Probiotics are selected live micro-organisms that can be ingested as supplements in order to achieve an effect on health(Reference Hill, Guarner and Reid1). Probiotics are commercially available in capsules or tablets and powder sachets. Although some foods contain probiotics, they are not classified as probiotics as they lack studies on their health benefits as stipulated by the definition of probiotics(Reference Zhang, Zhang and Li2). Studies have shown that probiotics maintain the intestinal barrier, accelerate energy metabolism, prevent the adhesion of pathogens from hosting cells, improve neurological diseases related to oxidative stress and improve the production of vitamins, SCFA and neurotransmitter molecules involved in gut communication with various physiological systems(Reference Zhang, Zhang and Li2).
It is stated that the consumption of probiotics is beneficial to the body according to strain, time and supplementation dose(Reference Jäger, Mohr and Carpenter3). Probiotic supplementation produces effects on the gastrointestinal tract, such as improving barrier function, and on the immune system by modulating the innate and adaptive immune response(Reference Bermon, Castell and Calder4), intensifying communication between both. For instance, a study carried out in humans demonstrated that the supplementation of Bifidobacterium animalis (B. animalis) subsp. lactis HN019 with 1 × 1011 colony-forming unit (CFU) twice daily for 6 weeks increases innate cell number and functionality(Reference Arunachalam, Gill and Chandra5). Monocytes and neutrophils showed greater phagocytic capacity after the supplementation of Lactobacillus acidophilus (L. acidophilus) 74–2 (9 × 108 CFU) and B. animalis subsp. lactis DGCC 420 (3 × 106 CFU) for 5 weeks, without changing the release of the large amounts of reactive oxygen species (oxidative burst) (Klein et al. 2008). Moreover, Roessler and collaborators demonstrated the supplementation with Lactobacillus paracasei (L. paracasei) Lpc-37, L. acidophilus 74–2 and B. animalis subsp. lactis DGCC 420 for 8 weeks increases the phagocytic activity; that is, it enhances the biological process by which phagocytes, such as monocytes and neutrophils, engulf and digest solid particles, such as bacteria, viruses, cellular debris or inanimate particles(Reference Roessler, Friedrich and Vogelsang6).
Monocytes are heterogeneous, highly malleable cells present in the bloodstream(Reference Yang, Zhang and Yu7). Depending on the environment in which they are inserted, they modify their phenotype and functionality. The main functions are related to phagocytosis and microbicidal actions(Reference Ginhoux and Jung8). Despite the evidence demonstrating the effectiveness of probiotic supplementation, the mechanisms involved in enhancing innate immune function still need to be well elucidated. It is believed that the benefits of probiotic supplementation occur through improved intestinal transit, re-establishment of the microbiota, preservation of intestinal epithelial integrity and increased immune functionality(Reference Jäger, Mohr and Carpenter3,Reference Vallianou, Stratigou and Christodoulatos9) .
Acute exercise reduces IL-1β and TNF-α production when lipopolysaccharide (LPS) challenges monocytes since the sensitivity of membrane receptors of the toll-like 4 receptor (TLR-4) to LPS is lower after exercise. This result shows a possible effect of exercise in reducing the ability of monocytes to produce pro-inflammatory cytokines since LPS is a molecule that is part of the cell wall of gram-negative bacteria and has a solid ability to stimulate monocytes to produce pro-inflammatory cytokines. Moderate and intense acute exercise can modify phagocytosis, antitumour activity, reactive oxygen and nitrogen species production and chemotaxis(Reference Walsh, Gleeson and Pyne10). On the other hand, the immunomodulatory effect of physical exercise is recognised(Reference Pals, Chang and Ryan11–Reference Zuhl, Lanphere and Kravitz13). Exercise performed at a moderate intensity of 50–75 % of VO2max and lasting up to 45 min is immunostimulatory, while prolonged strenuous exercise exceeding 90 min in length, such as marathon running, can be immunosuppressive(Reference Nieman, Henson and Austin12,Reference Gleeson14) . In this regard, dietary supplementation is used to reduce the stress generated by physical exercise on the body and improve the immune response of athletes(Reference Gleeson15).
In a recent publication by our research group, multi-strain probiotic supplementation, consisting of one billion CFU of each of L. acidophilus LB-G80, L. paracasei LPc-G110, Lactococcus lactis subsp. lactis LLL-G25, B. animalis subsp. lactis BL-G101 and Bifidobacterium bifidum (B. bifidum) BB-G90 for 30 d attenuated the incidence of upper respiratory tract infection symptoms and preserved cytokine production by monocytes(Reference Tavares-Silva, Caris and Santos16). However, little is known about the effects of supplementation with other strains and other critical cellular functions of monocytes, such as phagocytosis and hydrogen peroxide (H2O2) production.
The present study aimed to evaluate the effect of 30 d of probiotic supplementation on phagocytosis and H2O2 production in monocytes after a marathon race. The study hypothesises that probiotic supplementation of 1 × 1010 CFU of L. acidophilus and 1 × 1010 CFU of Bifidobacterium lactis can increase phagocytosis and H2O2 production and reduce the immunosuppressive effects of the marathon on these monocyte functions.
Methods
Sample
An online screening (via email) was performed to verify the inclusion and exclusion criteria of the study. The inclusion criteria were as follows: age between 25–45 years, history of at least one marathon race and at least 2 years of training. The exclusion criteria were as follows: consumption of alcoholic beverages more than twice a week; smokers; diagnosis of cardiovascular, pulmonary or metabolic disease; use of probiotics or consumption of foods enriched with probiotics; and use of drugs that could interfere with the results of the study, such as anti-inflammatories and antibiotics in the last 6 months. Finally, the sample consisted of twenty-seven male marathon runners divided into two distinct groups: placebo (PLA, n 13) and probiotic (PRO, n 14). The anthropometric characterisation of the runners was previously described(Reference Batatinha, Tavares-Silva and Leite17). Based on a study that assessed the alteration in IL-6 production by monocytes following probiotic supplementation (or placebo) in conjunction with a marathon, we conducted a sample calculation for the repeated-measures ANOVA test (2 groups × 5 time points) using the G*Power software. The significance level (alpha) was set at < 0·05 and the statistical power at > 0·80. The effect size calculated for internal factors was 0·47, and for between groups, it was 0·62. The correlation between repeated measurements was found to be 0·25. Consequently, it was recommended that a total of ten volunteers (five in each group) would be adequate to detect pre- and post-supplementation differences. Additionally, twelve volunteers in total (six in each group) were suggested to identify distinctions between the groups. Considering the experimental design’s nature and the potential for volunteer discrepancies, we initiated the study with a larger sample size, concluding with thirteen volunteers in the PLA group and fourteen in the PRO group. The sample size calculation drew inspiration from the study conducted by Tavares-Silva et al (Reference Tavares-Silva, Caris and Santos16).
Experimental design
This is a randomised, controlled and double-blind study (Fig. 1). As shown in Fig. 2, athletes were first attended to the laboratory for registration, which included a body composition analysis (BOD POD® body composition system; Life Measurement Instruments, Concord, CA, USA). Whether eligible for the study, athletes were allocated to their respective groups. Then, at baseline, it was collected a peripheral blood sample and provided supplements (probiotics or placebo). After 30 d of supplementation, athletes returned to the laboratory, and a new blood sample was collected 24 h before the competition (24 h-before). The supplementation was stopped at this time. On the day of the official marathon (42·195 m), a blood sample was collected 1 h after the end of the race (1 h-after). Another blood sample was collected 5 d after the marathon race (5 d-after).
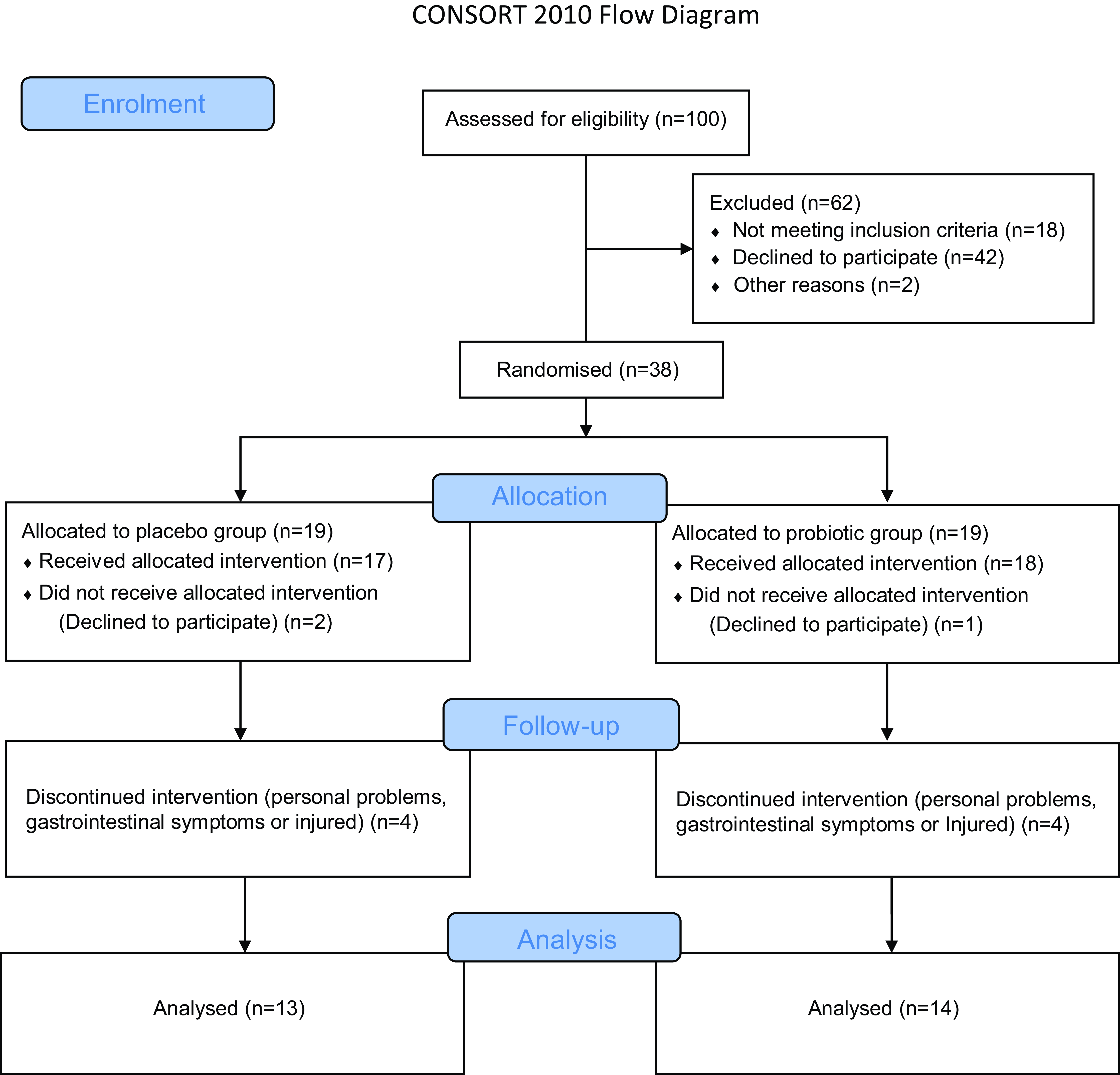
Fig. 1. CONSORT 2010 flow diagram.
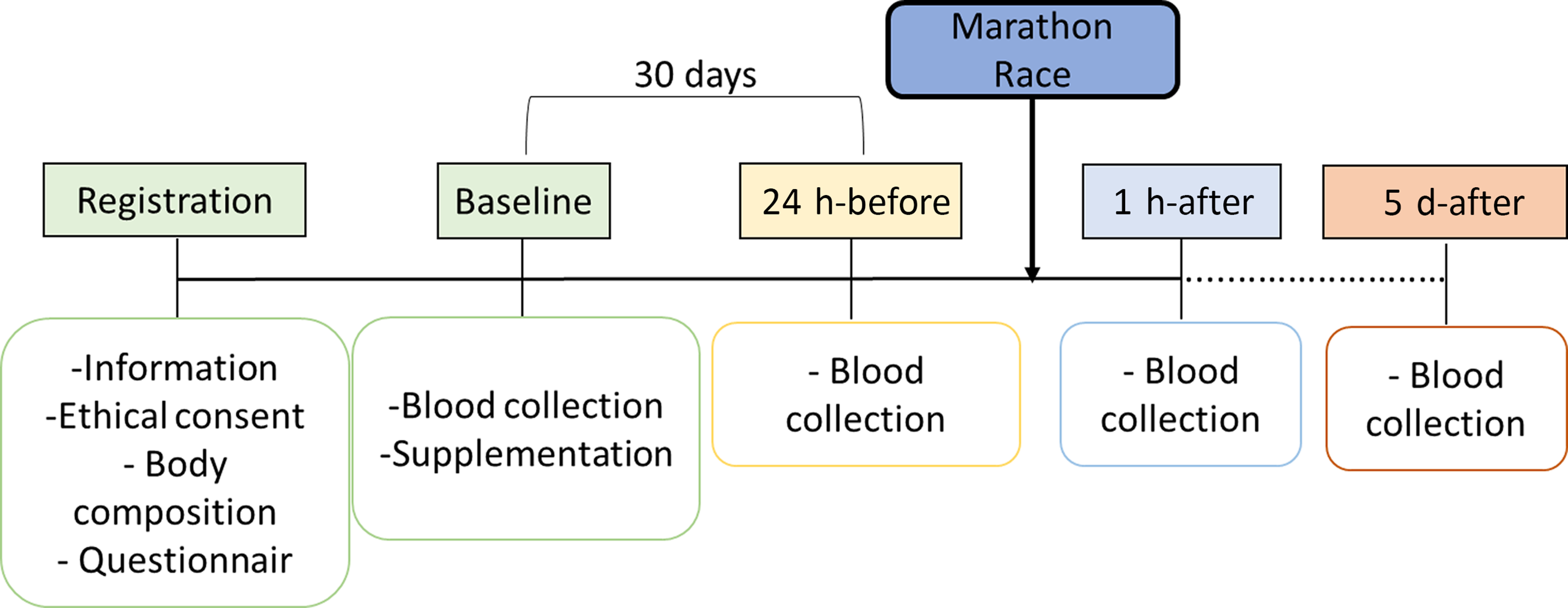
Fig. 2. Experimental design.
Supplementation
The study was double-blind concerning supplementation. A researcher outside the project offered the placebo or probiotic to the participants and monitored their intake. The researchers became aware of the division of groups and supplementation only after the statistical analysis had been carried out. Supplementation was ingested daily in sachets. All participants were informed to consume one sachet per d with water for 30 d at night before sleeping. The PRO group received thirty sachets containing 1 × 1010 CFU L. acidophilus and 1 × 1010 CFU B. bifidum subsp. lactis + 5 g of maltodextrin. The PLA group received thirty sachets containing 5 g of maltodextrin, with a similar colour, smell and taste of probiotic supplementation. The amount of maltodextrin consumed by the PLA group does not affect the variables studied. PRO and PLA supplements were manipulated in a pharmacy (Drogaderma). The probiotics were approved and certified by reports before the supplementation period, confirming the validity and identification of the species. The certification is produced by Lemma Supply Solutions Ltda.
Exercise protocol
Data was collected in three official marathon races – São Paulo International Marathon (42·195 m) in 2017 and 2018. The environment temperature before the race was 18·33°C (sd 3·05), with a relative humidity of 72·66 (sd 15·94), and it started between 06.00 and 07.00. The athletes were instructed to complete the route in the shortest possible time (Time Trial).
Blood collection
To measure the biochemical parameters, 30 ml of blood was collected at each moment following a 4-h fasting period, except on the day of the marathon when the athletes were instructed to eat as they usually do before long runs. After collection, 10 ml of whole blood was used for cellular function assays. The remaining 20 ml of blood was centrifuged at 400 g for 15 min at 4°C. Then, serum were stored at –80°C for further analysis.
Monocyte isolation
Peripheral blood mononuclear cells were isolated from fresh EDTA blood samples by centrifugation (400 g , 25°C, 30 min) with 3 ml of Histopaque 1077 and 3 ml of Histopaque 1119 and washed two times with PBS (40 g at 4°C for 10 min) and counted. 500 ul of diluted cells were plated per well with 2 ml of RPMI-1640 culture medium enriched with 2 mM glutamine, 1 ml of the volunteer’s serum and 650 µl of penicillin in a six-well culture plate. After 1 h of incubation at 37°C, the supernatant containing lymphocytes was collected, leaving the attached monocytes in the plate. 1·8 ml of enriched RPMI-1640 medium and 0·2 ml of LPS (5 μg/ml) were added to each well, and the plate was incubated for 24 h at 37°C and 5·0 % CO2. One billion cells were plated per well. All supernatants were aliquoted into 2 ml tubes and frozen in a − 80°C freezer until analysis. Brand reagents Sigma-Aldrich (Merck Group), San Luis, MI, USA.
Cytokine dosage
The concentration of the cytokines IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-15 and TNF-α in cell supernatant and serum was evaluated by the multiplex assay with Millipore kits (Darmstadt, Germany). Simultaneous analysis of multiple cytokines is performed using Luminex technology with magnetic beads in ninety-six-well plates. The test steps contained in the manufacturer’s manual were respected. The Luminex 200™ analyzer was used with the Magpix® system using Milliplex® Analyst 5.1 software.
Hydrogen peroxide (H2O2) production
H2O2 production was measured using the modified method described by Pick and Mizel(Reference Pick and Mizel18). Incubations were carried out in previously siliconized glass flasks (1 % silicone in acetone, v/v) and subsequently washed with distilled water. Monocytes were incubated at 37°C with shaking for a final volume equal to 1 × 106 cells in 1 ml of incubation medium. The incubation medium was composed (for final volume) of PBS with Ca, 2 % bovine serum albumin (v/v), 5 Mm glucose, 2 mM glutamine, 0·56 Mm phenol red, 10 µl phorbol myristate acetate (100 µg/ml PBS) and 100 µl peroxidase (1 mg/2 ml PBS). The formation of H2O2 was interrupted after 1 h of incubation with the addition of 20 µl of 1N sodium hydroxide. After 15 min, the total amount produced was quantified in a spectrophotometer at 620 nm.
Phagocytosis
To assess phagocytic capacity, the Vybrant® Phagocytosis Assay Kit from Molecular Probes (Thermo Fisher Scientific) was used. The Vybrant® Phagocytosis Assay Kit allows researchers to observe and quantify the process of phagocytosis following the internalisation of an antigen – dead Escherichia coli (E. coli) (strain K-12) cell that has been labelled with fluorescent dye. In addition to the lyophilised E. coli BioParticles® component, the kit contains a trypan blue solution (to quench the fluorescence of particles that have not been internalised). The instructions contained in the kit were followed, as well as the step-by-step instructions for carrying out the phagocytosis assay on a fluorescence microplate reader.
Immunophenotyping
100 µl of whole blood was separated in a test tube, and 2 ml of erythrocytes lysis solution, obtained from the company Qiagen®, was added. The tube was kept at 37°C for 10 min and centrifuged at 400 g for 10 min. The cell washing process was carried out twice with PBS (with centrifugation between washes). After the last centrifugation, whole blood was stained with the following monoclonal antibodies: 1 µl of cluster of differentiation (CD) 14 (BL-2) and 1·8 µl of CD16 (BL-3). The tubes were incubated in the absence of light for 30 min, washed with PBS and centrifuged to 400 g for 20 min, and the final pellet was resuspended with PBS 2 % bovine serum albumin. Samples were acquired in an Attune NxT Flow Cytometer (Thermo Fisher Scientific®). Data were analysed using FlowJo software version V10 (Treestar Inc.).
Statistical analysis
Normality was verified using the Shapiro–Wilk’s test, and normally distributed data are expressed as mean and standard deviation. Homogeneity was analysed with the Levene test, and the sphericity of the data was verified using the Mauchly test and Greenhouse–Geisser correction. Analysis of repeated measures by the general linear model was used according to the design, followed by Tukey’s post hoc. The significance level adopted was less than 5 %. Statistical analyses were performed using IBM SPSS Statistics 20 software.
Results
Both PLA and PRO showed a significant increase in total leukocytes and circulating monocytes 1 h-after the race when compared with their baseline (P < 0·01) and 24 h-before (P < 0·01) and a significant decrease when compared with 5 d-after (P < 0·01). No difference was found between the groups (Fig. 3). Immunophenotyping results demonstrated homogeneity between groups in the total number of classic (CD14++CD16-), intermediate (CD14++CD16+) and non-classical (CD14+CD16++) monocytes (Fig. 4).
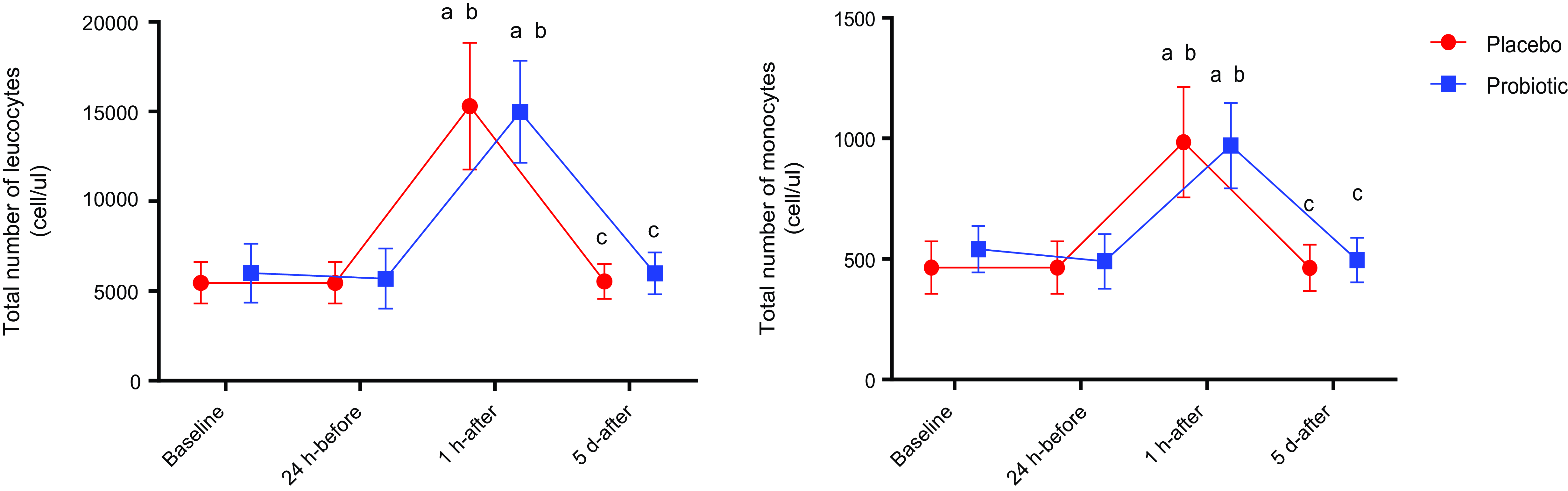
Fig. 3. Total number of leucocytes and monocytes. Data presented in mean ± sd. Placebo (n 13) and probiotic (n 14). The comparison between groups and moments was performed through the general linear model with post hoc Tukey. ‘a’ differs from the baseline condition of the same group. ‘b’ differs from the 24 h-before of the same group. ‘c’ different from 1 h-after of the same group.
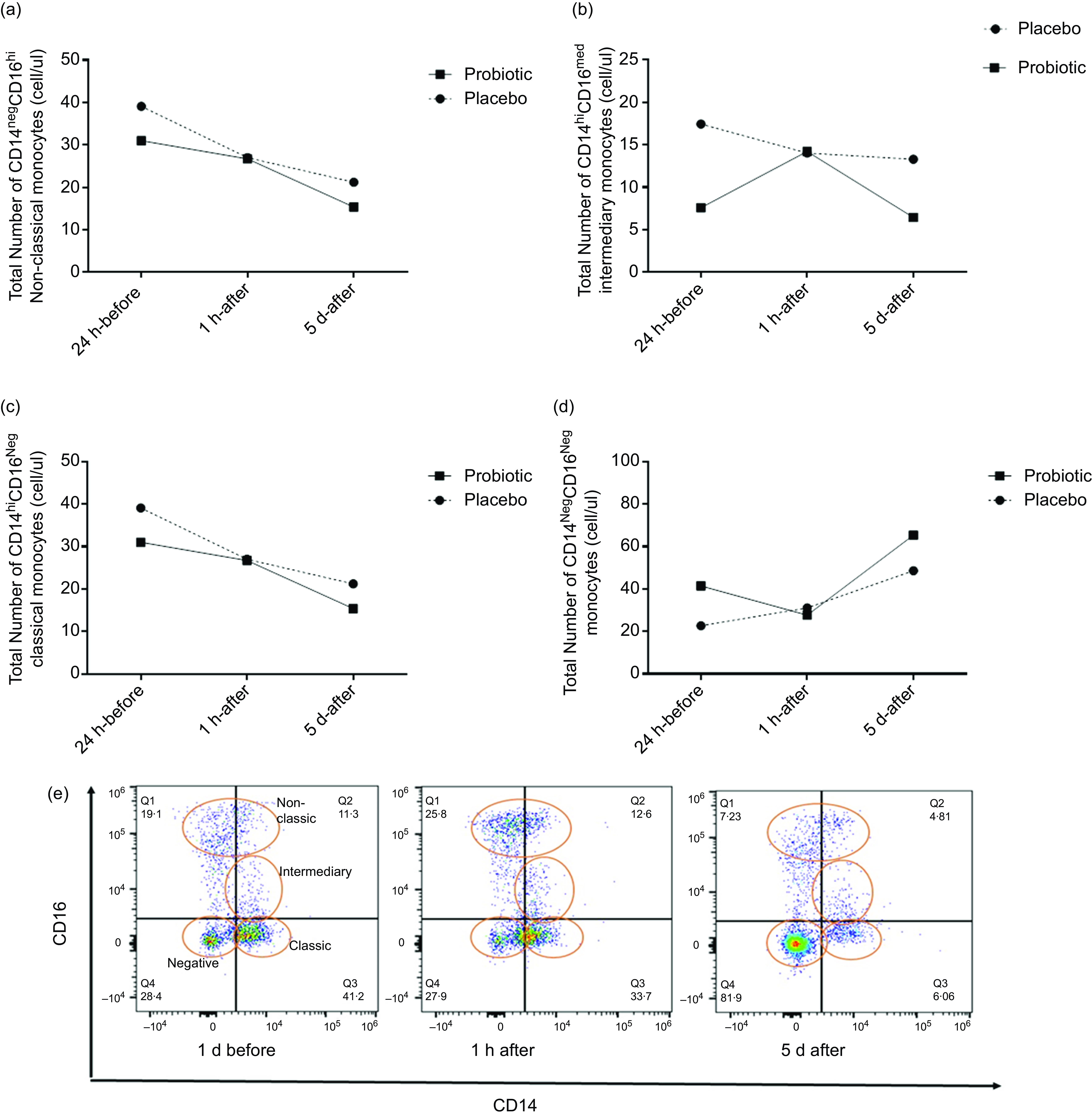
Fig. 4. Immunophenotyping. Data presented as mean ± sd. Significance P < 0·05. The absolute amount of non-classical monocytes CD14pos CD16pos (graph (a)), intermediate CD14pos CD14med (graph (b)), classic CD14pos CD16neg (graph (c)) and double negative CD14pos CD16neg (graph (d)) of both groups; in the pre-test, post-test and recovery moments. Graph (e) refers to the representation of the monocyte population from flow cytometry. Positive y-axis CD16 and positive x-axis CD14. A total of eight participants (placebo 4 and probiotic 4).
Regarding cell function, the PRO group showed an increase in phagocytosis 24 h-before compared with baseline (P < 0·01), followed by a decrease 1 h-after compared with 24 h-before (P = 0·03). At the 5 d-after, there was a significant increase compared with the 1 h-after (P < 0·01). In the PLA group, only an increase was observed in 5 d-after compared with 1 h-after (P = 0·03) (Fig. 5(a)). The H2O2 concentration did not differ significantly at any evaluated time point (Fig. 5(b)).
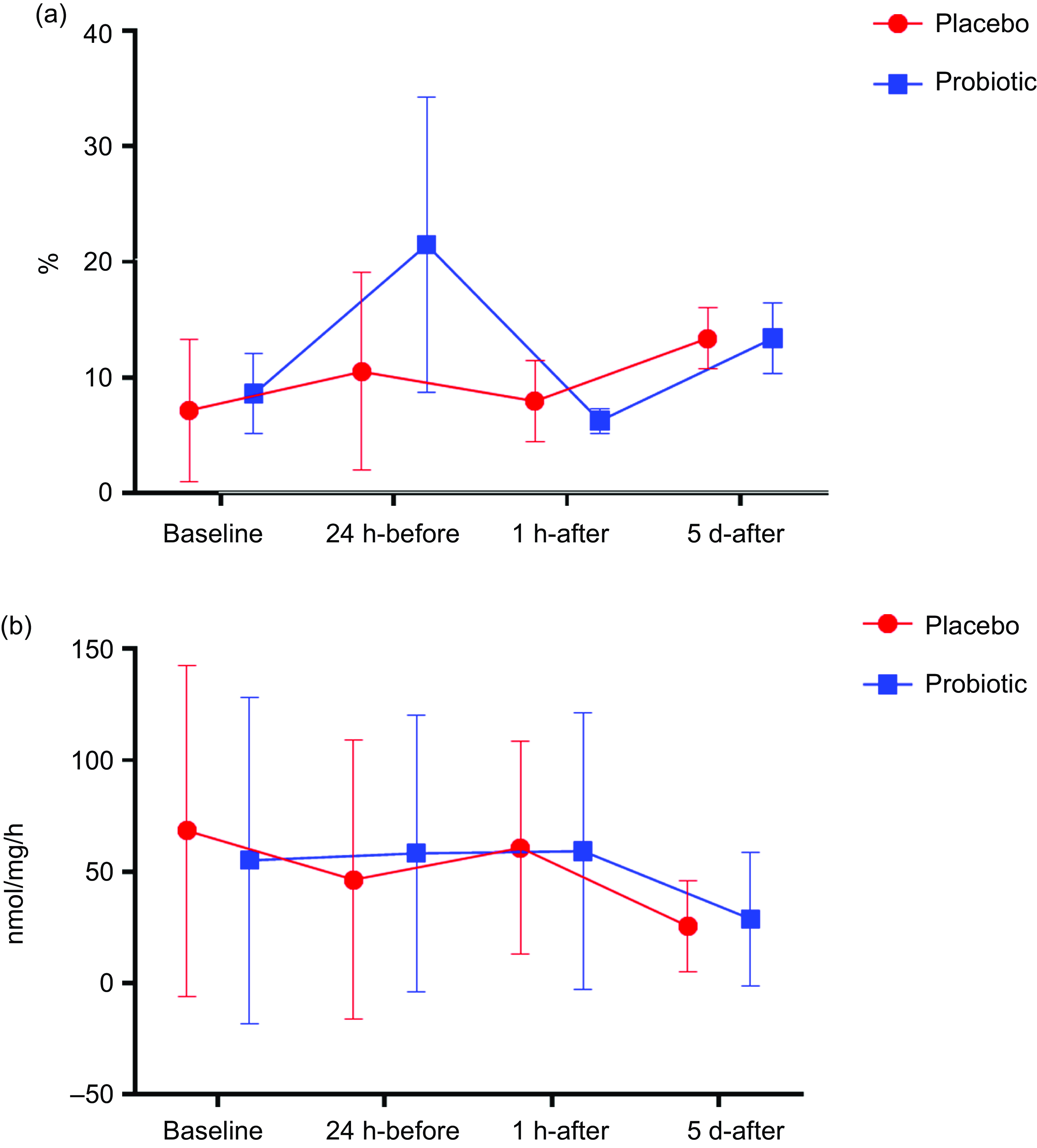
Fig. 5. Phagocytosis expressed in % (Fig. 5(a)) and H2O2 production expressed in nmol/mg per h (Fig. 5(b)). Data presented in mean ± sd. Placebo (n 13) and probiotic (n 14). The comparison between groups and moments was performed through the general linear model with post hoc Tukey. ‘a’ differs from the baseline condition of the same group. ‘b’ differs from the 24 h-before of the same group. ‘c’ different from 1 h-after of the same group.
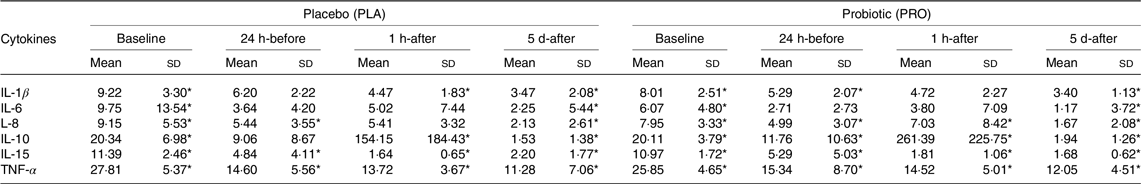
In both the PRO and PLA groups, IL-8 production by monocytes decreased 5 d-after compared with their respective 24 h-before (P < 0·001) and 1 h-after (P < 0·001). In the PRO group, IL-15 production increased 5 d-after compared with baseline (P < 0·001), 24 h-before (P < 0·01) and 1 h-after (P < 0·01), while the PLA group showed a significant increase in 5 d-after compared with baseline (P < 0·001). Besides, only the PLA group showed an increase in the production of TNF-α 5 d-after compared with the baseline (P = 0·02) (Table 1).
Table 1. Cytokine production by monocytes (mean values and sd)

PLA (n 13) and PRO (n 14) group. Values are presented as mean and standard deviation. The production of IL-1β, IL-6, IL-8, IL-10 and TNF-α was divided by 100.
* Difference between time points.
The serum concentration of IL-1β in the PRO group differed in 24-before (P < 0·01), 1 h-after (P < 0·01) and 5 d-after (P < 0·001) compared with baseline, whereas the PLA group showed a decrease at 1 h-after (P < 0·001) and 5 d-after (P < 0·001) compared with baseline and 5 d-after compared with 1 h-after (P = 0·03). IL-6 levels were lower 5 d-after compared with baseline in both PRO (P = 0·04) and PLA groups (P = 0·03). A significant reduction in IL-8 concentrations was observed 5 d-after in the PRO group compared with baseline (P < 0·001), 24 h-before (P < 0·01) and 1 h-after (P < 0·04). PLA group showed a decrease in 5 d-after compared with baseline (P < 0·001) and 24 h-before (P < 0·01). The anti-inflammatory cytokine, IL-10, from samples of the PRO group, showed an increase 1 h-after compared with baseline (P < 0·01) and 24 h-before (P < 0·01) and reduced 5 d-after compared with baseline (P < 0·001), 24 h-before (P < 0·02) and 1 h-after (P < 0·01). In the PLA group, this cytokine increased 1 h-after (P < 0·02) and reduced 5 d-after compared with 1 h-after (P < 0·02) and baseline (P < 0·001). Finally, both IL-15 and TNF-α decreased at 24 h-before (P < 0·01), 1 h-after (P < 0·001) and 5 d-after (P < 0·001) times compared with baseline on both groups (Table 2).
Table 2. Serum cytokines (mean values and sd)
PLA (n 13) and PRO (n 14) group. Values are presented as mean and standard deviation.
* Difference between moments.
Discussion
The main finding of the study was that 30 d of probiotic supplementation increased phagocytosis in monocytes compared with the PLA group. The increase in phagocytic capacity represents an improvement in the essential function of monocytes. It may shed light on the possibility of endurance athletes using probiotics to mitigate the immunosuppressive effects of strenuous exercise. Probiotics have been widely studied as dietary supplements capable of improving immune functions in endurance athletes, mainly runners(Reference Flannagan, Jaumouillé and Grinstein19,Reference Rosales and Uribe-Querol20) .
The phagocytosis results agree with previous research carried out in humans without exercise. Schiffrin et al.(Reference Schiffrin, Rochat and Link-Amster21) demonstrated that consumption of B. bifidum Bb 12 1 × 1010 or L. acidophilus LA1 (7 × 1010 CFU) for 3 weeks in fermented milk three times a day (120 ml dose) increases monocyte phagocytosis. This same result was found after daily supplementation with 300 g of yogurt enriched with L. acidophilus 74–2 (9·3 × 108 CFU) and B. animalis subsp. lactis DGCC 420 (3 × 106 CFU) for 5 weeks(Reference Klein, Friedrich and Vogelsang22) and Lactobacillus paracasei supplementation Lpc-37 (3·9 × 108 CFU), L. acidophilus 74–2 (2·9 × 104) and Bifidobacterium animalis subsp. lactis DGCC 420 (5·9 × 104) for 8 weeks(Reference Roessler, Friedrich and Vogelsang6).
The increase in the phagocytic rate in the supplemented group was not accompanied by an increase in H2O2 and cytokines production. H2O2 comes from cellular metabolism by oxidising organic substrates(Reference Nakagawara, Nathan and Cohn23). During infectious conditions and in the presence of inflammatory cytokines, there is an increase in the combination of H+ ions with O2 in immune cells, exhibiting antimicrobial and antitumour functions(Reference Nathan, Nogueira and Juangbhanich24–Reference Weiss, LoBuglio and Kessler26). Our results corroborate the study by Klein et al.(Reference Klein, Friedrich and Vogelsang22), in which they did not observe an increase in the cellular oxidative burst of monocytes after daily supplementation with L. acidophilus 74–2, 9·3 × 108 CFU and B. animalis subsp. lactis DGCC 420, 3 × 106 CFU for 5 weeks. However, Roessler et al.(Reference Roessler, Friedrich and Vogelsang6) observed an increase in the oxidative burst after 8 weeks with probiotic supplementation of L. paracasei Lpc-37 3·9 × 108 CFU, L. acidophilus 74–2 2·9 × 104 and Bifidobacterium animalis subsp. lactis DGCC 420 5·9 × 104 CFU. Furthermore, we did not observe significant changes in cytokine concentrations that could stimulate an increase in H2O2 production. Thus, the time of supplementation and dose of probiotics should be considered(Reference Jäger, Mohr and Carpenter3), suggesting in future studies the inclusion of L. paracasei, as in the Roessler study, in the probiotic supply to assess the oxidative response of monocytes.
In order to verify the cellular functionality, during the monocyte incubation, we used the volunteers’ serum to reproduce the cellular medium. This protocol has the characteristic of mimicking in vitro what may be happening in vivo, demonstrating the mechanisms that may be involved in the benefits of probiotics supplementation by maintaining the concentrations of energy substrates, hormones, metabolites and cytokines under the same conditions as they were in vivo at the time of collection(Reference Gstraunthaler, Lindl and van der Valk27). For this reason, our results should be compared with endurance exercise studies. Races of shorter duration and different intensities, such as 5 or 10 km, represent different physiological stress than marathon running and, therefore, different immunometabolic responses to supplementation and exercise. Interestingly, our results showed that the significant differences in the concentrations of IL-8 and IL-15 produced by monocytes were accompanied by changes in the concentrations of plasmatic cytokines after the marathon race.
Cytokine production by monocytes after LPS stimulation is closely related to pattern recognition receptors, mainly TLR-4 and activation of the NF-κB pathway(Reference Rossol, Heine and Meusch28). It is acknowledged that physical exercise promotes a decrease in the sensitivity of cell membrane receptors of the TLR-4 to LPS, both in short-term and high-intensity exercise(Reference Slusher, Zúñiga and Acevedo29) and in prolonged exercise(Reference Oliveira and Gleeson30). In the study by Oliveira and Gleeson(Reference Oliveira and Gleeson30), the expression of TLR-4 by monocytes was evaluated before and after an acute amount of prolonged physical exercise. Despite an increase in the number of circulating monocytes, there was a decrease in the expression of TLR-4 receptors. As in acute exercises, training also promotes a decrease in the expression of pattern recognition receptors and the production of inflammatory cytokines(Reference Gleeson, McFarlin and Flynn31).
In both groups, lower plasma concentrations of immunostimulatory cytokines were observed throughout the experiment (IL-1β, IL-15 and TNF-α) and a specific increase in IL-10 1 hr after the marathon compared with baseline. Plasma alterations were accompanied by a reduction in the production of IL-8, an increase in IL-15 5 d after the marathon race and a reduction in the phagocytic rate in the PRO group after the race. The imbalance in the concentrations of pro- and anti-inflammatory cytokines can affect cellular functionality(Reference Chung, Kim and Ma32–Reference Kapellos, Bonaguro and Gemünd34).
The mechanism of action of monocyte activation involves both LPS and cytokine receptors(Reference Rossol, Heine and Meusch28,Reference Justiz Vaillant, Sabir and Jan35) . In the presence of inflammatory cytokines or LPS, monocytes are activated, triggering a cascade response via Janus kinase-signal transducer and activator of transcription (JAK-STAT) or NF-κB to carry out gene transcription and initiate the immune response(Reference Barone, Catani and Ricci36). Each cytokine activates a specific JAK-STAT associated with the respective receptor, such as IL-2 activating JAK3-STAT5, IL-4 activating JAK3-STAT6 and IL-10 activating JAK1-STAT3(Reference Yoshimura, Nishinakamura and Matsumura37). Even in the presence of LPS, if there is an increase in the concentration of IL-10, there is activation of JAK1-STAT3, inhibiting the cellular response via NF-κB(Reference Yoshimura, Nishinakamura and Matsumura37).
It turns out that after binding of IL-10 to the receptor and activation of JAK1 and phosphorylation of STAT3, the NF-κB pathway is blocked by suppression of the IkappaB kinase complex, thus impairing the immune response(Reference Mosser and Zhang38,Reference Hutchins, Diez and Miranda-Saavedra39) . Furthermore, 5 d after the marathon race, we observed a significant decrease in plasma concentrations of IL-10 and a consequent increase in the production of inflammatory cytokines and the phagocytic capacity of monocytes. However, the mechanisms involved in the monocyte response after probiotic supplementation are still unclear.
The literature describes some non-exclusive hypotheses, which include the interaction of bacteria into Peyer’s patches and galactose-1-phosphate uridyltransferase, stimulating cell proliferation and maturation(Reference Klein, Friedrich and Vogelsang22). Probiotics are also associated with increased sensitivity of pattern recognition receptors and antigen presentation by dendritic cells, resulting in the maturation and proliferation of lymphocytes through the production of cytokines such as IFN-γ, IL-2 and IL-12 that induce a cellular immune response(Reference Morais, Schreiber and Mazmanian40), in addition to increased production of serotonin by enterochromaffin cells(Reference Kaur, Bose and Mande41,Reference Yano, Yu and Donaldson42) . Moreover, it is known that monocytes exhibit a high ability to stimulate cytokine production and increase phagocytic and oxidative capacity on exposure to serotonin(Reference Katoh, Soga and Nara43–Reference Herr, Bode and Duerschmied46). Therefore, probiotics act through different mechanisms, modulating the intestinal microbiota with the production of vitamins, increasing the bioavailability of nutrients and assisting in the homeostatic development of the resident microbial environment by stimulating immune functions(Reference Swanson, Gibson and Hutkins47).
Strenuous exercises such as marathons are potential stressors, causing changes in the concentrations of hormones such as cortisol and catecholamines, glucose, amino acids and fatty acids that are important for the metabolism of monocytes and other cells of the immune system. On the other hand, several nutritional actions may have immunonutrition actions in exercise(Reference Jäger, Mohr and Carpenter3,Reference Bermon, Castell and Calder4) . The relationship between microbiota, probiotics and the immune system during exercise still needs to be explored. Our results show that 30 d of probiotic supplementation had effects on phagocytosis. As for the production of cytokines by monocytes and cytokines in the bloodstream, the effects of exercise overlap with those of probiotics. Interestingly, the influence of exercise and supplementation was not able to change H2O2 production by monocytes. The regulation of immune system cells and inflammatory mediators occurs through several mechanisms, which are only sometimes detected in studies.
This study was carried out with young adults aged between 25 and 45 years old, as in this age group, there are few physiological differences due to age and because most of the population runs marathons and does strenuous exercise. Therefore, the results cannot be extrapolated to other age groups. In addition, this study was carried out only with men to avoid the influence of female and male sex hormones, as it was not possible to adequately control the phases of the menstrual cycle and the concentration of oestrogen and progesterone at the time of collection. The results should be extrapolated to women with great caution.
In conclusion, the probiotic supplementation of L. acidophilus 1 × 1010 CFU and B. animalis subsp. lactis 1 × 1010 CFU for 30 d prior to a marathon race was effective in increasing monocyte phagocytosis in marathon runners. However, changes in the concentration of plasma cytokines, including an increase in IL-10, impacted the cellular response. These outcomes reinforce the importance of investigating probiotics supplementation and probiotic food in the immunologic field.
Further studies with endurance athletes, including other immune cells and mechanisms not evaluated here, like plasma serotonin concentration, the sensitivity of Receptor Reserve Pool (RRP), maturation rate and cell proliferation, as well as adjusting for time, dose and composition of probiotics, are needed. Studies in this context with female runners should also be carried out in the future.
Acknowledgements
The authors thank the São Paulo Research Foundation and Federal University of São Paulo (UNIFESP) for the financial and technical support to carry out the study. The authors would also like to thank the marathon runners who voluntarily participated in the study.
This study was funded by the São Paulo Research Foundation case no. 2016/25821–5.
E. S.: hypothesis elaboration, experimental design, data collection, analysis, and discussion of results, writing of the manuscript. G. L.: Data collection, analysis and discussion of results and writing of the manuscript. H. B.: data collection, analysis and discussion of results and writing of the manuscript. A. R.: data collection, analysis and discussion of results and writing of the manuscript. V. de A. L. and C. M.: data collection and manuscript writing. A. H. L. Jr: hypothesis elaboration, experimental design and writing of the manuscript. J. R. N.: preparation of the hypothesis, experimental design and writing of the manuscript. R. T-S.: hypothesis elaboration, experimental design, data collection, analysis and discussion of results and writing of the manuscript.
The authors declare that there is no conflict of interest.
This study was approved by the Research Ethics Committee of UNIFESP/Hospital São Paulo (REC no. 000.007-2017) and complied with the norms established by Brazilian legislation in resolution no. 466/12 of the National Health Council and is following the guidelines established in the Declaration of Helsinki adopted in 1964. All participants agreed with the study by signing the free and informed consent form.










