Introduction
Various forms of competition during evolutionary time imply that species within a guild are likely to coexist through resource partitioning (Begon et al., Reference Begon, Harper and Townsend1996). In prey-rich areas predators may coexist by exploiting different prey species (Karanth & Sunquist, Reference Karanth and Sunquist2000; Odden et al., Reference Odden, Wegge and Eliassen2010), whereas in areas of limited prey predators may compete with each other (Koshkarev, Reference Koshkarev1989; Harihar et al., Reference Harihar, Pandav and Goyal2011). In the mountains of Central Asia the diversity and abundance of wild ungulates are generally low. Consequently, sympatric large carnivores such as the snow leopard Panthera uncia and the wolf Canis lupus may be expected to have a high potential for competitive interactions. However, diet overlap and competition may be low if the two species use different habitats and hunting tactics. For example, the stalking snow leopard largely uses steep, broken terrain (Jackson & Hunter, Reference Jackson and Hunter1996) and the cursorial pack-hunting wolf uses a greater diversity of topography, including open valleys and rolling hills (Viripaev & Vorobiev, Reference Viripaev and Vorobiev1983), leading to some level of separation of habitat, and possibly diet.
The snow leopard is categorized as Endangered on the IUCN Red List and is listed in Appendix I of CITES. Habitat degradation, poaching, retaliatory killing in response to livestock depredation and prey depletion are the key factors leading to population declines (Nowell & Jackson, Reference Nowell and Jackson1996; Mishra, Reference Mishra1997; McCarthy, Reference McCarthy2000; Lovari et al., Reference Lovari, Boesi, Minder, Mucci, Randi, Dematteis and Ale2009). In Kyrgyzstan, numbers declined from an estimated 600–700 individuals in the late 1980s (Koshkarev, Reference Koshkarev1989) to 150–200 individuals by 2000 (Koshkarev & Viripaev, Reference Koshkarev and Viripaev2000), putting the species at high risk of extinction in the country.
In contrast, wolves are common in Central Asia and are considered a pest animal in many countries, with governments offering financial incentives for killing them (Lescureux, Reference Lescureux2006; Mijiddorj, Reference Mijiddorj2011). According to a recent report of the Kyrgyz Hunting Agency (2010) 157 wolves were killed in 2010 and their population was estimated to be > 3,500.
Argali Ovis ammon and Siberian ibex Capra sibirica are the main prey of snow leopards and wolves in Kyrgyzstan (Viripaev & Vorobiev, Reference Viripaev and Vorobiev1983). In most of Central Asia the decline in populations of mountain ungulates has been attributed to poorly managed hunting programmes, poaching and overharvesting (Weinberg et al., Reference Weinberg, Fedosenko, Arabuli, Myslenkov, Romashin, Voloshina, Zheleznov and Shackleton1997). Marmots Marmota baibacina, which constitute a smaller, seasonal prey, have also faced considerable threats. Between 1950 and 1960 an estimated one million marmots were poisoned during an eradication programme to prevent the spread of plague (BSAP, 1996). Reoccurrence of plague may trigger a repeat of large-scale eradication programmes. What are the likely impacts of prey declines on snow leopards and wolves and on their niche relationships? Will populations of these carnivores decline in the same way as their prey populations, or will they be affected differently? To answer these questions and to understand intraguild interactions, we examined the diets of snow leopards and wolves during summer, when argali, Siberian ibex and marmots are available. We conducted our study in the Sarychat-Ertash Reserve in Kyrgyzstan. We predicted that the different hunting strategies of snow leopards and wolves would result in divergence in their diets, with snow leopards preying mainly on ibex and wolves on argali. In the case of marmots, their abundance is greater in foothills and valleys that are used by wolves, but in many areas they are reported to constitute an important seasonal prey for the snow leopard (Schaller et al., Reference Schaller, Junrang and Mingjiang1988). We therefore predicted that both carnivores would prey on marmots.
Study area
The 1,341 km2 Sarychat-Ertash Reserve is at an altitude of 2,000–5,500 m in the upper reaches of the Uch-Kol River basin in the Tien-Shan mountain range (Fig. 1). The flat river bottom, with steep slopes on both sides, is up to nearly 1 km wide. The climate is cold and continental, with mean monthly temperatures in June and January of +4.2 and −21.5 °C, respectively. Annual precipitation is 295 mm, with nearly half falling in June–August (SER, 2007).
Fig. 1 Snow leopard Panthera uncia range, adapted from Fox (Reference Fox, Fox and Jizeng1994), and the location of the study area, Sarychat-Ertash Reserve, in Kyrgyzstan.
Vegetation consists of three main types: arid grasslands, wet meadows, and tundra cushion plants (Zlotin, Reference Zlotin and Wielgolaski1997). Snow leopard, wolf and red fox Vulpes vulpes are the most common carnivores; brown bear Ursus arctos, lynx Lynx lynx, manul Felis manul and stone marten Martes foina are also present. In addition to ibex and argali, potential prey species include marmot, hare Lepus tolai, pika Ochotona roylei, and birds such as snowcock Tetraogallus himalayensis and chukar partridge Alectoris chukar. There are reports of four species of mustelids and four vole species in the area (SER, 2007).
Before the Reserve was established in 1995 the area had been under pressure from human activities. Three Soviet collective farms used the area for livestock grazing year-round, keeping c. 4,800 domestic sheep Ovis aries there during winter. Heavy livestock grazing, illegal hunting, road building, geological exploration and other activities took place. By 2012 only a small part of the Reserve's buffer zone was used for seasonal livestock grazing. There are two small villages near the Reserve. The nearest is Ak-Shiyrak, which has c. 25 households (70 people) and is 7 km south of the Reserve. Fourteen villagers from Ak-Shiyrak work as rangers in the Reserve, and livestock herding (sheep and horses) is the main source of income for the village.
The Reserve was part of the area covered by the marmot eradication programme in the 1950s, after epidemiologists discovered plague in the Central Tien-Shan (BSAP, 1996).
Methods
Scat collection
In 2009 we collected scats along 14 0.6–1.5 km transects spaced 3–5 km apart. The transects were along ridges, tributary streams (nullah) and the bases of cliffs, extending upwards from the flat valley bottom on both sides of the main river. We took samples four times between June and October, with at least 20 days between trips to allow fresh scats to be deposited. Only a part of the scat was collected. Uncollected material was left in its original location, and to avoid re-sampling the same scat in subsequent trips it was marked with red nail polish. We also collected scats opportunistically while travelling to and between transects, sampling proportionally more of the flat and grassy slopes closer to the main river. Small pieces of the fresh samples were collected in plastic tubes with silica gel for DNA analysis, and larger pieces were collected in paper envelopes and air-dried for microhistological diet analysis.
Genetic screening of scats
Recent studies have demonstrated that carnivore scats collected in the field are often misclassified (Janecka et al., Reference Janecka, Jackson, Yuquang, Diqiang, Munkhtsog and Buckley-Beason2008; Anwar et al., Reference Anwar, Jackson, Nadeem, Janecka, Hussain and Beg2011; Shehzad et al., Reference Shehzad, McCarthy, Pompanon, Purevjav, Coissac, Riaz and Taberlet2012; Wegge et al., Reference Wegge, Shrestha and Flagstad2012). When using scats for dietary analysis it is therefore necessary to control for species identity. Our scat samples were genetically screened at the American Museum of Natural History. Following extraction of genomic DNA, species identification was based on four mitochondrial DNA fragments (CytB, 12S, 16S and ATP6). Each identified target species was reconfirmed by genotyping three times. For further information on the laboratory procedure used at the Museum see Shehzad et al. (Reference Shehzad, McCarthy, Pompanon, Purevjav, Coissac, Riaz and Taberlet2012).
Reference key and laboratory analysis
We collected reference hair samples from the remains of ungulate species found in the study area and from specimens maintained at the Kyrgyz Academy of Sciences. Different types of livestock hair were taken from the villages near the study area. Hair types were selected from different body parts and fixed in envelopes marked with the species name. A photographic key of all potential prey species was prepared by taking pictures of hair structures with a microscopic camera at 40× magnification. As the hair scale patterns of ibex and argali are similar, we identified the species from cross-sectional cuttings of primary guard hairs, which were photographed at 60× magnification (Oli, Reference Oli1993). All scat samples were first oven-dried for 24 hours at 90 °C and then washed and sieved under running water. To extract hairs of ungulates we used a mesh size of 0.8 mm and for small mammal hair a mesh of 0.5 mm. Matter such as bones, stones and plant material was manually separated, dried and stored in annotated plastic bags.
To identify prey species we used a modified version of the point frame method (Stewart, Reference Stewart1967; Ciucci et al., Reference Ciucci, Tosoni and Boitani2004): instead of using drop pins to select the prey items we used tweezers to select the prey items at each intersection (n = 50) in a gridded tray (Wegge et al., Reference Wegge, Shrestha and Flagstad2012) and examined these under a dissecting scope, or a high-power microscope if necessary for reliable identification. The hair of marmot and hare was easily identified by colour, size, shape, length and scale patterns. Small rodents could not be identified to species and were therefore classified simply as rodents. Besides using the photographic key, important features for use in hair identification were taken from Brunner & Coman (Reference Brunner and Coman1974) and Oli (Reference Oli1993).
Abundances of main wild prey
Abundances of ungulate prey were estimated from total counts in 2007 (ibex) and 2009 (argali). The argali census was conducted shortly before the rut in late autumn, when animals aggregate at lower elevations. During a 10-day period a team of five observers, using binoculars and spotting scopes, traversed the central part of the Reserve, including all side-drainages, where scats were also sampled, and counted all animals seen. They were classified according to sex and age classes whenever possible. Landscape features, direction of movement, and herd composition helped to avoid multiple counting of the same groups. The ibex census was conducted by teams of well-trained Reserve rangers using the same method in the same area during winter, because ibex is more easily observed and counted during the snowy winter season. The results were extrapolated from the census report filed at the Reserve headquarters (SER, 2007).
Statistical analyses
Because smaller prey are composed of proportionally more indigestible material than larger prey, they occur in scats at higher relative proportions per unit ingested than larger prey (Floyd et al., Reference Floyd, Mech and Jordan1978). Based on feeding trials, correction factors have been developed to account for this. For the snow leopard we used the equation developed by Ackerman et al. (Reference Ackerman, Lindzey and Hemker1984) for the cougar Felis concolor (y = 1.980 + 0.035x), and for wolves we used the equation (y = 0.439 + 0.008x) developed by Weaver (Reference Weaver1993), where y is the biomass of prey consumed (kg) to produce a single scat, x is the mean body weight of the prey species (kg), and x/y represents the total number of scats produced by consumption of a single individual of a species.
Mean weights of prey species were taken from Iyanushevich et al. (Reference Iyanushevich, Aizin, Kidiraliev, Umrihina, Fedianina and Shukurov1972). The use of correction factors to calculate estimates of biomass and number of prey consumed from the amount of remains in scats is an established technique in carnivore scat analysis (Link & Karanth, Reference Link and Karanth1994; Bagchi et al., Reference Bagchi, Mishra and Bhatnagar2004; Müller, Reference Müller2006; Wegge et al., Reference Wegge, Odden, Pokharel and Storaas2009). To compare selection of main prey by snow leopards and wolves we generated expected frequencies from the mean density estimates of argali and ibex, with coefficients of variation assumed to be 50%. We applied such large coefficients of variation to allow for errors, given that the density estimates were not robust and did not report variances. Using these mean densities and their variances we applied a parametric bootstrap procedure to generate random prey densities according to a normal distribution. For each likelihood ratio test, 500 bootstrap simulations were run to generate sets of densities, hypothesized scat frequencies and G 2 statistics. The mean P-values associated with G 2 statistics from these simulations were used to infer predator selectivity patterns. Simulations were run for all three principal prey species (argali, ibex and marmot), for both snow leopards and wolves. Details of this statistical methodology were described by Link & Karanth (Reference Link and Karanth1994).
We used SCATMAN (USGS, Laurel, USA; Hines & Link, Reference Hines and Link1994) to analyse the diets. The scat production rate was estimated as x/y, and the variability of this rate was considered to be a constant 40%. This was done to incorporate the large variation between the body masses of different age/sex classes of each of the primary prey species. As the physiologies of the two predators are different, the numbers of scats they produce after consuming the same prey species of the same size will vary. This results in different proportions of scats for each predator for a given density of prey. Diet diversity was estimated using the standardized Levin's index (B a; Colwell & Futuyma, Reference Colwell and Futuyma1971), and diet overlap was estimated using Pianka index (Pianka, Reference Pianka1973).
Results
We collected 98 fresh scats in an area of c. 700 km2. Of 59 putative snow leopard scats, 39 (67%) were confirmed by genetic analysis to be from snow leopard. The others were from red fox (12), stone marten (3) and unknown species (5). Of 50 putative wolf scats, 36 (72%) were confirmed to be from wolf. The others were from brown bear (4) and unknown species (2), and eight were from snow leopard, raising the sample size of this species to 47 scats. Accumulation curves showed that the verified samples were sufficient for the diet analysis because the relative contribution of prey species to the diets stabilized at 30–35 scats.
The diets of the two carnivores are given in Fig. 2, showing the composition based on the frequency of occurrence of prey remains in scats and based on percentage contribution of biomass of each specific prey consumed (plant material is omitted). Argali was the most important prey in the diet of both carnivores, constituting c. 50% of the diet of snow leopards and > 60% of the diet of wolves. Ibex constituted a larger percentage of the diet of snow leopards (c. 30%) than of wolves (c. 10%), and marmots were more commonly found in the wolf scats (c. 20% of samples) than in the snow leopard scats (c. 8% of samples). Remains of the shrub myricaria Myricaria bracteata were found in 45% of the snow leopard scats. Livestock remains were not detected in any samples. Other samples consisted mainly of rodents and unknown species.
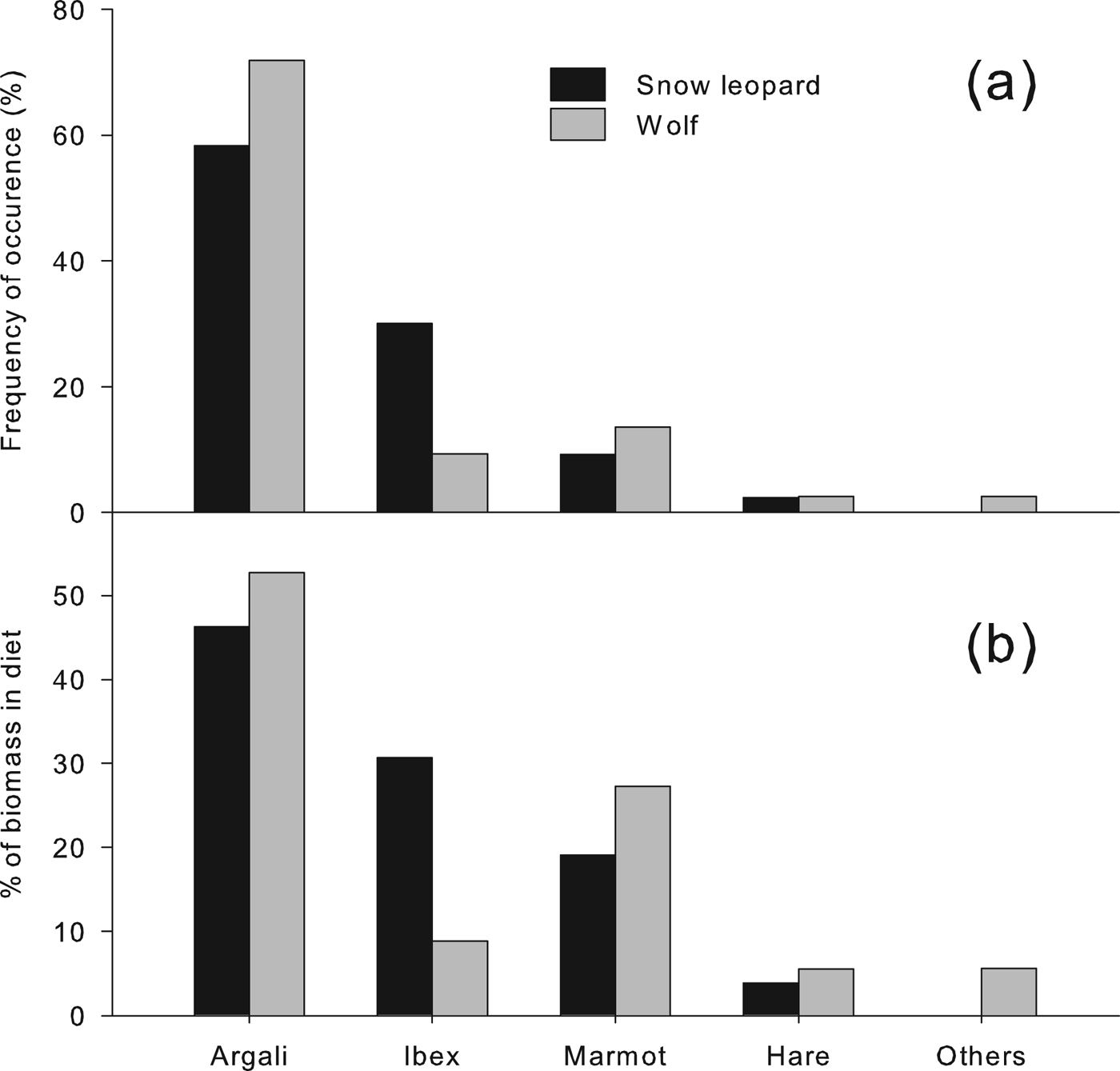
Fig. 2 Composition of summer diets of snow leopards and wolves in Sarychat-Ertash Reserve (Fig. 1) in terms of (a) frequency of occurrence and (b) biomass consumed, derived from analysis of scats.
Diet diversity was higher in snow leopards than in wolves. Based on the number and biomass of individuals consumed, Levin's indices were 0.35 and 0.25, respectively, for snow leopard; for wolf they were 0.31 and 0.17. Pianka's index was 0.91, indicating a large overlap in the diets of the two predators.
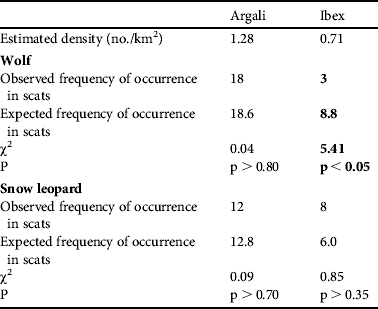
The crude densities of the two main prey species were estimated to be 0.67 and 1.79 individuals km−2 for ibex and argali, respectively. Observed and expected distributions of these prey species in the scats are shown in Table 1. The observed frequencies of argali were as expected in both snow leopard and wolf. In wolf, ibex occurred less frequently than expected, whereas in snow leopard ibex occurred according to their availability in the Reserve.
Table 1 Selection of primary prey species (argali Ovis ammon and ibex Capra sibirica) by wolf Canis lupus and snow leopard Panthera uncia in Sarychat-Ertash Reserve (Fig. 1) during summer 2009, based on scat analysis. Significant preference or avoidance is indicated in bold font.
Discussion
Three prey species, argali, Siberian ibex and marmot, accounted for > 90% of the food of both predators, with argali alone constituting > 50% of the diet for both. The narrow food base was not surprising, considering that livestock were unavailable and there are large differences in biomass between the two highly profitable ungulates and the smaller potential prey species. Although relatively abundant in the Reserve, hares, rodents and gallinaceous birds made up only a small proportion of the diets of the two carnivores. Except for studies in Nepal (Oli et al., Reference Oli, Taylor and Rogers1993) and China (Schaller et al., Reference Schaller, Junrang and Mingjiang1988), our results agree well with what has been reported for snow leopards elsewhere (Anwar et al., Reference Anwar, Jackson, Nadeem, Janecka, Hussain and Beg2011; Shehzad et al., Reference Shehzad, McCarthy, Pompanon, Purevjav, Coissac, Riaz and Taberlet2012).
The low diversity and large overlap in diets, especially with respect to argali, suggest that the two carnivores may compete for food. Conversely, the primary prey species may be sufficiently abundant for both predators to exploit them without competitive interference. There is some evidence in support of the latter scenario: as smaller prey was readily available in the Reserve, the dominance of only two ungulate species and marmots in the diets indicates that the principal food resources (argali and ibex) were not critical for either snow leopards or wolves. This interpretation is supported by the relatively high crude density of 1.8 argali km−2 recorded during the autumn survey, which was probably an underestimate, according to a recent test of survey methods for this species (Wingard et al., Reference Wingard, Harris, Ambalanbaatar and Reading2011). Productivity was also relatively high, as evidenced by the composition of subsamples of observed herds (n = 33): lambs constituted c. 18% of the autumn population, at a ratio of lambs to adult ewes of 0.52, which is similar to the results from surveys conducted in the same general area a few years earlier (Klich & Magomedov, Reference Klich and Magomedov2010). A probable explanation for the lack of competition between the two carnivores in spite of similar and specialized diets is that the range quality for wild ungulates increased significantly following the termination of widespread and heavy grazing by livestock 13 years previously. This, combined with virtual cessation of hunting, could have facilitated an increase in the ungulate population. The population of wolves, which are persecuted when roaming outside the Reserve during winter, and the originally sparse population of snow leopards did not increase as quickly as that of their primary prey species (Reserve staff, pers. comm.). This scenario may allow wolves and snow leopards to share the same food resources without competition.
The alternative scenario is that competition is taking place, mainly because of food limitation among snow leopards. Ibex occupies the same rugged habitat as snow leopards and is a principal prey of this predator. In the Reserve the abundance of this prey may be limited relative to the numbers of snow leopards, leading the leopards to prey heavily on argali, thus competing with wolves for this resource. Although argali mainly occupy open ground away from ibex habitat (Schaller, Reference Schaller1976; Dzieciolowski et al., Reference Dzieciolowski, Krupka, Bajandelger and Dziedzic1980; Namgail et al., Reference Namgail, Fox and Bhatnagar2004), small family groups of argali could be vulnerable to snow leopard attack when they graze within critical distances of snow leopard habitat during summer. As wolf packs rarely venture into the steep, rugged terrain of ibex when hunting (Viripaev & Vorobiev, Reference Viripaev and Vorobiev1983), competition with snow leopard for this species occurs less frequently.
We collected carnivore scats in summer, when the prey base is more diverse than in winter. We found that marmots comprised a significant portion of the summer diets of both snow leopards and wolves, but especially of wolves. In winter, when marmots are unavailable because of hibernation, predation on the two ungulates would be expected to intensify, although wolves reportedly also obtain food by preying on domestic stock (mainly horses and sheep) outside the Reserve. Therefore, to further assess food competition and identify predator–prey interactions, the diets of the two carnivores also need to be monitored and compared during winter.
Ongoing trophy hunting outside the Reserve could potentially lead to population declines of ibex and argali. We would expect a reduction in the ibex population to have a direct negative effect on snow leopards, whereas the wolf is likely to be indirectly affected through more intense competition resulting from elevated predation on argali by snow leopards.
Our study area was more or less free of livestock grazing, making it atypical of the snow leopard's range, but the number of livestock is now increasing in the communities near the Reserve. This may pose a number of threats to ongoing conservation efforts, including escalation of human–carnivore conflicts, overgrazing and range deterioration, and transfer of disease to wild ungulate prey. It is therefore important that good management and protection of the Reserve be maintained in the coming years.
Acknowledgements
The study was funded by Panthera and the Snow Leopard Trust. The Sarychat-Ertash State Reserve provided logistical and personnel support for the fieldwork. We are grateful to Musaev Mukhtar (Director of the Sarychat-Ertash Reserve), Tomas McCarthy, Jennifer S. Rullmann and Raghunandan Singh Chundawat for assistance in various phases of the study. Laboratory facilities for the scat analysis were provided by the Norwegian University of Life Sciences.
Biographical sketches
Kubanychbek Jumabay-Uulu is now the coordinator of snow leopard conservation work in Kyrgyzstan, following a study of snow leopards and wolves in the Sarychat-Ertash Reserve. Per Wegge's research interests include predator–prey relationships of large mammals, mainly in Nepal, and the impact of commercial forestry on boreal forest grouse. The research and conservation activities of Koustubh Sharma and Charudutt Mishra are centred on carnivores and wild and domestic ungulates in the Indian Himalayas, with a focus on mitigating predator–human conflicts.





