Introduction
Listeria monocytogenes is a Gram-positive facultative anaerobic bacterium. Although L. monocytogenes is ubiquitous and commonly found in the environment, humans get infected mostly through ingestion of contaminated food.
Human infection with L. monocytogenes, listeriosis, is a rare disease which may be severe and even fatal in at-risk populations. Symptoms of listeriosis appear to be host-dependent and can range from a mild self-resolving gastro-intestinal illness to an invasive form of the disease, which can cause bacteraemia and meningitis. Persons particularly at risk for invasive disease include pregnant women, elderly and immunosuppressed individuals, with an overall case fatality rate of 20–30% [1, Reference Jensen2]. Among infected pregnant women, bacteria may cross the placenta and infect the foetus, leading to premature birth, abortion or neonatal listeriosis. L. monocytogenes is classified in four lineages (I, II, III and IV) and 13 serotypes. The most common circulating strains are from lineage I (serotype 4b and 1/2b) and lineage II (serotype 1/2a and 1/2c) [Reference Chenal-Francisque3]. The bacterium is salt tolerant and can grow at a wide range of pH and at extreme temperatures (ranging from −0.4 to 45 °C [Reference Junttila, Niemelä and Hirn4]), which are significant advantages for its survival and persistence in the environment and hence, its ability to contaminate.
Listeriosis has been a notifiable disease, by clinicians as well as laboratories, in Israel since 1996 [Reference Siegman-Igra5]. All cases are notified to the relevant district office and the L. monocytogenes isolates are sent to the National Reference Laboratory for confirmation and subtyping. Surveillance has shown that its national incidence in Israel varies from 0.2 to 0.8/100 000 population per year [6]. The rate of pregnancy-associated listeriosis in Israel was found to be among the highest worldwide with up to 25.2 cases per 100 000 live births [Reference Junttila, Niemelä and Hirn4].
Listeriosis rates in the Tel Aviv District were observed to be higher compared to overall national rates [Reference Elinav7].
We report epidemiologic, clinical and molecular findings of listeriosis to further understand the high rates of morbidity and exposure.
Materials and methods
A case of listeriosis was defined as a patient from whom L. monocytogenes was isolated from a normally sterile site (e.g., blood or cerebrospinal fluid or, less commonly, joint, pleural or pericardial fluid). Pregnancy-associated cases were defined as cases with a L. monocytogenes isolation from a pregnant woman, her foetus, or from a newborn up to 1-month old. A mother–neonate pair was defined as a pair for which L. monocytogenes was isolated from both the mother and the neonate. The study only included residents of the Tel Aviv District.
Each case of culture-confirmed listeriosis is notified to the Tel Aviv District Health Office. A trained nurse contacts the patient or a close relative and initiates an epidemiologic investigation. A structured questionnaire [8] is used to clarify the disease presentation, underlying medical conditions and food consumptions up to 2 months preceding disease onset. The questionnaire includes a list of popular foods that were recalled in Israel for L. monocytogenes contamination such as hummus, raw, smoked and cured fish, deli meat and dairy products. The list is passed thereafter to the District Food Service for food sampling in the retail stores where the food was purchased by the patient. In the case of packaged food, samples are taken from sealed packages only. Restaurants in which cases consumed food during the 2 months preceding illness onset undergo sanitary inspections and relevant food samples are taken. According to the results of the inspection and/or if positive food samples are identified, the restaurant, retail store or food factory undergo corrective measures, repeated inspections and the district physician is informed. Additionally, the Israeli food safety legislation requires food factories and restaurants to undergo routine investigations and sampling, as it is mandatory to provide food free of L. monocytogenes.
Clinical and socio-demographic data regarding patients were collected from hospital files. Patients were classified as having either neuro-invasive disease (NID) which included meningitis, encephalitis or meningo-encephalitis, blood stream infection (BSI) or infection in another bodily site with L. monocytogenes. Deaths that occurred within 1 month of diagnosis were considered as illness associated. Hospital-acquired disease was defined as a case for which exposure to food, during the entire 2 months prior to disease onset, was in a hospital or a nursing home. Possible hospital-acquired infection was defined as a case having been exposed to food anytime in the 2 months prior to disease onset in a hospital or nursing home.
Laboratory analysis
Each isolate of L. monocytogenes identified by the diagnostic laboratory is sent to the National Listeria Reference Laboratory for confirmation and serotype identification. Serotyping was performed with commercial antisera (Denka Seiken, Tokyo, Japan).
Since 2007 L. monocytogenes from all clinical cases and most of the positive food samples are subtyped by pulse field gel electrophoresis (PFGE). The PulseNet standardised PFGE protocol for L. monocytogenes was performed with two restriction endonucleases, XbaI and AvrII, in two separate experiments [Reference Halpin9]. PFGE patterns were analysed by the BioNumerics software (Applied Maths) followed by unweighted pair group method with arithmetic mean clustering analysis. Clones were assigned a number according to the international PulseNet criteria.
Statistical analysis
All the data collected were evaluated using IBM SPSS Statistics 23.0 Armonk, NY: IBM Corp. Given our relatively small sample, analysis of continuous variables was done using Kruskal–Wallis H test, and categorical variables with a χ 2 test. A two-tailed value of P inferior or equal to 0.05 was considered statistically significant.
Results
Epidemiologic characteristics
During the years 2010–2015, 102 cases of culture-confirmed listeriosis were notified to the Tel Aviv District Health Office. All cases were hospitalised. Among them, 69 (68%) cases were women. Of all cases, 23 (23%) cases were pregnancy associated. There were 26 (25%) deaths associated with the illness.
During the studied years, rates of listeriosis in the Tel Aviv District were grossly twice as high as the overall national rates (0.8–1.8/100 000 vs. 0.4–0.9/100 000 population per year). Compared to the other Israeli districts, rates of listeriosis were consistently higher in the Tel Aviv District (Fig. 1).
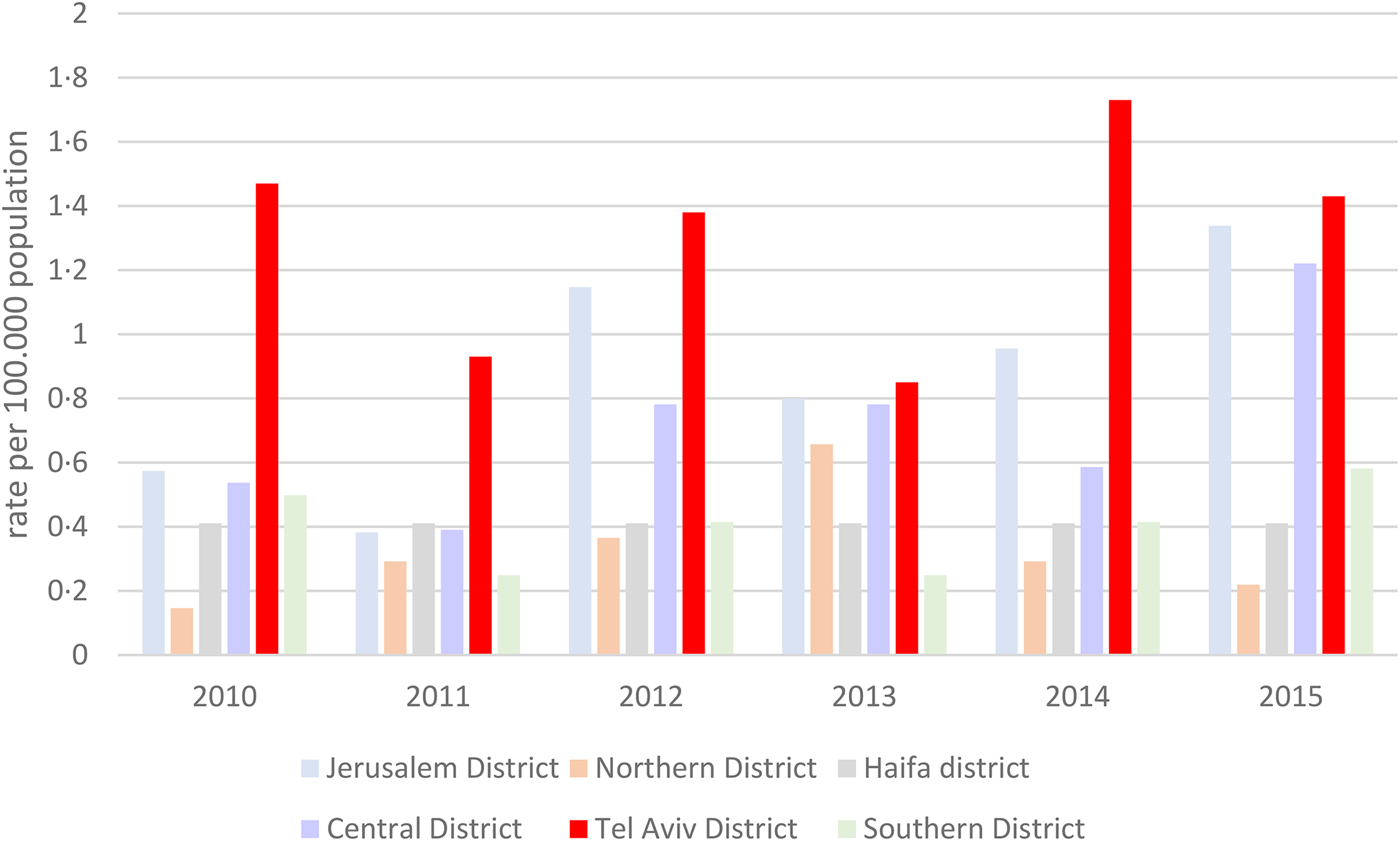
Fig. 1. Rates of listeriosis per 100 000 population in the different districts in Israel, 2010–2015.
Age-specific disease rates in the Tel Aviv District were also higher in all age categories compared to national rates (Fig. 2).
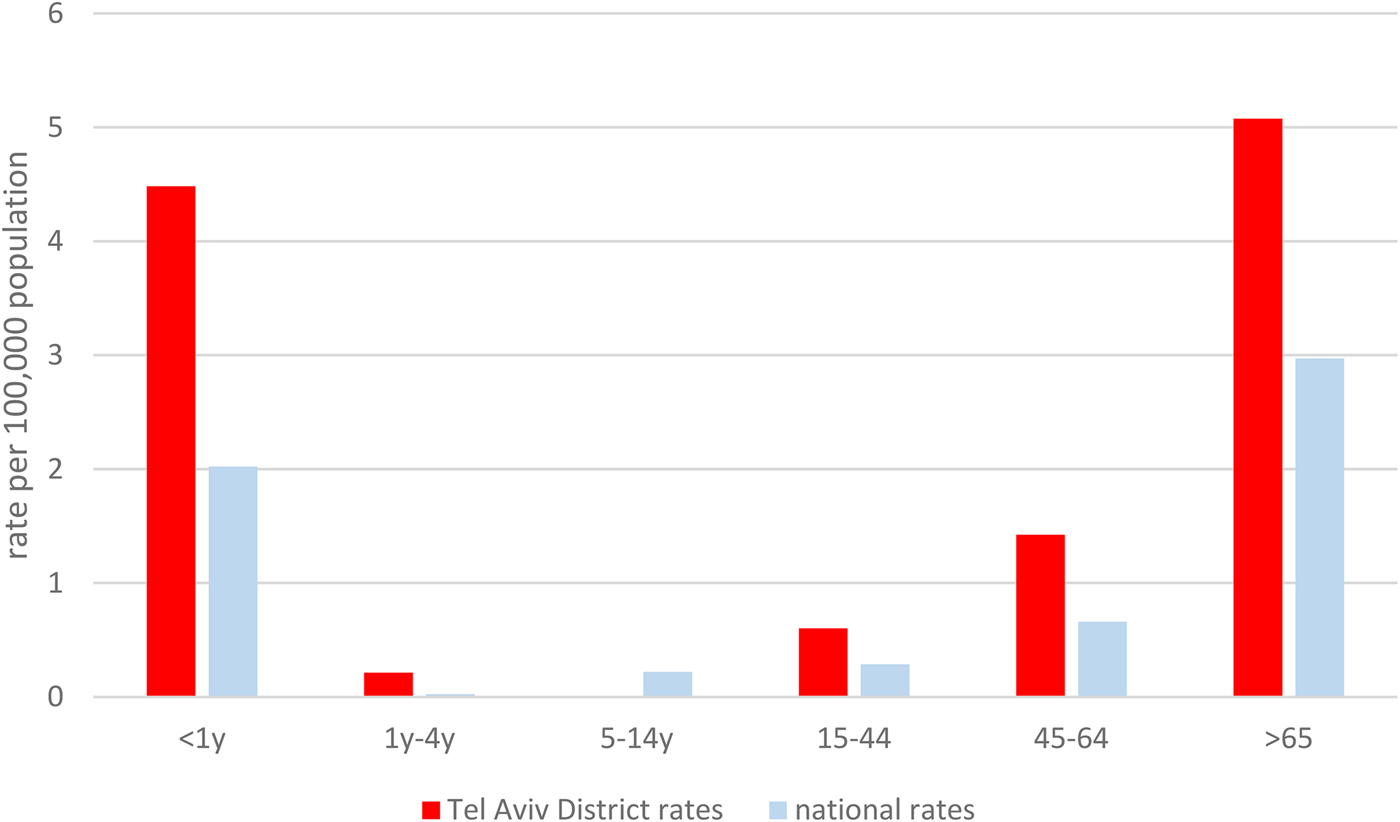
Fig. 2. Age-specific listeriosis rates in the Tel Aviv Districts and national age-specific listeriosis rates, 2010–2015.
We did not find a seasonal trend in illness onset in the Tel Aviv District but we did find that disease peaked in May and September, in which two major Jewish holidays (the Jewish New Year and the Israeli Independence Day) occur (Fig. 3).

Fig. 3. Monthly distribution of the number of cases of listeriosis during the years, Tel Aviv District, Israel, 2010–2015.
Non-pregnancy-associated cases
Among 79 notified cases, median age was 76 years old (range 4–100). There were 31 men (39%) and 48 women (61%). Seven cases were nursing home residents. Fifteen patients (19% of cases) had possible or proven hospital acquisition.
Median duration of hospitalisation was 10 days (range 1–54). Seven cases (9%) were hospitalised in the intensive care unit, two of which required mechanical ventilation. All patients had at least one underlying medical condition (Table 1). Eighteen cases (23%) had NID, 54 (68%) had BSI. Three patients had L. monocytogenes infection in an isolated body site (one patient with wound infection, one with arthritis and one with peritonitis).
Table 1. Demographic and clinical characteristics of non-pregnancy-associated listeriosis cases, Tel Aviv District, Israel, 2010–2015
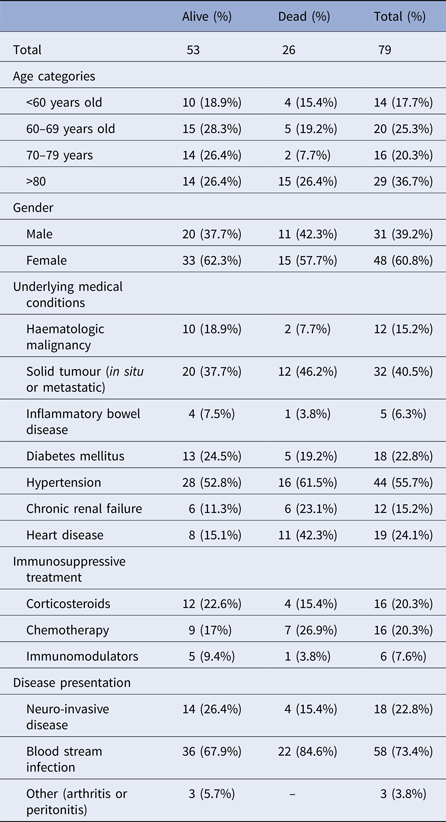
The occurrence of NID was not significantly associated with age, gender or L. monocytogenes serotype. Mean duration of hospitalisation was significantly longer for patients with NID compared with patients with BSI (Table 2).
Table 2. Clinical and bacterial serotype characteristics of non-pregnancy-associated cases, Tel Aviv District, Israel, 2010–2015
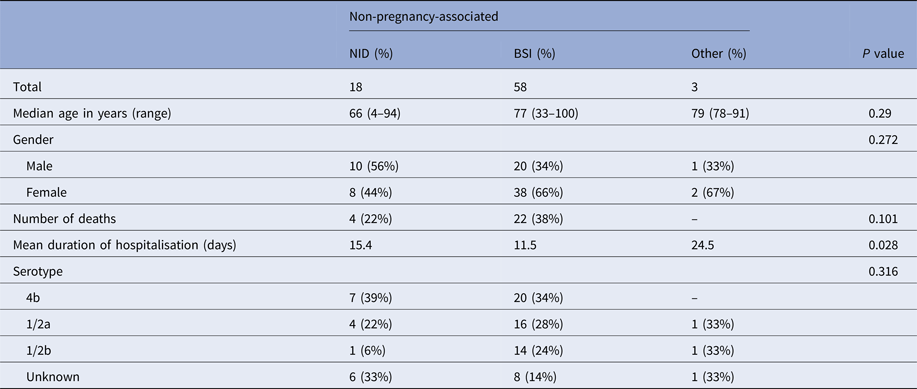
All 26 deaths observed in this study were non-pregnancy associated (case fatality rate in this category was 33%). There was no significant association between death occurrence and L. monocytogenes serotype or clinical presentation (P > 0.05).
The most common food histories included consumption of pre-packed commercial hummus (23%), cured or smoked fish (20%), deli meats (17%) and sausages (9%). Seven patients (9%) reported having eaten in restaurants during the 2 months prior to their illness onset.
Pregnancy-associated cases
Twenty-three pregnancy-associated cases were observed in this study, approximately 15 cases per 100 000 live births in the Tel Aviv District. Seventeen (74%) were pregnant women and six (26%) were newborns. Among them, there were three identified mother–neonate pairs. Their most common food consumptions included deli meats, hummus and sushi. Ten women reported having eaten in various restaurants.
Among pregnant women, mean age was 31 years old (range 19–40). Pregnancy outcomes included seven (30%) miscarriages, five (22%) pre-term births, five (22%) cases of neonatal listeriosis and six (26%) normal ongoing pregnancies.
We found a statistically significant association between trimester of pregnancy in which disease was diagnosed and pregnancy outcome. There were significantly more cases of neonatal listeriosis and pre-term births when disease was diagnosed in the third trimester, and more miscarriages when diagnosed in the first or second trimester (P = 0.012) (Table 3).
Table 3. Pregnancy-associated listeriosis outcome characteristics, Tel Aviv District, Israel, 2010–2015

Laboratory results
The bacterial serotype was identified for 84 isolates. Fifty-two per cent were serotype 4b, 25% were serotype 1/2a and 23% were serotype 1/2b (Fig. 4). All three subtypes were isolated among non-pregnancy-associated cases, but among pregnancy-associated cases bacterial isolates solely belonged to lineage I (serotypes 4b and 1/2b). There was no statistically significant association between bacterial serotype and number of deaths or duration of hospitalisation. Moreover, there was no significant association between serotype and pregnancy outcome (P > 0.05).
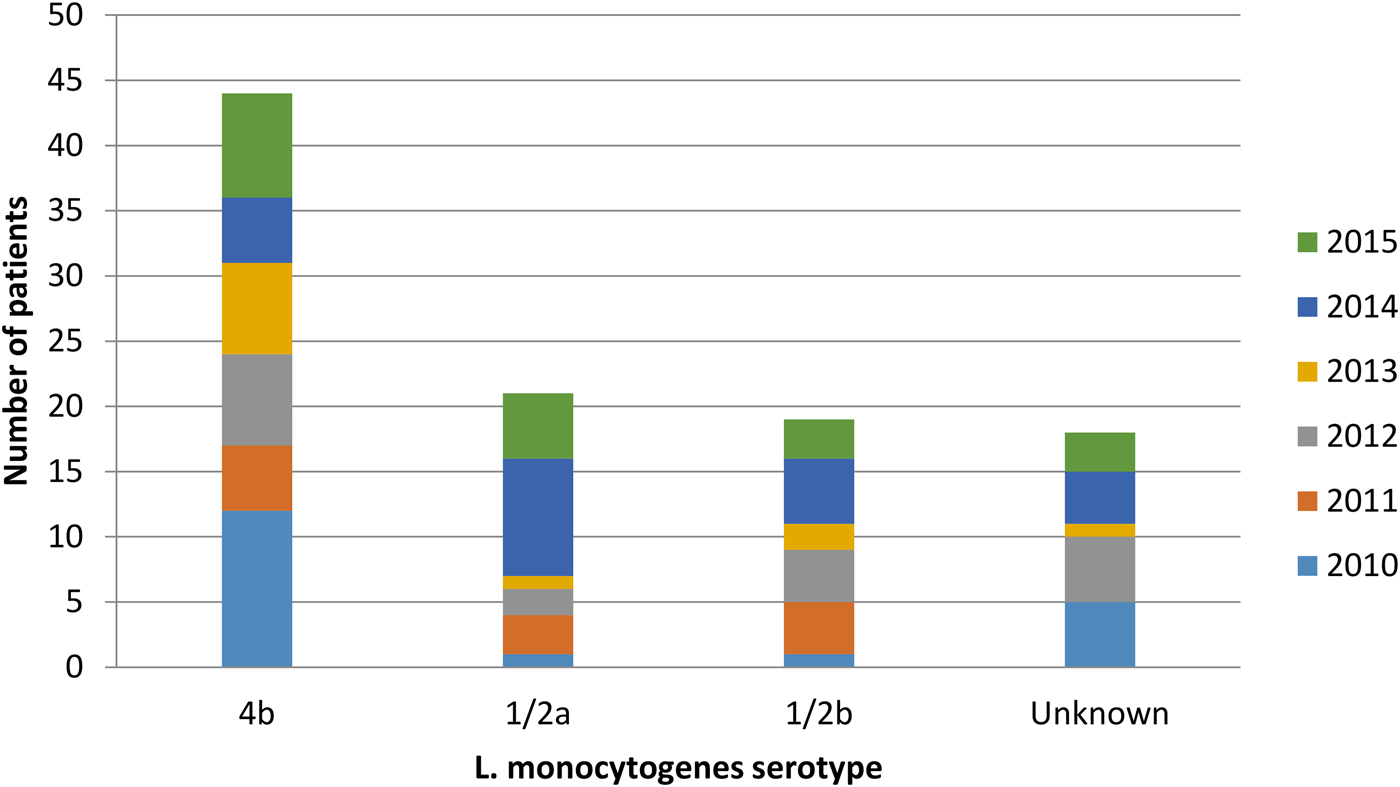
Fig. 4. Listeria monocytogenes serotypes isolated from patients during the years, Tel Aviv District, Israel, 2010–2015.
Trace-back and molecular epidemiology
During the studied period, a total of 35 molecular clusters were identified in the Tel Aviv District. Six molecular and epidemiologic clusters are described for the purpose of this study, entailing 38 patients (Table 4; Fig. 5). Smaller clusters of identical molecular clones were found but were not described as we could not find an epidemiologic link between the patients.
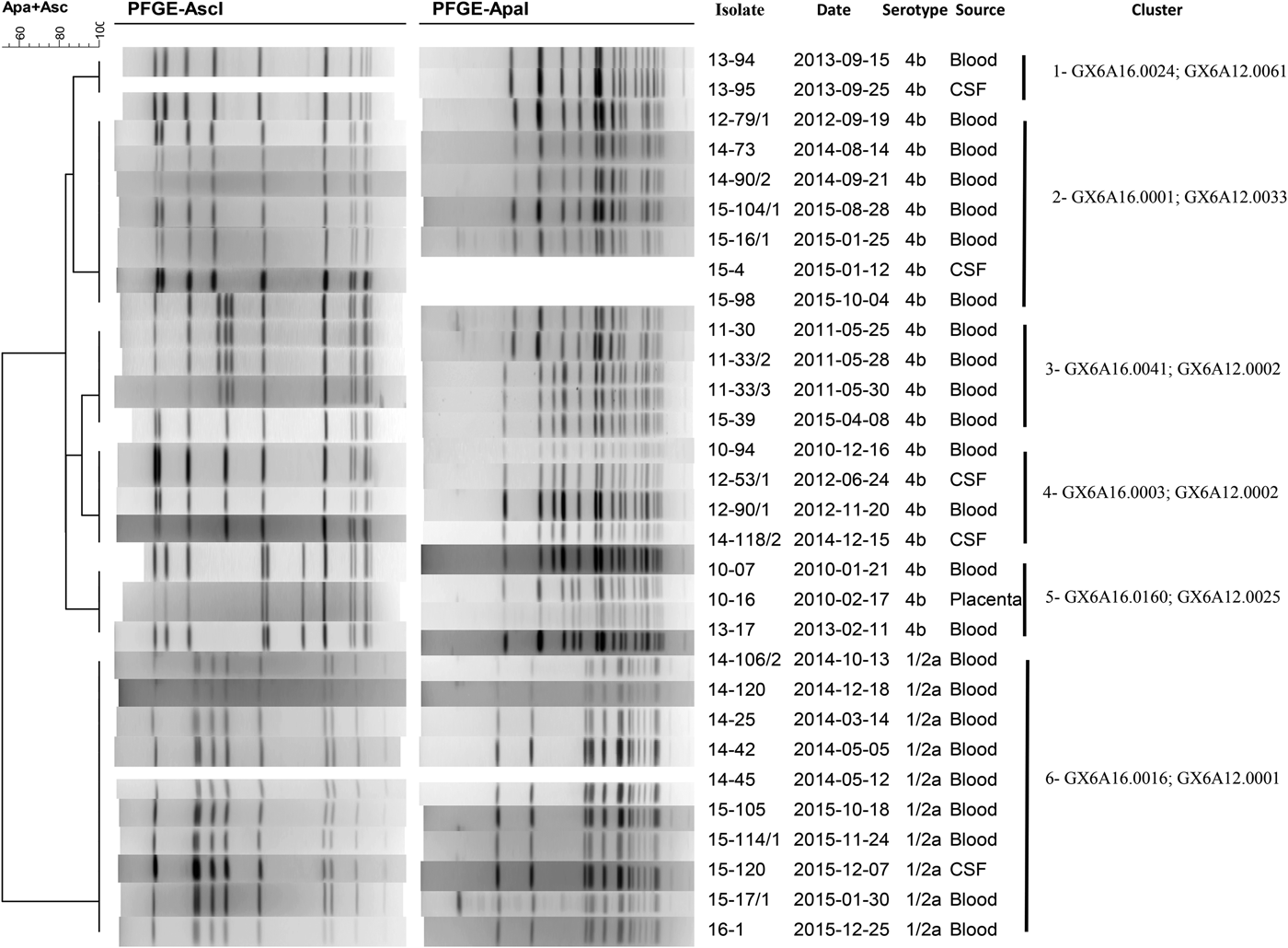
Fig. 5. Molecular clusters of Listeria monocytogenes isolates in the Tel Aviv District, Israel, 2010–2015.
Table 4. Selected clusters of clonal Listeria monocytogenes in the Tel Aviv District, Israel, 2010–2015

Cluster 1 consisted of two newborns infected with the same clone of L. monocytogenes. The two newborns were born on the same day in the same hospital and assisted by the same midwife. The first newborn to develop symptoms was born to a mother that developed fever during pregnancy and delivered at 36 weeks gestational age (GA). The mother was given antibiotics during delivery and her cultures were negative for L. monocytogenes. The newborn had BSI, improved with antibiotic treatment and was discharged in good condition. The second case was born spontaneously to an asymptomatic mother at 39 weeks GA, was discharged home and was readmitted a week post-discharge with NID. The mothers did not report common exposures and did not live in the same city. The identical L. monocytogenes genotype was isolated in raw fish in that same year. However, the women did not report having eaten fish during their pregnancy. One of the mothers reported having eaten vegetarian sushi at a Japanese restaurant, which later underwent a sanitary inspection during which food samples were taken but were all found to be negative for the presence of L. monocytogenes.
Clusters 2 and 5 included both pregnancy and non-pregnancy-associated cases. In both clusters, the same L. monocytogenes pulsotype was not isolated from any food source.
Cluster 2 involved three cases living a few blocks apart in the same city, two of which fell ill 1 month apart. The third was diagnosed 3 years later. The two non-pregnancy-associated cases died as a result of the disease and no food histories were collected. The pregnancy-associated case reported having consumed deli meats as well as frozen fish at home and uncooked salads at a restaurant, which underwent a sanitary investigation during which salads were found to be kept above the recommended temperatures but samples were negative for the presence of L. monocytogenes.
In cluster 5, most cases were pregnancy-associated (six out of eight cases). Pregnancy outcomes included one normal ongoing pregnancy and five abnormal outcomes (two pre-term births, two cases of neonatal listeriosis and one miscarriage). Five out of eight patients reported having eaten in various restaurants in Tel Aviv, but all food samples taken were negative for listeria.
Cluster 3 included seven cases, one of which was pregnancy-associated. Two cases had NID, the remainder had BSI. Among non-pregnancy-associated cases, all patients were older than 60 years (mean age was 77.67) and had underlying medical conditions. Two patients died during hospitalisation. All five cases for which data on food consumption were available reported having eaten a popular brand of pre-packed deli meat. Incidentally, the identical L. monocytogenes pulsotype had been isolated from that same brand of pre-packed deli meat during routine sampling. The factory underwent corrective measures as well as repeated sampling, and the foodstuff was recalled, shortly before the occurrence of the cluster. This pulsotype was also found in unrelated routine samples of cakes (in 2010 and 2013).
Clusters 4 and 6 consisted of non-pregnancy-associated cases.
Cluster 4 included three tenants of the same nursing home who fell ill a few days apart. They exclusively ate food that was served at the nursing home, which was sampled by the District Food Service as part of the epidemiological investigation. L. monocytogenes was isolated from cooked ground chicken which was served at the nursing home but it was not identical to the bacteria that were isolated from the patients. The three patients died as a result of the illness. The additional case reported having eaten various packaged condiments, which were sampled but were not found to be contaminated with L. monocytogenes. This pulsotype has also been found in unrelated isolates of ready-to-eat salads since 2008.
The pulsotypes demonstrated in clusters 3 and 4 have been among the most commonly isolated genotypes in Israel, ever since 2006.
In cluster 6, epidemiologic inquiries were carried out for eight out of nine cases. Four patients reported having eaten cured or smoked fish. One sample of smoked salmon taken by the District Food Service in a restaurant as part of the investigation was positive for the L. monocytogenes of the same pulsotype. In addition, the same pulsotype was also found in other various smoked and cured fish across the country. All the factories which provided the contaminated foodstuffs recalled their fish products, underwent sanitary investigations and were required to provide repeated food sampling to prove the end of the L. monocytogenes contamination.
Discussion
This study describes the epidemiology and molecular findings of listeriosis patients in the Tel Aviv District during the 6-year period from 2010 to 2015.
We found an average rate of 1.3 per 100 000 population, which is considerably higher than the reported EU average of 0.6 per 100 000 population [10]. Those high rates concur with previous findings [6, Reference Elinav7] and can be attributed to a number of factors. The Tel Aviv District has the highest proportion of elderly population in Israel, with 15% being older than 65 years old [11] compared with the national median of 11%. However, this can only partly explain the high disease rates as age-specific listeriosis rates were still higher in the Tel Aviv District compared to overall national rates. Furthermore, the Tel Aviv District's socio-economic level is one of the highest in Israel and enables better access to medical care, with perhaps higher diagnosis rates.
The Tel Aviv District is a metropolitan district, which attracts over 1 million tourists yearly. It has a high supply, and a high demand, of restaurants and food markets, as well as a high consumption of ready-to-eat foods, which are often imported. The restaurant turnover is particularly high, with a needed adaptation time to learn basic hygienic measures, which hinders the proper handling of food. We found that disease peaked during two major holidays in which it is customary to consume meat and fish. Further study is needed to establish if this trend is consistent and if so, periodic recommendations to thoroughly cook fish and meat as well as for the handling of raw food to avoid cross-contamination should be published.
In our study, 23% of cases were pregnancy-associated. Although the Tel Aviv District does not have a higher birth rate compared with other districts [12], it has been previously shown that it has the highest proportion of pregnancy-associated cases compared with other districts [Reference Elinav7]. Pregnant women often reported having eaten foods at higher risk for listeria contamination such as deli meats, pre-packed condiments or cured fish [Reference Vongkamjan13, Reference Wu14], which have been repeatedly recalled in Israel due to L. monocytogenes contamination [15], but we were often left with no laboratory evidence for the suspected exposure. Further study is needed to elucidate the sources of infection in this particular category, as few women seem to be changing their eating habits during pregnancy, and more targeted prevention methods should be issued, notably through the attending physician.
In terms of molecular findings, the L. monocytogenes serotype distribution was stable during the studied period, with 4b being the most widely distributed among clinical isolates. The pulsotypes were very diverse and the high rates of disease could not be attributed to a specific strain. We identified a case of possible nosocomial infection, in a nursery, suggesting contamination by a healthcare worker. This is a rare but known form of L. monocytogenes transmission [Reference Hof, Lampidis and Bensch16–Reference Colodner18]. Such cases emphasise once again the importance of hand hygiene and maintaining a therapeutic environment that is as sterile as possible. Another interesting find was the positive food samples taken during the epidemiologic investigations. Unfortunately, even after finding a L. monocytogenes isolate in the food sample with a corresponding serotype to the case (cluster 4, Table 4), the isolate was not genetically identical to the human isolates, accentuating the difficulty of finding the food source.
In our study, dissimilar PFGE profiles were more informative than identical ones, as they were able to exclude suspicious food sources or common exposures. Identical profiles could only be informative if a link could be found in the epidemiologic inquiry. In those cases, whole-genome sequencing of the isolates could potentially elucidate the occurrence of indistinguishable but not epidemiologically linked PFGE clusters. We are implementing this state of the art, high-resolution standardised method for all L. monocytogenes analysis at the reference laboratory.
There were a few limitations to our study.
L. monocytogenes is a very common environmental bacterium and food sampling is carried out a long time after the exposure. As the incubation period can be as long as 2 months, the contaminating food source might not be available for sampling or might be contaminated with another clone of bacterium when sampled, as was found in our study. Furthermore, there is an inevitable recall bias in the epidemiologic inquiries. The diagnosed patients are those who develop severe disease, with underlying medical conditions and a relatively high mean age. This hampers recollection of food and the interview is often nebulous. A case–control study might be able to elucidate some of the differences in the food exposures but would not eliminate this limitation altogether.
Enhanced public health intervention, such as targeted messaging to at-risk populations, upgraded guidelines and reminders for practitioners, should be applied to increase awareness and prevention among susceptible populations, as the bacteria are widespread and disease burden is heavy.
As many countries are reporting increasing listeriosis rates [19], partly explained by longer life spans, changing food-handling methods and global exposure to foods from all over the world, enhanced and sustained routine sampling and molecular analysis of foodstuffs, as well as surfaces in food factories and restaurants, should be implemented, as it is the most helpful tool during epidemiologic investigations to unravel the source exposures.











