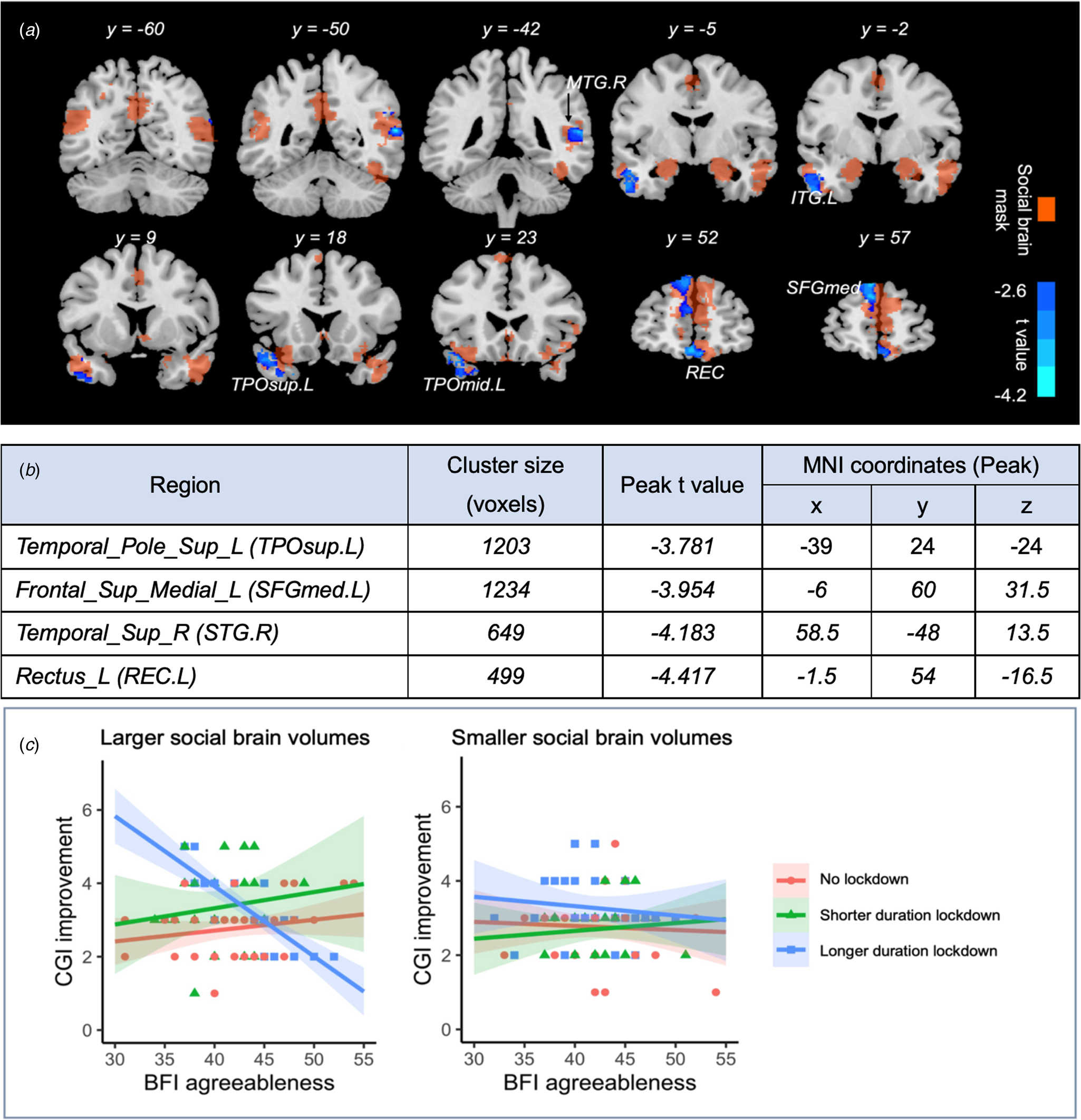
Figure 3. Pre-pandemic grey matter volume interacted with parental agreeableness, and lockdown status on CGI improvement. (a—b) The significant brain regions with grey matter volume interacted with parental agreeableness, and longer duration strict lockdown status after FDR correction. (c) The larger the pre-pandemic brain volume, the stronger the protective effect of parental agreeableness on CGI-I score during the longer duration strict lockdown.
Discussion
Our study had three important and clinically relevant findings. First, we found that the longer duration strict lockdowns were associated with a reduction in clinical improvement among children with ASD, and that this association was most prominent in social cognition. Second, parental agreeableness was a protective factor for children with ASD during the lockdowns. Third, larger grey matter volumes in brain regions of the social network in ASD children enhanced the effect of parental agreeableness. Therefore, our study suggests that social brain development interacts with the environmental social factor of parental agreeableness in promoting better improvement in clinical symptoms in ASD children during strict, longer duration lockdown.
Our findings provide novel evidence that a longer duration strict lockdown is associated with increased behavioral problems in ASD children. Previous studies have reported behavioral changes (such as social withdrawal [Mutluer et al., Reference Mutluer, Doenyas and Aslan Genc2020] and social communication [Tokatly Latzer, Leitner, & Karnieli-Miller, Reference Tokatly Latzer, Leitner and Karnieli-Miller2021]) in ASD patients during the COVID-19 pandemic. However, the heterogeneous degrees of lockdown stringency made it difficult to differentiate the effects of the policy (lockdown) from the direct effects of the pandemic itself (Adams et al., Reference Adams, Zheng, Taylor and Bishop2021; Fong et al., Reference Fong, Cornish, Kirk, Ilias, Shaikh and Golden2021; Vasa et al., Reference Vasa, Singh, Holingue, Kalb, Jang and Keefer2021). Inclusion of policy stringency is crucial because stricter restrictions are associated with greater impairments in mental health, and policies to control COVID-19 varied greatly worldwide (Aknin et al., Reference Aknin, Andretti, Goldszmidt, Helliwell, Petherick, De Neve and Zaki2022). In this study, an equally strict policy as measured with the Stringency Index was implemented only in lockdown districts, making it possible to compare the effects of different lockdown durations and also no lockdown. However, in this Shanghai cohort, a strict lockdown did not result in reduced clinical improvement or poor social cognition in ASD children unless it lasted for 35 days or more. Strict, longer duration lockdown may reduce parental agreeableness and increase discord within the family. Changes in social cognition were previously associated with social isolation during the COVID-19 lockdown in adults (Bland et al., Reference Bland, Roiser, Mehta, Sahakian, Robbins and Elliott2020) and adolescents (Orben, Tomova, & Blakemore, Reference Orben, Tomova and Blakemore2020). Our study extends this association to ASD children under strict, longer duration lockdown. This change in social cognition is likely to be due to difficulties in attending school and in gaining access to clinical interventions which typically focus on social cognition. The longer this interruption was sustained, the more damage it had on clinical improvement. Another explanation for this difference may be the disruption of parent–child interaction during the lockdown. Recent evidence has indicated that enhanced parent–child interaction can improve the social cognition of children with ASD (Li et al., Reference Li, Wu, Ren, Shen, Xue, Yu and Li2022).
For the first time, we identified higher levels of parental agreeableness as protective for ASD children during a longer duration, strict lockdown. Prior studies indicated that parents with high agreeableness may have a positive impact on the pro-social behaviors of typically developing children (Bradley & Corwyn, Reference Bradley and Corwyn2019; Hipson, Gardiner, Coplan, & Ooi, Reference Hipson, Gardiner, Coplan and Ooi2017), but its influence on children with ASD children was not determined. Our results suggest that there is a lockdown-dependent association between parental agreeableness and pediatric ASD symptom improvement, which was significantly amplified in the setting of a prolonged lockdown. We hypothesize that in the setting of a sudden interruption in social support, family-related factors have a prominent role in ASD intervention. Agreeable parents have more positive interactions with each other and probably with their children, and these parent–child interactions can help to improve ASD symptoms (Bradley & Corwyn, Reference Bradley and Corwyn2019; Li et al., Reference Li, Wu, Ren, Shen, Xue, Yu and Li2022; Prinzie, Stams, Deković, Reijntjes, & Belsky, Reference Prinzie, Stams, Deković, Reijntjes and Belsky2009).
Notably, we found that better social brain development was protective for children with ASD during a longer duration strict lockdown. The social brain refers to the brain structures that subserve social processes, such as social cognition and theory of mind (Kennedy & Adolphs, Reference Kennedy and Adolphs2012; Redcay & Schilbach, Reference Redcay and Schilbach2019). Brain regions across the medial superior frontal gyrus, the superior temporal gyrus, and the temporal pole are typically involved during mentalizing processing, when one is trying to understand the intentions and emotions of other people. The superior temporal gyrus is also activated during both observed and self-produced action to support observational learning and promote imitation. Structural alterations in the social brain, such as decreased thickness of the temporal pole, the parahippocampus, and left superior temporal gyrus have been observed in ASD patients (van Rooij et al., Reference van Rooij, Anagnostou, Arango, Auzias, Behrmann, Busatto and Buitelaar2018). ASD-specific morphometric variation correlates with symptom severity and larger volume in ventromedial prefrontal cortex and anterior temporal lobe correlated with better social cognition (Aglinskas, Hartshorne, & Anzellotti, Reference Aglinskas, Hartshorne and Anzellotti2022) and the degree of richness of social interaction (Kiesow et al., Reference Kiesow, Dunbar, Kable, Kalenscher, Vogeley, Schilbach and Bzdok2020). Our finding that the pre-pandemic social brain may play a protective role may be explained by the ASD child being able to take greater advantage of family interactions or parent-initiated interventions during the lockdown. This is supported by previous findings in the literature. Active parent–child interactions increased interpersonal neural synchronization in the social brain network between ASD children and their parents (Wang et al., Reference Wang, Han, Hu, Feng, Wang, Liu and Yi2020). Behavioral interventions targeted toward the social brain, such as PACT and the Early Start Denver Model, which emphasizes social imitation and dyadic engagement, could enhance the parent–child interactions of children with ASD (Green et al., Reference Green, Charman, McConachie, Aldred, Slonims, Howlin and Pickles2010; Pickles et al., Reference Pickles, Le Couteur, Leadbitter, Salomone, Cole-Fletcher, Tobin and Green2016; Vivanti & Rogers, Reference Vivanti and Rogers2014).
Our findings broaden our insight into the elements that influence the outcomes of autistic children in times of unintentional life events, such as a pandemic-induced lockdown. Our findings suggest that two elements, pre-pandemic brain development of the child and parental personality, can help us identify vulnerable ASD children during a lockdown. The child's clinical improvement was directly related to how their parents coped. A large body of research has suggested that personality traits can and do change (Lucas & Donnellan, Reference Lucas and Donnellan2011; Roberts & Mroczek, Reference Roberts and Mroczek2008; Roberts, Walton, & Viechtbauer, Reference Roberts, Walton and Viechtbauer2006). Recent studies of e-health technologies have suggested that ‘agreeableness’ can be trained by interventions delivered via smartphone applications (Stieger et al., Reference Stieger, Flückiger, Rüegger, Kowatsch, Roberts and Allemand2021; Thielmann & de Vries, Reference Thielmann and de Vries2021). To the best of our knowledge, agreeableness training for parents is not part of the current ASD interventions, although our study suggests that it should be. Online health care consultations (Fernandes & Kwak, Reference Fernandes and Kwak2022; Summers et al., Reference Summers, Baribeau, Mockford, Goldhopf, Ambrozewicz, Szatmari and Vorstman2021) can stress the importance of parental agreeableness for greater symptom improvement in ASD children, and it could be added to existing ASD therapies. Future studies should examine the effects of training parental agreeableness on the clinical symptoms, social cognition, and brain development of the social network in children with ASD.
The strengths of current study are: (1) this is a cohort study with comprehensive pre-pandemic clinical information and neuroimaging data, which enabled us to investigate the effects of lockdown on children with ASD and the factors that modulated the child's response to the lockdown; (2) a strict policy was implemented, making it possible to evaluate the effects of different lockdown durations. This study should be interpreted in light of following limitations. This study only involved autistic children admitted to a single center, which might affect the generalizability of our findings. Future multi-center studies with larger sample sizes are required to confirm the findings. In addition, future studies with longer follow up are needed to investigate whether the negative effects of this strict lockdown on children with ASD may dissipate over time.
Conclusion
By analyzing a longitudinal neuroimaging cohort of children with ASD who had a follow-up assessment right after the omicron lockdown, we discovered that parental personality and social brain development before that pandemic was able to mitigate against the negative effects of a strict longer duration lockdown.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723002908
Data availability statement
The data of this study are available under reasonable and ethically approved request to the corresponding authors.
Author contributions
F. L. and Q. L. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: F. L., Q. L., L. Z., H. H., T. R. Acquisition, analysis, or interpretation of data: L. Z., T. R., H. H., F. L., Q. L., L. H., R. H., Y. X., L. Zh., H. T., J. C., D. W., H. Y., J. Y., Y. D., and Y. P. Drafting of the manuscript: L. Z., T. R., H. H., Q. L., F.L. Critical revision of the manuscript for important intellectual content: B. J. S., C. L., X. W., S. S.
Consent for publication
Not applicable.
Funding statement
This study was supported by grants from the National Natural Science Foundation of China(Nos 82125032, 81930095, and 81761128035 to F. L.; 81873909 to Q. L.; 82001771 to L. Z.; 82204064 to H. H.), the National Key Research and Development Program of China (No. 2019YFA0709502), the Science and Technology Commission of Shanghai Municipality (Nos 19410713500 and 2018SHZDZX01 to F. L.; 20ZR1404900, 20DZ2260300, 23XD1423400, and 20JC1413400 to Q. L.), the Shanghai Municipal Commission of Health and Family Planning (Nos GWV-10.1-XK07, 2020CXJQ01, 2018YJRC03 to F. L.; 20214Y0125 to L. Z.), the Shanghai Clinical Key Subject Construction Project (No. shslczdzk02902 to F. L.), the Shanghai Municipal Science and Technology Major Project (Nos: 2018SHZDZX01 and 2021SHZDZX0103 to Q. L.), the Guangdong Key Project (No. 2018B030335001 to F. L.), and innovative research team of high-level local universities in Shanghai (No. SHSMU-ZDCX20211100 to F. L.), the Shanghai Pujiang Program (No. 22PJD045 to H. H.) and the Fundamental Research Funds for the Central Universities (to Q. L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
None.
Ethical standards
The study was approved by the Ethics Committee of Xinhua Hospital affiliated to the Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from the parent or legal guardian of each participant.









