DHA (22 : 6n-3) and EPA (20 : 5n-3), the so-called n-3 long-chain PUFA (LC-PUFA), have been reported to play important roles in reproductive performances of animals( Reference Gulliver, Friend and King 1 , Reference Elis, Freret and Desmarchais 2 ), especially marine fish( Reference Asturiano, Sorbera and Carrillo 3 – Reference Bruce, Oyen and Bell 13 ), which require LC-PUFA as essential fatty acids( Reference Tocher 14 ). However, despite the wide studies on the effects of n-3 LC-PUFA on animal reproduction, little information has been published about the different effects between DHA and EPA. The present study was aimed at investigating the different efficacy between DHA and EPA in regulating reproductive processes, in a typical marine teleost, tongue sole (Cynoglossus semilaevis).
In animal reproduction, synthesis of sex steroid hormones is one of the most primary processes( Reference McKenna 15 ). Steroid hormones activate the gonadal development and play important regulating roles all through the reproductive period, even including ovulation( Reference Ogino, Sato and Iguchi 16 ). Compared with spawning performance and offspring quality, the regulation of steroid hormones by fatty acids has been relatively neglected, although a few studies have investigated the interactions between body fatty acid accumulation and steroid production( Reference Baeza, Mazzeo and Vílchez 17 , Reference Baeza, Peñaranda and Vílchez 18 ). Therefore, the present study focused on the regulatory effects of DHA and EPA on steroid hormone synthesis. In both terrestrial animals and fish species, less information has been available about the modulation of steroidogenesis by DHA and EPA( Reference Wade, Van Der Kraak and Gerrits 19 – Reference Luo, Ai and Li 22 ). Moreover, contradictory results have been reported in fish studies( Reference Mercure and Van Der Kraak 23 – Reference da Silva, Støttrup and Kjørsvik 26 ). The present study will provide new insight into the regulatory effects of DHA and EPA on animal gonadal steroidogenesis. The regulation on key proteins involved in the key processes of sex steroid synthesis, that is, the response to gonadotrophins, the delivery of cholesterol substrate and biosynthetic reactions, in terms of gene expressions, was also investigated in this study, in order to elucidate the involved mechanisms.
In a previous study of our laboratory with tongue sole (C. semilaevis), we have studied the response of gonadal steroidogenesis to arachidonic acid (ARA, C20 : 4n-6), the most important n-6 LC-PUFA( Reference Xu, Cao and Zhang 27 ). We found that dietary ARA was differentially accumulated in gonads and differentially regulated the gonadal steroidogenesis depending on fish sex and maturation stage. Interestingly, in this study, we found that DHA and EPA were also differentially accumulated between ovaries and testis. Therefore, as a follow-up study, the present study investigated the different effects of DHA:EPA on gonadal steroidogenesis between females and males. Tongue sole is a typical fish with sexual dimorphism. The females are several times bigger than males, making it a perfect species to study the sex-based difference in animal nutrition. This study will provide useful information for sex-specific nutritive strategies in domestic teleost, and these nutritive strategies may also be inspiring for other animals.
Methods
Experimental diets
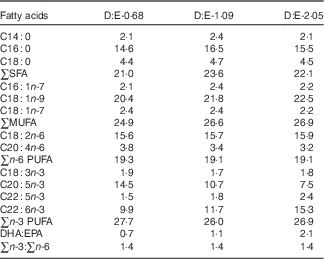
Three isonitrogenous and isolipidic experimental diets were formulated to contain different levels of DHA:EPA ratio (Table 1). The basal diet was formulated using fishmeal, casein and wheat meal as protein sources, and soya lecithin, soyabean oil and olive oil as lipid sources. Different levels of EPA-enriched oil (containing 11·2 % DHA and 52·0 % EPA, in the form of TAG; Xi’an Renbang Biological Science and Technology Co., Ltd) and DHA-enriched oil (containing 69·5 % DHA and 6·6 % EPA, in the form of TAG; Xi’an Renbang Biological Science and Technology Co., Ltd) were added to the basal diet to obtain different DHA:EPA ratios. The diets were made, packed and stored according to the standard procedures in our laboratory( Reference Xu, Mu and Zhang 28 ). The DHA:EPA ratio in the three experimental diets was 0·68, 1·09 and 2·05, respectively (Table 2). The corresponding diets were designated as D:E-0·68, D:E-1·09 and D:E-2·05, respectively.
Table 1 Formulation and proximate composition of the experimental diets (g/kg DM)
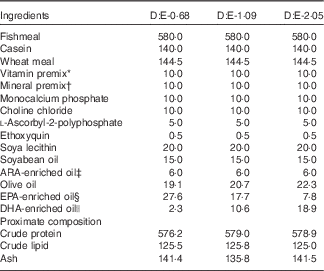
ARA, arachidonic acid.
* Vitamin premix (mg or g/kg diet): thiamin, 25 mg; riboflavin, 45 mg; pyridoxine HCl, 20 mg; vitamin B12, 0·1 mg; vitamin K3, 10 mg; inositol, 800 mg; pantothenic acid, 60 mg; niacin, 200 mg; folic acid, 20 mg; biotin, 1·2 mg; retinol acetate, 32 mg; cholecalciferol, 5 mg; α-tocopherol, 120 mg; wheat middling, 13·67 g.
† Mineral premix (mg or g/kg diet): MgSO4.7H2O, 1200 mg; CuSO4.5H2O, 10 mg; ZnSO4.H2O, 50 mg; FeSO4.H2O, 80 mg; MnSO4.H2O, 45 mg; CoCl2.6H2O (1 %), 50 mg; NaSeSO3.5H2O (1 %), 20 mg; Ca(IO3)2.6H2O (1 %), 60 mg; zoelite, 13·485 g.
‡ ARA-enriched oil: containing 41·0 % ARA (of total fatty acids), in the form of TAG; Jiangsu Tiankai Biotechnology Co., Ltd.
§ EPA-enriched oil: containing 11·2 % DHA and 52·0 % EPA (of total fatty acids), in the form of TAG; Xi’an Renbang Biological Science and Technology Co., Ltd.
|| DHA-enriched oil: containing 69·5 % DHA and 6·6 % EPA (of total fatty acids), in the form of TAG; Xi’an Renbang Biological Science and Technology Co., Ltd.
Table 2 Fatty acid compositions of the experimental diets (% total fatty acids)
Experimental fish and feeding procedure
Tongue sole C. semilaevis broodstock, 3-years old, which have been reared with formulated feeds from the early juvenile stage, were used in the present study. The average initial body weight of females and males was 3206 and 253 g, respectively. Before the start of the feeding trial, experimental fish were reared in concrete tanks (25 m3) and fed the control diet for 7 d to acclimate to the experimental conditions. At the onset of the feeding trial, experimental fish were distributed into nine polyethylene tanks (diameter: 230 cm, height: 100 cm) and each diet was randomly assigned to triplicate tanks. Each tank had twenty-five fish (ten females and fifteen males) and the tanks were supplied with flowing filtered seawater at a rate of 50 litre/min. Fish were hand-fed to apparent satiation twice daily. The feeding trial lasted for 90 d, from July to October, which is the natural spawning season of tongue sole. Fish were reared under the natural photoperiod and ambient temperature of Haiyang, Shandong, China (N36°41', E121°07'). During the experiment, the water temperature ranged from 20 to 26°C; salinity was 30–32; pH was 7·4–8·5; and dissolved O2 was 5–7 mg/l. The ponds were cleaned daily by siphoning out residual feed and faeces.
Sampling
At the end of the feeding trial, serum, liver, muscle and gonad samples from five mature females and five mature males per tank were collected. The maturity of female and male fish was confirmed by spontaneous ovulation and the release of milt when handled, respectively. After being anaesthetised with eugenol (1:10 000), blood was drawn from all fish via the caudal vein to collect serum samples. Fish were then dissected to collect liver, muscle and gonad samples. All samples were frozen with liquid N2 immediately, and then stored at −80°C before analysis. All sampling protocols, as well as fish rearing practices, were reviewed and approved by the animal care and use committee of the Yellow Sea Fisheries Research Institute.
Proximate composition analysis and fatty acid analysis
The proximate composition analyses of experimental diets were performed in accordance with the standard methods of the Association of Official Analytical Chemists (AOAC). The fatty acid compositions of diet and fish tissue lipids were analysed via a gas chromatograph, using a flame ionisation detector. Fatty acids in freeze-dried samples were esterified first with KOH–methanol and then with HCl–methanol, on a 72°C water bath. Fatty acid methyl esters were extracted with hexane and then separated via gas chromatography (HP6890; Agilent Technologies Inc.) with a fused silica capillary column (007-CW; Hewlett Packard). The column temperature was programmed to rise from 150°C up to 200°C at a rate of 15°C/min, and then from 200°C to 250°C at a rate of 2°C/min. Both the injector and detector temperatures were 250°C. Results were expressed as the percentage of each fatty acid with respect to total fatty acids (TFA).
Quantitative real-time PCR analysis and the analysis of oestradiol and testosterone in serum
Total RNA in gonads was extracted using RNAiso Plus (TaKaRa Biotechnology (Dalian) Co., Ltd) and reverse-transcribed with PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa Biotechnology (Dalian) Co., Ltd) according to the user manual.
Specific primers for key proteins in steroidogenesis and the reference gene β-actin were designed on the basis of the sequences available in the GenBank database and synthesised by Sangon Biotech (Table 3). The real-time PCR was carried out with SYBR Green Real-time PCR Master Mix (TaKaRa Biotechnology (Dalian) Co., Ltd) in a quantitative thermal cycler (Mastercyclereprealplex; Eppendorf). The detailed program was similar to that of Xu et al.(
Reference Xu, Dong and Ai
29
). The mRNA expression levels were studied by quantitative real-time PCR method:
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
(
Reference Livak and Schmittgen
30
).
$2^{{{\minus}\Delta \Delta C_{t} }} $
(
Reference Livak and Schmittgen
30
).
Table 3 Sequences of the primers used in this work

FSHR, follicle-stimulating hormone receptor; StAR, steroidogenic acute regulatory protein; P450ssc, cholesterol side-chain cleavage enzyme; P450c17, 17α-hydroxylase; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase.
Serum oestradiol and testosterone concentrations were assayed in collaboration with the affiliated hospital of Qingdao University, using an electrochemiluminescence method. COBAS-6000 (e 602 module) automatic electrochemiluminescence immunoassay analyzer (Roche Diagnostics) and affiliated commercial kits (Elecsys Estradiol III and Testosterone II) supplied by Roche were used in this assay. The standard curve for oestradiol was between 18·4 and 11 010 pmol/l, and for testosterone it was between 0·087 and 52 nmol/l. To validate the assays for tongue sole, an assay of serial dilutions of various tongue sole plasma samples was conducted. The dilutions were found to be parallel to the standard assay curve. The validation of the recovery was also conducted by the assay of added steroids in tongue sole plasma. The recovery of oestradiol and testosterone was 90·5 % and 92·6 %, respectively. The steroid values were corrected for recovery losses. The inter- and intra-assay CV for the oestradiol assay were 9·3 (n 7) and 4·3 % (n 9), respectively, and those for the testosterone assay were 9·6 (n 7) and 5·2 % (n 9), respectively.
Statistical methods
All data were subjected to one-way ANOVA in SPSS 16.0 for Windows. All percentage data were arcsine transformed before analysis. Significant differences between the means were detected by Tukey’s multiple-range test. The level of significance was chosen at P<0·05. The results are presented as means of triplicate groups.
Results
Concentrations of oestradiol and testosterone in serum
In female fish, the oestradiol concentration in group D:E-0·68 was significantly (P<0·05) lower than that in group D:E-1·09, whereas no significant difference was observed either between groups D:E-0·68 and D:E-2·05 or between groups D:E-1·09 and D:E-2·05 (Fig. 1(A)). In male fish, the testosterone concentration in group D:E-0·68 was significantly (P<0·05) lower than that in group D:E-2·05, whereas no significant difference was observed either between groups D:E-0·68 and D:E-1·09 or between groups D:E-1·09 and D:E-2·05 (Fig. 1(B)).

Fig. 1 Effects of dietary DHA:EPA ratios on the serum concentrations of oestradiol in females (A) and testosterone in males (B). Values are means of triplicate groups, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P<0·05).
Gonadal mRNA expressions of sex steroid-synthesising proteins
In mature ovaries (Fig. 2(A)), the mRNA expressions of follicle-stimulating hormone receptor (FSHR) and 17α-hydroxylase (P450c17) were significantly higher (P<0·05) in group D:E-1·09 than in the other two groups, whereas no significant difference was observed between the other two groups. The mRNA expressions of steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD) in group D:E-1·09 were significantly higher (P<0·05) than those in group D:E-0·68, whereas no significant difference was observed either between groups D:E-0·68 and D:E-2·05 or between groups D:E-1·09 and D:E-2·05. No significant difference was observed in mRNA expressions of cholesterol side-chain cleavage enzyme (P450ssc), 17β-hydroxysteroid dehydrogenase (17β-HSD) and aromatase among dietary groups.
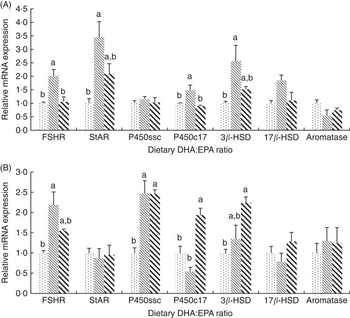
Fig. 2 Effects of dietary DHA:EPA ratios on the relative mRNA expressions of sex steroid-synthesising proteins in ovaries (A) and testes (B). Values are means of triplicate groups, with their standard errors represented by vertical bars. ![]() , 0·68;
, 0·68; ![]() , 1·09;
, 1·09; ![]() , 2·05. FSHR, follicle-stimulating hormone receptor; StAR, steroidogenic acute regulatory protein; P450ssc, cholesterol side-chain cleavage enzyme; P450c17, 17α-hydroxylase; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase. a,b Mean values with unlike letters were significantly different (P<0·05).
, 2·05. FSHR, follicle-stimulating hormone receptor; StAR, steroidogenic acute regulatory protein; P450ssc, cholesterol side-chain cleavage enzyme; P450c17, 17α-hydroxylase; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase. a,b Mean values with unlike letters were significantly different (P<0·05).
In mature testis (Fig. 2(B)), the mRNA expression of FSHR was significantly higher (P<0·05) in group D:E-1·09 than in group D:E-0·68. The gene expression of P450ssc in group D:E-0·68 was significantly lower (P<0·05) than that in groups D:E-1·09 and D:E-0·68, whereas no significant difference was observed between the latter groups. The mRNA expression of P450c17 was the highest in group D:E-2·05, significantly higher (P<0·05) than that in groups D:E-0·68 and D:E-1·09. The mRNA expression of 3β-HSD was significantly higher in group D:E-2·05 compared with group D:E-0·68, whereas no significant difference was observed either between groups D:E-0·68 and D:E-1·09 or between groups D:E-1·09 and D:E-2·05. The gene expression of StAR, 17β-HSD and aromatase showed no significant difference among dietary groups.
Tissue fatty acid profiles
In all tissues of female and male fish, except the muscle of male fish, the EPA content significantly decreased (P<0·05) and the DHA content significantly increased with increasing dietary DHA:EPA ratios (Tables 4–6). No significant difference was observed in DHA content in muscle of male fish (Table 6). The DHA:EPA ratios in fish tissues always significantly increased with increasing dietary DHA:EPA ratios.
Table 4 Gonad fatty acid compositions of tongue sole fed the experimental diets (% total fatty acids)Footnote *

a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Data represent the mean of triplicate groups.
Table 5 Liver fatty acid compositions of tongue sole fed the experimental diets (% total fatty acids)Footnote *

a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Data represent the mean of triplicate groups.
Table 6 Muscle fatty acid compositions of tongue sole fed the experimental diets (% total fatty acids)Footnote *

a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Data represent the mean of triplicate groups.
Compared with ovaries, testes had lower average EPA content but higher average DHA content (Table 4). On the contrary, compared with female livers, male livers had higher average EPA content but lower average DHA content (Table 5). Testes had much lower average C18 : 1n-9 content but higher average C16 : 0 content than ovaries. In muscle, both EPA and DHA contents were higher in males than in females. The ARA contents were higher in all tissues of male fish compared with those of female fish.
Group D:E-0·68 had significantly lower (P<0·05) testis C16 : 0 content than group D:E-1·09. Females from group D:E-2·05 showed significantly higher (P<0·05) liver C18 : 2n-6 content but significantly lower (P<0·05) ovary C18 : 1n-7 content compared with group D:E-0·68.
Discussion
The present study showed that both oestradiol in females and testosterone in males had the lowest concentrations in the group with the lowest dietary DHA:EPA ratio (0·68). This indicated that a low DHA:EPA ratio in the diet may have inhibitory effects on gonadal steroidogenesis in tongue sole. This inhibitory effect could partly be attributed to the high EPA levels in diets. In in vitro studies with gonadal tissues from goldfish and rainbow trout, EPA has been shown to inhibit the gonadotrophin-stimulated testosterone production( Reference Wade, Van Der Kraak and Gerrits 19 , Reference Mercure and Van Der Kraak 23 ). High levels of EPA could competitively inhibit the production of ARA-derived eicosanoids such as 2-series PG (PGE2), which play important roles in gonadal steroidogenesis and maturation, and consequently inhibit the gonadal steroidogenesis and maturation( Reference Tocher 14 , Reference Wade, Van Der Kraak and Gerrits 19 , Reference Asturiano, Sorbera and Zanuy 20 , Reference Stacey and Goetz 31 – Reference Sargent, Bell and Tocher 35 ). In this regard, altered EPA:ARA ratios could also modulate the gonadal steroidogenesis and maturation of fish. In this study, although the dietary ARA level was relatively constant among groups, the EPA:ARA ratio varied among diets with different DHA:EPA ratios, being 3·82, 3·14 and 2·34, respectively, in the three experimental diets. The highest EPA:ARA level (3·82) in the lowest DHA:EPA diet could probably exert inhibition on the steroidogenesis in tongue sole via the regulation of PG production.
With respect to ARA, although the ARA content was designed to be constant among experimental diets, a slight increase in actual dietary ARA content (3·2, 3·4 and 3·8 % of TFA) existed with decreasing dietary DHA:EPA ratios. Moderate levels of ARA and its metabolites, PGE2, have been widely demonstrated to stimulate gonadal steroidogenesis and maturation( Reference Sorbera, Asturiano and Carrillo 4 , Reference Wade, Van Der Kraak and Gerrits 19 , Reference Asturiano, Sorbera and Zanuy 20 , Reference Mercure and Van Der Kraak 23 , Reference Van Der Kraak and Chang 32 , Reference Wade and Van Der Kraak 33 , Reference López-Ruiz, Choi and Rose 36 – Reference Norambuena, Estévez and Sánchez-Vázquez 40 ). A recent study in our lab investigating the effects of dietary ARA on the gonadal steroidogenesis in tongue sole showed that, compared with the group without ARA supplementation, 15·44 % ARA (of TFA) in the diet enhanced the testosterone production in males, but 5·14 % was ineffective( Reference Xu, Cao and Zhang 27 ). With regard to this, inhibitory effects of the diet with the lowest DHA:EPA ratio on gonadal steroidogenesis in this study may be less attributed to the slight ARA increment.
DHA is also an important regulator of fish gonadal steroidogenesis and maturation( Reference Baeza, Peñaranda and Vílchez 18 ). Similar to EPA, the inhibitory effects of high levels of DHA on gonadotropin-induced steroidogenesis have also been observed in in vitro studies with fish gonadal tissues( Reference Wade, Van Der Kraak and Gerrits 19 , Reference Mercure and Van Der Kraak 23 ). Besides steroidogenesis, the gonadotropin-induced oocyte maturation in marine teleost (European sea bass Dicentrarchus labrax) was also observed to be inhibited by high levels of DHA( Reference Sorbera, Asturiano and Carrillo 4 ). The inhibitory effects of high DHA levels could partly contribute to the inhibitory effects of the highest DHA:EPA ratio on oestradiol production in female tongue sole in this study. The group with highest DHA:EPA (2·05) showed a decrease in oestradiol production in females compared with the group with the moderate DHA:EPA ratio (1·09). However, in testes of male tongue sole, the highest testosterone production was observed in the group with the highest DHA:EPA ratio. This indicated that, compared with females, male tongue sole may require more DHA and less EPA for the gonadal steroidogenesis. Although in vitro studies stated above showed the inhibitory effects of high levels of both EPA and DHA on gonadotropin-induced steroidogenesis( Reference Wade, Van Der Kraak and Gerrits 19 , Reference Mercure and Van Der Kraak 23 ), a very high level of DHA significantly stimulated the PGE production in testis cell of European sea bass at all the times of incubation( Reference Asturiano, Sorbera and Zanuy 20 ). To some extent, this might provide a clue for the relatively high DHA requirement in steroidogenesis and maturation of male fish. A significantly positive correlation between testis DHA content and serum testosterone concentrations has been reported in mature male European eel (Anguilla anguilla)( Reference Baeza, Peñaranda and Vílchez 18 ), indicating that DHA might play positive roles in testosterone synthesis.
The difference in effective DHA:EPA ratio between female and male tongue sole was in good accordance with the different accumulation of DHA and EPA between ovaries and testes. Testes had more DHA (average 18·5 v. 13·9 % of TFA) but less EPA (average 3·6 v. 4·3 %) accumulation than ovaries. Interestingly, in the liver of tongue sole, a reverse observation was made; that is, males had less DHA (average 17·7 v. 21·7 %) but more EPA (average 6·9 v. 5·4 %) than females. Preferable LC-PUFA were probably transferred to gonads from other tissues during maturation to meet the reproductive requirements( Reference Huang, Yin and Shi 41 ). Baeza et al. ( Reference Baeza, Mazzeo and Vílchez 17 ) reported that in the testis of European eel all primary LC-PUFA, that is, DHA, EPA and ARA, remained constant during the maturation process. De novo synthesis of EPA and DHA in the liver and probable subsequent transfer of them to the testis was also observed in that study. Differently, Japanese eel (Anguilla japonica) testes seemed to have a higher preference for EPA( Reference Rupia, Shen and Wu 42 ). In addition, in the muscle of tongue sole the contents of both DHA and EPA were higher in males than in females. Higher muscle LC-PUFA contents in males than in females were also observed in mountain trout (Salmo trutta macrostigma)( Reference Akpinar, Görgün and Akpinar 43 ). The sex-specific fatty acid preference in fish tissues must be closely correlated with the sex-specific bio-functions of fatty acids, and this could be regulated by the different sex hormone secretion between female and male fish( Reference Baeza, Peñaranda and Vílchez 18 , Reference Alessandri, Extier and Astorg 44 – Reference Marks, Kitson and Stark 47 ).
Besides the sex-specific effects of DHA:EPA, another interesting observation of this study was that the DHA:EPA ratio inducing the highest sex steroid production in both females and males was >1·0. This indicated that DHA may be superior to EPA in promoting fish steroid hormone synthesis. Very little information has been available about the relative effectiveness of DHA and EPA in regulating fish steroidogenesis, although several in vivo studies with fish species such as gilthead seabream (Sparus aurata), silver pomfret (Pampus argenteus) and European eel have investigated the effects of total LC-PUFA or n-3 LC-PUFA on sex steroid production( Reference Wassef, Wahbi and Shalaby 24 – Reference da Silva, Støttrup and Kjørsvik 26 ). A feeding trial with Siberian sturgeon (Acipenser baeri) showed that serum oestradiol was higher in females fed the diet with a DHA:EPA ratio of 1·9:1 compared with the ratio of 1:1·9( Reference Luo, Ai and Li 22 ), providing another evidence for the superiority of DHA to EPA in inducing sex steroid production. Similar to steroidogenesis results, some studies have also indicated the superior effect of DHA to EPA on other reproductive parameters. In a study with European sea bass, the PUFA-enriched diets with higher DHA content but lower EPA content induced the better-quality eggs and larvae (although reduced the spawning parameters) compared with the control diet( Reference Asturiano, Zanuy and Ramos 48 ), whereas another study with male European sea bass showed that the wet diet containing a higher EPA content resulted in lower spermiation performance and lower sperm quality as compared with the PUFA-enriched diet with a lower EPA content( Reference Asturiano, Sorbera and Carrillo 3 ). However, contradictory results were also observed in other fish species. A study with domesticated common sole (Solea solea) breeders showed that egg EPA concentration was positively correlated with larval viability, but egg DHA concentration was negatively correlated with hatching rate and larval viability( Reference Parma, Bonaldo and Pirini 49 ). To date, the relative effects of DHA and EPA on steroidogenesis and other reproductive parameters is highly unknown, and more future studies are needed.
Regarding the regulation of sex steroid-synthesising proteins by dietary DHA:EPA ratios, in tongue sole ovaries the gene expression of FSHR, StAR, P450c17 and 3β-HSD was significantly influenced by dietary treatments; however, the gene expression of 17β-HSD and aromatase was not significantly different among experimental groups. The effects of diets on 17β-HSD and aromatase may be masked by the endogenous inhibition of these enzymes in the time point of sampling. Tongue sole has group-synchronous ovarian development. Shifts in gonadal steroidogenesis in the group-synchronous gonadal development have been observed in several fish species such as European sea bass, rainbow trout (Oncorhynchus mykiss) and striped bass (Morone saxatilis)( Reference King, Berlinsky and Sullivan 50 – Reference Asturiano, Sorbera and Ramos 53 ). At the very beginning of ovulation, during which the samples were obtained in the present study, maturation-inducing progestagens may reach peaks, whereas the oestradiol production may already be decreasing( Reference Asturiano, Sorbera and Ramos 53 ). Inhibition of 17β-HSD and aromatase might be involved in the endogenous down-regulation of oestradiol production at this time point, considering that these two enzymes are two of the most important and rate-limiting enzymes in oestradiol synthesis.
In the testes, although dietary DHA:EPA ratios influenced gene expressions of several proteins such as FSHR, P450ssc, P450c17 and 3β-HSD, only the regulation of 3β-HSD correlated well with the testosterone concentration results. This was in agreement with our previous studies investigating the steroidogenesis-regulating effects of ARA in this fish( Reference Xu, Cao and Zhang 27 ). These results indicate that 3β-HSD, which converts pregnenolones to progesterones, may play a key role in the regulation of testosterone synthesis by fatty acids( Reference Wang and Stocco 54 , Reference Midzak, Chen and Papadopoulos 55 ). Peñaranda et al.( Reference Peñaranda, Morini and Tveiten 56 ) have reported the gene expression of cyp11a1 (P450ssc) and cyp17-I (P450c17), which are important enzymes initiating the testosterone synthetic pathway, correlated well with the testosterone synthesis during the maturation of European eel testis. However, in the present study, only a general positive correlation was observed between testosterone production and P450ssc or P450c17 gene expression. As in ovaries, the aromatase gene expression in testes was also not influenced by dietary DHA:EPA ratio, but this may be related to the fact that the aromatase mRNA was less abundant in the testis( Reference Deng, Chen and Xu 57 ).
Regarding the difference between sexes in effective DHA:EPA ratio inducing the highest gene expression of steroidogenic proteins, the results were consistent with the steroid concentration results for some proteins such as FSHR, StAR, P450c17 and 3β-HSD in ovaries and P450c17 and 3β-HSD in testes; that is, the DHA:EPA ratio of 1·09 induced the highest gene expressions in ovaries, whereas the ratio of 2·05 induced the highest values in testes. This, to some extent, confirmed that males may require more DHA and less EPA than females for steroid synthesis. The difference in gonadal steroidogenesis between males and females may contribute to the sex-specific modulation of sex steroid-synthesising proteins by dietary fatty acids; however, very little information has been available about the difference in steroidogenesis between ovaries and testes of this fish. The commonly occuring sex reversal in juveniles of this fish makes this issue more complicated( Reference Shao, Li and Chen 58 , Reference Jiang and Li 59 ). In this study, the gene expression of StAR, the rate-limiting transport protein translocating cholesterol from the outer mitochondrial membrane to the inner mitochondrial P450scc site, was regulated by experimental diets in females but not in males. This difference could be related to the fact that ovaries and testes may use cholesterol substrate supplied in different ways( Reference Hu, Zhang and Shen 60 ). In mammals, for the steroid synthesis, the ovaries preferentially use cholesterol supplied from plasma lipoprotein via the scavenger receptor class B type I-mediated endocytic pathway, whereas the testicular Leydig cells rely heavily on the use of endogenously synthesised cholesterol( Reference Azhar and Reaven 61 – Reference Goldstein and Brown 63 ). In this regard, the difference in cholesterol substrate abundance between ovaries and testes may interfere with regulation of StAR by fatty acids. Contrary to StAR, the gene expression of P450ssc was regulated by diets in males but not in females. This may be related to the fact that the gene expression of P450ssc (cyp11a1) correlates better with testosterone production( Reference Peñaranda, Morini and Tveiten 56 ).
With respect to the tissue fatty acid compositions, besides DHA and EPA, ARA also differently accumulated in the females and males, as observed in our previous studies with this fish( Reference Xu, Cao and Zhang 27 ). Males had much higher ARA contents than females in all tissues analysed. Male average v. female average was 6·0 v. 2·5 % (of TFA), 7·2 v. 5·6 % and 4·2 v. 2·5 % in gonad, liver and muscle, respectively. Several studies have indicated that ARA was metabolised differently in male and female fish and suggest that ARA may be more important for the reproductive success of males( Reference Norambuena, Estévez and Sánchez-Vázquez 40 , Reference Norambuena, Morais and Estévez 64 ). Higher n-6:n-3 PUFA ratios have also been shown to be more beneficial to male fish than to female fish( Reference Asturiano, Sorbera and Carrillo 3 , Reference Bruce, Oyen and Bell 13 , Reference Cerdá, Zanuy and Carrillo 65 ).
Besides the LC-PUFA, oleic acid (C18 : 1n-9, OA) was another important fatty acid with different accumulation between female and male tongue sole. For all tissues, females had obvious high OA contents than males. Female average v. male average for OA was 22·1 v. 11·1 % (of TFA), 15·0 v. 11·6 % and 15·1 v. 14·0 % in gonad, liver and muscle, respectively. Gonad had higher OA content variation between sexes (content in ovary is two times that in testis) than liver and muscle, and on the other hand ovaries had the highest OA content among all the tissues. OA is a major energy source during egg and larval development( Reference Van Der Meeren, Klungsoyr and Wilbelmsen 66 ). Significant positive correlations between the OA content of fish eggs and egg viability or hatching percentages have been observed in gilthead seabream broodstock( Reference Fernández-Palacios, Izquierdo and Robaina 67 ). Therefore, the high OA accumulation in tongue sole ovaries must be a preparation for the energy demand in egg and larval development. Different from OA, palmitic acid (C16 : 0, PA), another important energy fatty acid, had no significant difference between sexes in the liver and muscle contents. However, testes had much higher PA content than ovaries (average 23·7 v. 15·9 % of TFA). De novo synthesis of PA has been observed in the liver of male European eel during spermatogenesis( Reference Baeza, Mazzeo and Vílchez 17 ). PA might have special physiological or energetic roles in the testosterone synthesis or spermatogenesis in male fish, but the precise mechanisms need to be elucidated by future studies.
In conclusion, the present results suggest that the dietary DHA:EPA ratio, possibly combined with the dietary EPA:ARA ratio, differentially regulated sex steroid hormone synthesis in mature female and male tongue soles. Females seemed to require more EPA but less DHA than males for gonadal steroidogenesis. The LC-PUFA accumulations in fish gonads and the response of gene expressions of sex steroid-synthesising proteins to dietary DHA:EPA confirmed the regulation of sex steroid hormone production by dietary DHA:EPA.
Acknowledgements
The authors thank Y. M. Yang and Q. H. Li for their help in fish rearing.
This work was supported by the National Natural Science Foundation of China (31402309), Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS (20603022017018) and a Grant from the Modern Agro-industry Technology Research System (CARS-50-G08, Government of China).
H. X. and M. L. designed the study. H. X. analysed the data and wrote the manuscript. L. C. carried out the feeding trial and the real-time PCR. Y. W. and Y. Z. were involved in the real-time PCR and the fatty acid analysis. All the authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.










