INTRODUCTION
In April 2009, a novel strain of influenza A (H1N1) emerged in Mexico and the USA [1, 2]. Antigenically these newly detected swine-lineage influenza viruses were distinct from seasonal human influenza A (H1N1) [Reference Garten3]. There was soon evidence of sustained transmission in North America together with rapid global spread of the virus. The UK was one of the first countries affected in Europe and one of the few to experience a substantial first wave in spring and summer 2009. Worldwide, pandemic (H1N1) 2009 influenza has mainly affected children and young adults causing a generally mild illness, although deaths have occurred, mainly in people aged <65 years [Reference Garten3].
In response to the emergence of this novel virus, the Health Protection Agency (HPA) in England and the health protection organizations of the Devolved Administrations of Scotland, Wales and Northern Ireland strengthened their national surveillance of acute respiratory illness in travellers returning from affected areas. The first UK confirmed cases of pandemic (H1N1) 2009 influenza were in a Scottish couple returning home from Mexico at the end of April 2009 [4, 5]. Until 1 July 2009, each possible case (see Table 1 for definition) detected in the UK was tested virologically. All confirmed cases and their close contacts were offered antiviral drugs as treatment or prophylaxis, respectively (arranged by local HPA Health Protection Units) in an attempt to reduce morbidity and minimize secondary spread – known as the UK ‘containment phase’ [6]. After 1 July 2009, virological testing became discretionary based on clinical need and antiviral drugs were offered as treatment [through the National Health Service (NHS)] to all cases meeting the clinical criteria – the ‘mitigation phase’.
Table 1. Clinical, laboratory and epidemiological criteria for classifications of pandemic (H1N1) 2009 influenza cases, adapted from FF100 protocol [ 9]

A detailed investigation of at least the first 100 confirmed cases of pandemic influenza had previously been suggested by the World Health Organization as part of the comprehensive assessment of any new pandemic [7]. As part of the UK pandemic preparedness programme, a generic protocol for the First Few Hundred (FF100) surveillance system had been developed around follow-up of confirmed cases in the early stages of an influenza pandemic together with tracing of their close household and non-household contacts [Reference McMenamin8]. Following detection of the first UK cases, the pre-pandemic FF100 system was modified and implemented in order to gain a rapid understanding of the main clinical, epidemiological, and virological features of pandemic (H1N1) 2009 influenza [9], to facilitate real-time modelling efforts to make predictions of the future course of the UK epidemic and to underpin guidance development and policy decisions to manage cases and reduce the spread of infection in the UK.
Initial reports of these cases have already been published early in the pandemic [4, 5]. This paper provides a final descriptive analysis of the cases followed up as part of the ‘pandemic’ FF100 in the UK which ran from May to June 2009; analyses of the transmission from cases to contacts are presented separately.
METHODS
After detection of the novel virus in North America [2], the pre-pandemic FF100 protocol and data-collection questionnaires were rapidly modified for the 2009 pandemic. An online database was built, to enable collaborative data collection and entry across the HPA in England and corresponding health protection organizations in the Devolved Administrations of Scotland, Wales and Northern Ireland.
Data collection was coordinated from the HPA national Centre for Infections, London. Local HPA Health Protection Units gathered initial case information; latterly Flu Response Centres undertook this, with support and direction from HPA regional epidemiologists in England. The 2-week follow-up of cases was coordinated from the HPA Centre for Infections. Equivalent processes took place in the Devolved Administrations. An attempt to follow-up all confirmed cases in the FF100 database was made until the database was closed to new cases on 21 June 2009. Cases were identified and classified according to agreed definitions (Table 1). Only virologically confirmed cases were included.
Information on cases was collected at two time points; the first as soon as possible after a positive laboratory result was reported and the second, 14 days later. Data were collected through interviews with the cases, and/or their parent/guardian or healthcare worker. Cases were also asked to provide details of their close contacts (household and non-household) who were interviewed as well; the contacts were followed-up prospectively and those who became ill and tested positive for pandemic (H1N1) 2009 influenza were included as cases. To ensure all FF100 contacts testing positive for pandemic (H1N1) 2009 influenza were identified, matching was undertaken with the FF100 database and laboratory reports of cases from HPA regional and national laboratories.
Case questionnaires
Clinical and epidemiological information was collected from the FF100 cases, including demographic details, clinical illness history (e.g. date of onset, signs and symptoms and their severity), medical history, including whether the case suffered from a pre-defined underlying medical condition (chronic heart disease, diabetes, HIV/immunodeficiency, kidney disease, liver disease, lung disease, malignancy, organ or bone marrow recipient, seizure disorder, pregnancy) and seasonal trivalent influenza vaccination status. Follow-up information on cases was collected to determine the occurrence of any medical complications, final outcome (e.g. death, recovery) and the use of antiviral drugs and antibiotics. For the 14-day follow-up, telephone calls were attempted over a minimum of three consecutive days.
Analysis
Descriptive analyses of the FF100 cases were undertaken relating to patient characteristics: age, sex, probable source of infection (e.g. UK or abroad), occupation (in particular whether cases were healthcare workers), clinical symptoms (as reported at initial presentation and at any time during illness), underlying medical condition, seasonal trivalent influenza vaccination status, duration of illness (as defined by onset date and date of symptom resolution provided by the case at 14-day follow-up), contact with the NHS and use of antiviral drugs including side-effects. Data were provided at different time points and any positive response was taken even if a negative response was given at another time. Data were deemed to be missing or unknown if no answer was given at all time points.
The English FF100 cases were matched to a dataset of all virologically confirmed pandemic (H1N1) 2009 influenza cases in England reported by HPA reference and regional laboratories. The characteristics (including sample date, region, age, sex) of the FF100 cases were compared with all the virologically confirmed cases over the same time period.
The Scottish FF100 cases were also matched to a dataset of all virologically confirmed cases in the same time period in Scotland.
Multivariable analyses were performed to investigate independent predictors of individual symptoms reported at any time during illness. Factors examined were: gender, age group, antiviral treatment given within or after 48 h of symptom onset, seasonal trivalent flu vaccination and underlying medical condition. These were examined in mutually adjusted and unadjusted analyses.
The proportion of the English FF100 cases who suffered from an underlying medical condition was compared to the proportion in the general population using Fisher's exact test. As the population data were only available for England, non-English FF100 cases were excluded from this analysis. The population proportions were calculated from the total number of patients aged between 6 months and 64 years registered with English general practitioners (GPs) (based on data from 96·2% of all general practices) and the numbers eligible for seasonal influenza vaccine by risk group, which was available through the Department of Health–HPA influenza vaccine uptake monitoring system [Reference Gates10].
The proportion of female English FF100 cases who were pregnant and aged 15–44 years were compared to the population proportion using Fisher's exact test. The point prevalence of women who were pregnant was calculated with the estimated English mid-2007 female population aged 15–44 years [source: Office for National Statistics – (ONS)] as the denominator. According to ONS, an estimated 658 771 maternities (live and still births) occur each year in England. It was assumed that 4% of the female population of child-bearing age (15–44 years), experience a miscarriage or abortion in one year [11] and using the mid-2007 estimate of the female population aged 15–44 years, an annual figure of 421 924 miscarriages/abortions was calculated. To calculate the number of women who are pregnant at any one time, 9/12 of the annual number of maternities (assuming these pregnancies have 9 months' duration) was added to 3/12 of the annual number of miscarriages/abortions (assuming these pregnancies have average 3 months' duration).
Laboratory methods
Laboratory confirmation of pandemic (H1N1) 2009 influenza virus was performed using respiratory swabs collected into virus transport medium. All samples were tested either at the Respiratory Virus Unit, HPA Centre for Infections, London, the local HPA Regional Microbiology Network Laboratories in England or the equivalents in Scotland and Northern Ireland, using real-time RT–PCR assays for detection of influenza A, and subtyped for pandemic H1N1 2009 viruses [Reference Ellis, Curran, Stephenson and Warnes12, Reference Ellis13].
Ethical considerations
This was an observational surveillance system carried out under NHS Act 2006 (section 251), which provides statutory support for disclosure of such data by the NHS, and their processing by the HPA for communicable disease control. Health Protection Scotland remains imbedded as part of the NHS in which the sharing of outbreak and investigation data is undertaken as part of their role in the coordination of national outbreaks.
Data
Data on cases and contacts were collated and stored in an internet-based data capture system built by the HPA. Data were entered via a PHP/Ajax web interface and captured in a relational PostgreSQL database. Data submission was via HTTPS and personal identifier information data were TripleDES-encrypted within the database (database schema and further database-related information is available at http://www.hpa-bioinformatics.org.uk/supplementary_information/FF100_HYG1000136/). Data quality assurance was undertaken through implementing standard data entry checks, manual checking of entered data against hard-copy questionnaires and internal and external data consistency checks.
Data extraction and analysis were undertaken using specific scripts, Microsoft Excel 2007 and Stata release 11.0. (StataCorp, USA).
RESULTS
The FF100 online database was established within 1 week of detection of the first UK laboratory-confirmed cases of pandemic (H1N1) 2009 influenza at the end of April 2009. By the time it closed to new cases in the week ending 21 June 2009, 392 cases had been included (373 from England, 18 from Scotland, one from Northern Ireland). The 392 cases reported illness onset dates from 16 April 2009 to 13 June 2009. Follow-up data were available for 321 (81·9%) cases.
It was possible to match 369 (98·9%) of 373 English FF100 cases to laboratory records of confirmed cases in the laboratory database. The first collection date for a respiratory sample for FF100 cases was 27 April 2009 and the final date was 14 June 2009. Six hundred and forty other virologically confirmed cases were reported in the same time period (Fig. 1): thus, 36·6% of the 1009 English confirmed cases in the same period were included in the FF100 project. Initially most laboratory-confirmed cases were included (up to the end of May 2009, 80·3% of cases were included). As case numbers began to increase rapidly from June, the proportion of cases included decreased to 17·2% (Fig. 1).
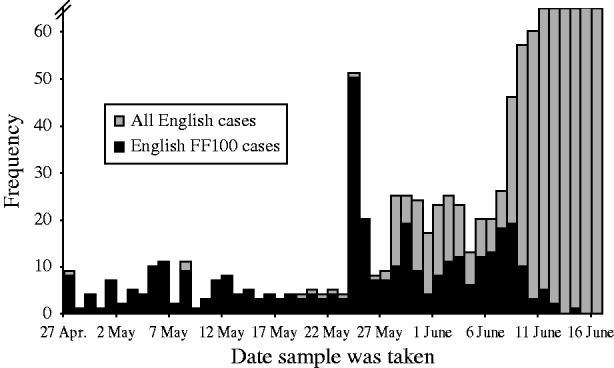
Fig. 1. English FF100 cases (369) and all other cases of pandemic (H1N1) 2009 influenza by sample date between April and June 2009.
There was a total of 498 Scottish cases in the same time period, of which 18 (3·6%) were included.
Demographic characteristics of cases
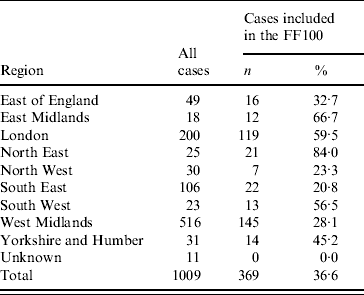
Demographic features of the English FF100 cases were compared to English laboratory-confirmed cases to assess their representativeness. The majority of English FF100 cases were reported from London (32·2%) and the West Midlands (39·3%) regions reflecting the distribution of cases in England during the first pandemic wave. The proportion included in the FF100 varied by region, ranging from 20·8% (South East) to 84·0% (North East) (Table 2). There was no evidence of a significant difference between the distribution of English FF100 cases and total laboratory-confirmed cases up to 14 June 2009, by sex (χ2P=0·91) or age (χ2P=0·45).
Table 2. Regional distribution of all English cases and English FF100 cases of pandemic (H1N1) 2009 influenza with sample date to 14 June 2009
There was an approximately equal distribution of the UK FF100 cases between the sexes (47·2% female). UK FF100 cases ranged between 0 and 73 years with a median age of 15 years [interquartile range (IQR) 10–27 years]. Most cases were children aged 6–15 years (Fig. 2) with 204 (52·0%) cases aged <16 years.
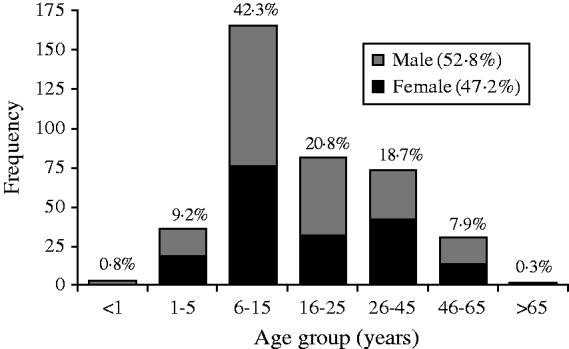
Fig. 2. Age and sex distributions of UK FF100 cases of pandemic influenza (H1N1) 2009.
Source of infection
Ninety-seven (24·7%) of all UK FF100 cases were recorded as imported cases, having acquired their infection abroad. Of these, 66 had travelled from the USA, 26 from Mexico, four from both the USA and Mexico, and one from Canada.
At least 210 UK FF100 cases were part of 18 clusters of illness affecting more than one person in a closed setting [one aeroplane cluster, five school clusters (several of which have been described in more detail [Reference Calatayud14–Reference Kar-Purkayastha16]) and 12 family/household clusters]. These clusters varied in size from two to 84 cases included in the FF100 (additional cases may have occurred in each of the clusters which were not included in FF100). The largest cluster occurred in a school where there was an outbreak of pandemic (H1N1) 2009 influenza in May 2009 which was recognized late [15].
Nine cases were reported to be healthcare workers. Four were assessed to have most likely acquired their infection through occupational exposure: two school nurses involved in caring for children during a school outbreak of pandemic influenza, one GP who cared for a suspected case and one hospital doctor. Three are assumed to have acquired their infection from other non-occupational sources and for two cases the source of infection was unknown.
Clinical features
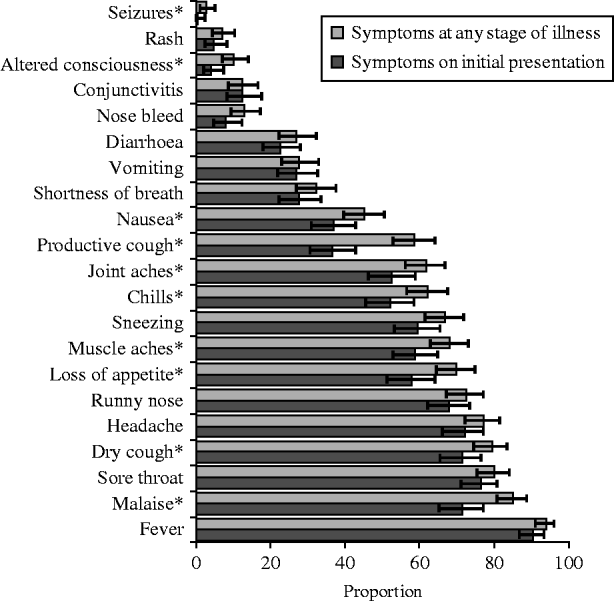
The symptoms reported by the UK FF100 cases at initial presentation and at any point during their illness are summarized in Figure 3. The most commonly reported symptom at any time was fever, which was reported by 348/371 cases (93·8%, 95% CI 90·8–96·0), followed by malaise (85·0%, 95% CI 80·6–88·7) and sore throat (79·9%, 95% CI 75·4–84·0). Vomiting was reported by 92/333 cases (27·6%, 95% CI 22·9–32·8) and diarrhoea by 89/330 (27·0%, 95% CI 22·3–32·1); only 42 cases experienced both diarrhoea and vomiting. A smaller proportion of people reported most symptoms at initial presentation compared to at any time. The greatest difference was for productive cough which was reported by 90/247 (30·4%) of cases on their initial interview compared to 190/325 (58·5%) cases at any time (Fisher's exact P value for difference in proportions <0·001). Malaise, muscle aches and joint aches were also >10% more common at any time of illness compared to initial presentation (P<0·05).
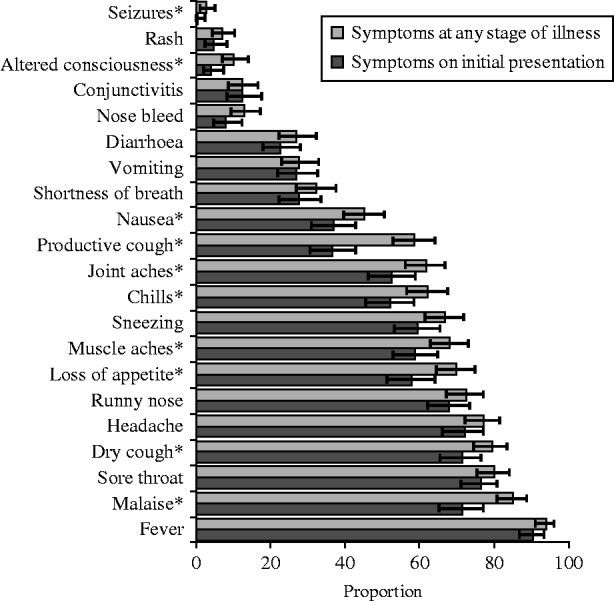
Fig. 3. Proportion (%) of all UK FF100 cases of pandemic (H1N1) 2009 influenza reporting symptoms at any stage of illness and at initial presentation, with binomial exact 95% confidence interval. * Symptoms with Fisher's exact P<0·05 for difference in proportions indicated.
Children (aged <16 years) exhibited a different symptom pattern to adults (reported at any time during illness), with a significantly higher probability of vomiting, nose bleed and conjunctivitis, and a lower probability of several other symptoms including muscle aches, shortness of breath and chills (Table 3). Starting antiviral treatment within 2 days of symptom onset had little significant effect on most symptoms compared to starting later (P>0·05), although vomiting was reported significantly more frequently in people who took antivirals after 48 h (34·4% vs. 20·4%, P=0·01). There was no evidence of a significant difference in any symptom by gender, presence of underlying medical condition or 2008/2009 seasonal trivalent influenza vaccine status in unadjusted or mutually adjusted analyses.
Table 3. Symptoms reported at any stage during illness by UK FF100 cases that differed by age group
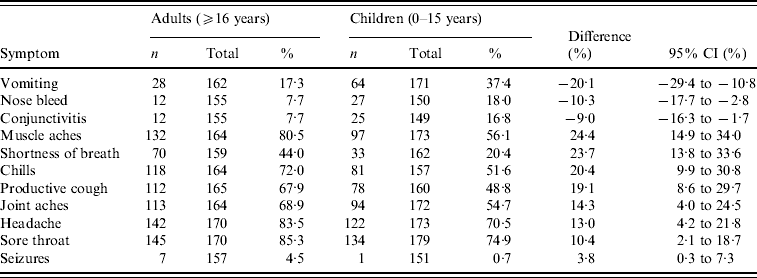
CI, Confidence interval.
* Fisher's exact test used to compare proportions.
Underlying risk factors
Of all UK FF100 cases, 44 (11·2%) had at least one underlying medical condition (excluding pregnancy). The commonest reported condition was chronic respiratory disease (28 cases, 7·1%), of whom 17 had asthma. Five cases reported immunodeficiency, five were diabetic and four cases were pregnant. There was no evidence of a significant difference in the proportion of English FF100 cases aged <65 years with an underlying condition compared to the general population, except for chronic respiratory disease, which was significantly more common in FF100 cases (7·6% vs. 4·4%, Table 4).
Table 4. Underlying conditions reported from all UK FF100 cases, English FF100 cases and the general population in England aged 6 months to 65 years in a risk groupFootnote *
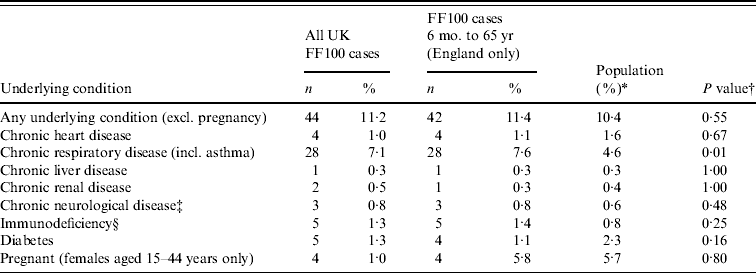
* According to HPA/DH 2008/2009 seasonal influenza vaccine uptake monitoring survey, except in the case of pregnancy [Reference Gates10].
† Fisher's exact test used to compare proportion of England FF100 cases and the English general population.
‡ For FF100 cases this only includes ‘seizure disorder’.
§ For FF100 cases this includes ‘HIV/other immunodeficiency’, ‘Malignancy’ and ‘Organ or bone marrow transplant’.
Twenty (10·2%) of 197 English FF100 cases, for whom data were available, had been vaccinated with the most recent (2008/2009) seasonal trivalent influenza vaccine which is similar to the proportion in the general population in England aged 6 months to 65 years (7·2%, χ2P=0·12) [Reference Begum and Pebody17]. Vaccinated cases tended to be older, with a median age of 27 years (IQR 17–43) compared to unvaccinated cases (continuity corrected χ2 test for difference in medians, P=0·02). Ten of the 20 (50·0%) vaccinated cases were reported as suffering from an underlying medical condition; two with cancer, four with diabetes, one with a seizure disorder, one with chronic heart disease and five with respiratory disease (including three with asthma). Three cases suffered from two underlying conditions.
Antiviral treatment
Of the 365 UK FF100 cases with information available, 335 (91·8%) reported receiving treatment with the antiviral drug oseltamivir (Roche, USA). The time from onset to treatment date was available for 315 cases and ranged from 0 to 46 days (mean 3·8 days, median 3 days). Only 89 (26·6%) of the cases started antiviral treatment within 48 h of onset of illness.
Forty-one (12·2%) of the 335 cases who took antiviral drugs reported adverse events (side-effects) which they attributed to antiviral treatment. More adults (n=25) than children (n=16) reported an adverse event. Of the 17 who had specified the severity of the adverse event, 15 (88·2%) graded it as moderate and two as severe: one reported headaches and nausea and the other diarrhoea. Gastrointestinal symptoms were the most commonly reported adverse events, reported by 30/41 cases reporting side-effects [11 (37·0%) children and 19 adults]; other adverse events included fatigue (two children, four adults), headache (two adults), nose bleed (one child) and earache (one child).
Disease severity
Two hundred and twenty-five (57·4%) of the UK FF100 cases had sufficient information recorded to calculate the duration of illness. This ranged from 0 to 34 days (median 7 days, IQR 4–12 days, mean 8·7 days). Children experienced a significantly shorter illness than adults (median of 6 days vs. 9 days, P=0·01). Those admitted to hospital had a significantly longer illness (median 12 days vs. 7 days for non-hospitalized, P=0·01). Duration was significantly shorter when antiviral treatment was given within 48 h of onset compared to those who received it after this time (median 5 days vs. 9 days, P=0·01); this difference persisted after adjusting for age (adults: 5 days vs. 10 days, P=0·03; children: 4·5 days vs. 7 days, P=0·06).
There was no difference in duration of illness according to presence of underlying medical condition or seasonal influenza vaccination status (Table 5).
Table 5. Duration of illness (in daysFootnote *) of all UK FF100 cases, age, sex, underlying condition, vaccination status, hospital admission and antiviral treatment
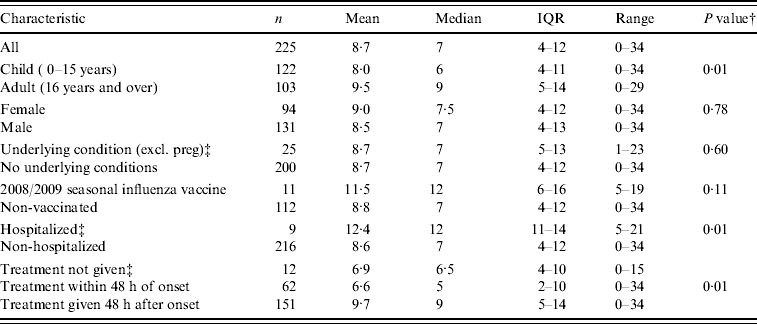
* Cases missing onset or symptom resolution date, or when symptom resolution date was recorded as less than 24 h after onset date were excluded.
† Continuity corrected Pearson χ2P value for differences in medians displayed.
‡ Missing data were excluded from the analysis except for underlying condition, hospital admission and antiviral prophylaxis or treatment where missing data was assumed to be ‘No’.
Of the 302 cases providing information, 276 (91·4%) contacted the NHS. Twenty-six cases reported no contact with the NHS, of whom 25 reported that they had taken the antiviral drug oseltamivir as treatment arranged by their local HPA Health Protection Unit or equivalent in Scotland or Northern Ireland.
Two hundred and twenty-one of 286 (77·3%) cases reported visiting or telephoning their GP; 84/238 (35·3%) called NHS Direct/24 (the nurse-led telephone advice line open 24 h a day in England, Wales, and Scotland), 35/230 (15·2%) attended accident and emergency departments and 14/240 (5·8%) were hospitalized. The age range of the 14 cases who were hospitalized was 8–45 years (median 23·5 years, mean 24 years). Four of the 14 hospitalized cases reportedly had an underlying medical condition, two had received the 2008/2009 seasonal influenza vaccine and three were recorded as receiving antibiotics. All 14 hospitalized cases had received oseltamivir as treatment, although only three (21·4%) within 48 h of onset. However, the proportion was similar in cases who were not reported to have been hospitalized (29·8%, Fisher's exact P=0·76).
Five of the hospitalized cases had documented medical complications: three required mechanical ventilation and two cases had pneumonia with X-ray diagnosis. All five cases were young adults, of whom three had underlying medical conditions.
Fifteen of 132 (11·3%) confirmed cases with completed follow-up information had received antibiotics.
No cases followed-up in the FF100 project were reported to have died.
DISCUSSION
The pandemic FF100 project was rapidly established and collected and analysed data on the first cases of pandemic (H1N1) 2009 influenza in the UK. The cases were predominantly children and young adults. They represented either the original introductions from Mexico and the USA or those who acquired their infection in the UK early in the pandemic through transmission in household and school settings. A small number of the cases were healthcare workers who were infected through nosocomial transmission from their patients. Most cases experienced a typical influenza-like illness with little evidence that presence of an underlying medical condition affected clinical presentation. The reported duration of illness in cases was similar to seasonal influenza – with evidence that prompt administration of antivirals shortened length of symptoms, although it was apparent that a significant proportion of cases received antiviral therapy later than the recommended 48 h time window. Although a modest number of cases were hospitalized, the impact of illness was otherwise generally low with no deaths reported.
The FF100 was rapidly established in the first days after the arrival in the UK of pandemic (H1N1) 2009 influenza. It captured and synthesized information on almost 400 of the first UK cases in the first 7 weeks of the pandemic. This involved the rapid establishment of an internet-based database to store information on these early cases and their close contacts. Standard outputs of data were established that were used by a network of mathematical modellers to estimate key epidemiological parameters [Reference Ghani18] and to provide reports to international partners monitoring the global pandemic such as the World Health Organization and the European Centre for Disease Prevention and Control. Epidemiological analyses were shared with national policy-making bodies and formed a key component of information used to inform decisions on the initial clinical and public health management of the pandemic.
The cases captured by the FF100 were mainly children; an age distribution that has been reported consistently throughout the pandemic in the UK and globally [Reference Dawood19]. The FF100 cases were either returning travellers from affected countries or were linked to school outbreaks/clusters and to transmission in household settings. The key role in transmission played by children presumably reflects their high susceptibility, as evidenced by H1N1-specific serological surveys [20, Reference Miller21] and age-specific mixing patterns. Indeed schools became one of the main amplifiers of spread of infection in the community [Reference Ghani18].
The disease presentation observed in FF100 cases was generally mild, with clinical symptoms similar to what has been previously reported for seasonal influenza [Reference Nicholson22]. Of note are the differences observed in the pattern of symptoms as reported initially compared to at any time, presumably reflecting the natural history of the infection with the later onset of symptoms such as cough. The duration of illness in cases of pandemic (H1N1) 2009 influenza was slightly longer than that reported in a review of seasonal influenza by Nicholson [Reference Nicholson22] where acute symptoms (in particular fever) were reported to last up to 5 days. However, variation in duration of illness according to influenza virus strain is well recognized. Gastrointestinal symptoms were reported to be more common than in seasonal influenza: a feature observed also in the USA [Reference Dawood19]. The explanation for this observation is unclear. Although antiviral use may partly explain this pattern of symptoms, with several cases reporting these symptoms as adverse events attributed to antiviral use, it does not explain the similar picture in the USA, where antiviral use was much less widespread.
Although none of the FF100 cases died; a small proportion were hospitalized (5·8%), and three cases required mechanical ventilation. This compares to a case-hospitalization rate of about 9% in the USA at the time [Reference Dawood19]. It is well recognized that hospitalization rates are generally higher in the USA compared to the UK, and presumably reflects differences in healthcare utilization. The case-hospitalization ratio is somewhat higher than case-hospitalization ratios that have subsequently been reported [Reference Presanis23]. This difference may be due to initial uncertainty about the new virus, with a more cautious approach being adopted to hospitalization. Indeed some of the UK hospitalized cases were known to have been hospitalized for isolation purposes rather than severe illness. However, a small number of young adult hospitalized cases, particularly those with underlying clinical disease, had severe infection requiring mechanical ventilation.
Until 1 July 2009, as part of the UK's ‘containment’ approach to slow or limit transmission in the community and reduce morbidity, antiviral drugs were offered to all confirmed cases and their close contacts as treatment or prophylaxis [6]. Consequently almost all FF100 cases were taking antiviral drugs. This high level of antiviral use may have modified the natural history of the illness. For optimal effectiveness in the management of seasonal influenza, it is recommended that antivirals are administered within 48 h of onset [24]. However, a significant proportion of cases, received antiviral therapy later than this recommended time. We were able to demonstrate that early treatment does significantly shorten reported duration of illness. This supports previously published evidence from seasonal influenza [Reference Aoki and Boivin25], and highlights the value of antiviral treatment. This is particularly relevant for those individuals with an underlying medical condition, who are at risk of more severe outcome [Reference Donaldson26].
There were a number of limitations to this study. At this early stage in the pandemic, people were tested for pandemic (H1N1) 2009 influenza according to whether they fulfilled certain clinical and epidemiological criteria. The influenza virus can also cause mild illness or asymptomatic infection. As a result, many individuals who experienced an illness due to pandemic (H1N1) 2009 infection may have been missed if they did not meet the case definition. Data from a study of a large outbreak in a school in England suggested that approximately one third of those with serological evidence of infection had a case-defining influenza-like illness, one third had a mild non case-defining illness and one third had no illness at all (C. Ihekweazu, personal communication). This case definition may also have affected the symptoms reported by the cases as people without fever and respiratory symptoms will have been less likely to have been tested. However, the follow-up and testing of contacts allowed inclusion of cases who may not have fulfilled the case definition. In the latter stages of the FF100 project as overall case numbers increased, the proportion of cases that were followed up dropped. This may have biased the results; this can be seen especially in Scotland where only 4% of cases were included due to pressure on local staff to deal with numerous cases in a short amount of time. Data were often collected by different people and some discrepancies were introduced, particularly in the reported date of illness onset. This may be due to recall or recording errors. In this analysis, data from the FF100 questionnaires were favoured over any other data sources, unless a data item was missing, but available elsewhere or unlikely to be correct (e.g. date of symptom resolution date before onset date). These sorts of errors and discrepancies are unlikely to have introduced significant bias in the results.
In conclusion, the epidemiological and clinical picture suggests that pandemic (H1N1) 2009 influenza in the UK has a similar picture to seasonal influenza, with a generally mild clinical presentation. In-depth investigation of the early pandemic cases provided information on the key clinical and epidemiological characteristics of pandemic (H1N1) 2009 influenza and has helped inform national policy decisions.
ACKNOWLEDGEMENTS
The authors thank Anjana Roy, Heather Northend, Kathlyn Slack, Babatunde Olowokure, Tim Brooks, Oliver Blatchford, Sharon Hutchison, Eva van Velzen, John Love, Kirsty Roy, Katy Sinka, Beth Cullen, Maureen O'Leary, Norah Palmateer, Amanda Weir, Allan McLeod, Glenn Codere, Lelsey Wallace, Hamish Innes, David Goldberg and all staff across the Health Protection Agency, Regional Microbiology Network, Health Protection Scotland and Public Health Agency Northern Ireland who assisted in data collection, sample collection and testing. We are also grateful to Paragi Patel for assisting with data management, to Neill Keppie for provision of data on all pandemic flu cases in England and to Fateha Begum for provision of data from the DH vaccine uptake programme. P.W. thanks the MRC for funding.
DECLARATION OF INTEREST
J.S. N-V-T has received funding to attend influenza-related meetings, lecture and consultancy fees and research funding from several influenza antiviral drug and vaccine manufacturers and is a former employee of SmithKline Beecham plc (now GlaxoSmithKline), Roche Products Ltd, and Sanofi-Pasteur MSD. M.Z. chairs the Neuraminidase Inhibitor Susceptibility Network which is funded by GSK and Roche (Zambon and Hayden, 2001 Antiviral Research). J.E. has received funding from Sanofi-Pasteur MSD to attend a meeting. HPA receives funding from vaccine manufacturers for work undertaken in the influenza laboratory.