Introduction
Ototoxicity is a severe side effect of aminoglycoside antibiotics and may result in sensorineural hearing loss. This hearing loss may be permanent and bilateral, and there is no specific therapy for it.Reference Shi, An, Wang, Gao and Yang 1 , Reference Polony, Humli, Ando, Aller, Horvalth and Harnos 2 Histological studies have revealed that aminoglycoside-induced ototoxicity begins in the outer hair cells at the basal portion of the cochlea and subsequently affects the inner hair cells. The degeneration of nerve fibres, spiral ganglion neurons and supporting cells is secondary to hair cell damage.Reference Berkiten, Slturk, Topaloglu and Ugras 3 , Reference Guthrie 4 Many reports have discussed possible prevention methods for ototoxicity, such as anti-inflammatory drugs, antioxidants, apoptosis inhibitors, neuroprotective compounds, neurotrophic factors and gene therapeutic approaches.Reference Polony, Humli, Ando, Aller, Horvalth and Harnos 2 , Reference Blakley, Alsaleh, Dewji, Qureshy, Berard and Xie 5 , Reference Kocyigit, Vural, Unal, Sipahioglu, Yucel and Aydemir 6 In recent decades, stem cells have also been used to replace deficient cell types in order to restore hearing.Reference Kelly and Lalwani 7
Serum is a complex mixture of factors and molecules involved in cell growth. Growth factors are biologically active polypeptides produced by the body that can stimulate cell division, growth and differentiation.Reference Fortier, Barker, Strauss, McCarrel and Cole 8 In addition, healing factors such as nerve growth factor, epidermal growth factor, transforming growth factor β, platelet-derived growth factors and insulin-like growth factor-1 are present in both diluted and undiluted serum.Reference Wehling, Moser, Frisbie, McIlwraith, Kawcak and Krauspe 9 Nerve growth factor is the best-known neurotrophic factor; it is responsible for the growth and survival of developing neurons, and the maintenance of mature neurons.Reference Lang, Gallinat, Danker-Hopfe, Bajbouj and Hellweg 10 , Reference Matsumoto, Dogru, Goto, Ohashi, Kojima and Ishida 11 Recent studies have indicated the efficacy of autologous serum in the treatment of neurotrophic keratopathy, osteoarthritis, chronic graft-versus-host disease and tympanic membrane perforations.Reference Matsumoto, Dogru, Goto, Ohashi, Kojima and Ishida 11 – Reference Kakehata, Hirose, Kitani, Futai, Maruya and Ishii 13 In addition to treatment, autologous serum should be able to prevent or at least decrease cell damage via its anti-apoptotic factors.Reference Bomstein, Yuklea, Radnay, Shapiro, Afanasyev and Yarkoni 14 It is these potentially beneficial substances that may provide medical benefits to diseased or damaged inner-ear cells and nerves, in a therapeutic and protective manner.Reference Matsumoto, Dogru, Goto, Ohashi, Kojima and Ishida 11 , Reference Kaevalin, Jongkhajornpong, Choubtum and Chuckpaiwong 15
Apoptosis can be induced in cells by application of aminoglycosides. The terminal deoxynucleotidyl transferase-mediated dUTP (
![]() $\acute{2}$
-deoxyuridine,
$\acute{2}$
-deoxyuridine,
![]() $\acute{5}$
-triphosphate) nick-end labelling (‘TUNEL’) technique has been extensively used in the detection and quantification of apoptosis in histological tissue sections.Reference Duan, Garner, Williams, Funckes-Shippy, Spath and Blomme
16
In addition, transient evoked otoacoustic emissions (TEOAEs) provide an important and useful electrophysiological technique for monitoring ototoxicity by reflecting changes in the functions of outer hair cells damaged by aminoglycosides, particularly in animal experiments.Reference Kocyigit, Vural, Unal, Sipahioglu, Yucel and Aydemir
6
,
Reference Derinsu and Akdas
17
$\acute{5}$
-triphosphate) nick-end labelling (‘TUNEL’) technique has been extensively used in the detection and quantification of apoptosis in histological tissue sections.Reference Duan, Garner, Williams, Funckes-Shippy, Spath and Blomme
16
In addition, transient evoked otoacoustic emissions (TEOAEs) provide an important and useful electrophysiological technique for monitoring ototoxicity by reflecting changes in the functions of outer hair cells damaged by aminoglycosides, particularly in animal experiments.Reference Kocyigit, Vural, Unal, Sipahioglu, Yucel and Aydemir
6
,
Reference Derinsu and Akdas
17
In this study, we hypothesised that autologous serum would have beneficial therapeutic and/or protective effects on ototoxicity generated by amikacin in guinea pigs. Its efficacy was evaluated by TEOAE and immunohistopathological examination.
Materials and methods
This study was approved by the Ethical Committee for Animal Experimentation at Celal Bayar University, Manisa, Turkey (reference number: 77.637.435-14). All surgical procedures were completed in accordance with the National Institutes of Health guidelines on the care and use of laboratory animals for research purposes.
In total, 24 female albino guinea pigs, with body weights ranging from 700 to 900 g, were used in the study. These were equally separated into a therapeutic group (group A) and a protective group (group B), with positive and negative controls created within each group. Only the right ears of the guinea pigs were used.
Transient evoked otoacoustic emissions (TEOAEs) were recorded using an Echoport ILO292 USB-I device with ILO version 5 software (Otodynamics, Hatfield, Herts, UK). The TEOAEs were evoked using clicks of 63 ± 2 dB at a rate of 50 per second, with a click duration of 80 μs. Response frequency bands were in half octaves, centred at 1, 1.4, 2, 2.8 and 4 kHz. Signal-to-noise ratio is the amplitude of otoacoustic emissions above the noise floor and is measured in decibels of sound pressure levels; signal-to-noise ratio was recorded at these centre frequencies. Reproducibility and probe stability percentage were also recorded. Reproducibility was measured as the percentage correlation between two averages during data collection. Stability indicates the stability of the stimulus peak level during the otoacoustic emission recording. It shows the stability of the probe fit during measurement. The stability of the response is high if all the stimuli remain almost the same throughout the test.
Autologous serum was collected from the lateral saphenous vein, under anaesthesia with intraperitoneal injections of xylazine hydrochloride (10 mg/ml) (Bioveta, Ivanovice na Hané, Czech Republic) and ketamine (50 mg/ml) (Pfizer, Istanbul, Turkey). Blood samples were centrifuged for 5 minutes at 3000 revolutions per minute.Reference Kakehata, Hirose, Kitani, Futai, Maruya and Ishii 13 The resulting serum was placed in a dental syringe and stored at 4°C. In order to create ototoxicity, amikacin (Amikozit; Eczacibasi-Zentiva, Istanbul, Turkey) was administered at a dose of 0.2 cc (80 mg/ml) via the intratympanic and intramuscular routes, respectively.
Therapeutic group
Prior to the procedure, the TEOAE responses of the guinea pigs in group A were recorded. In the first week, eight guinea pigs (the study group) received 0.2 cc of intratympanic amikacin three times to generate hearing loss. The positive control group of two guinea pigs received 0.2 cc of amikacin; the negative control group, also composed of two guinea pigs, received 0.2 cc of saline. The TEOAE responses were evaluated in both the study and positive control groups after amikacin application. In the second week, 0.2 cc of autologous serum was administered three times to the study group, beginning after the 3 days of amikacin application. In the third week of the study, the TEOAE responses of the guinea pigs were obtained and the animals were sacrificed. The guinea pig cochleae were removed for immunohistochemical examination.
Protective group
The TEOAE responses of the guinea pigs in group B were obtained initially. Ten guinea pigs (eight from the study group and two from the positive control group) received 0.2 cc of amikacin intramuscularly every day of the week. Two guinea pigs (the negative control group) received 0.2 cc of 0.9 per cent saline. Autologous serum (0.2 cc) was applied intratympanically three times in a week, at the same time as the intramuscular amikacin administration. One week after the last autologous serum application, the TEOAE responses of the guinea pigs were recorded and the animals were sacrificed for immunohistochemical examination.
Histopathology
Cochlear samples were fixed in 10 per cent formalin for 24–48 hours and then embedded in paraffin blocks after routine processing. A rotary microtome was used to cut the samples into 5 µm sections. Haematoxylin and eosin staining was used to examine the tissue morphology using standard protocols. Apoptotic cells in the tissue were determined using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling method.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling assay
The paraffin sections were rehydrated after deparaffinisation in xylene. After washing with phosphate-buffered saline, they were incubated with 20 µg/ml of proteinase K for 10 minutes. The sections were then incubated with 3 per cent hydrogen peroxide for 15 minutes. After adding equilibration buffer for 10–15 minutes, terminal deoxynucleotidyl transferase enzyme was added and the sections were incubated in a humidified atmosphere at 37 °C for 60 minutes. The slides were washed with stop/wash buffer at room temperature for 10 minutes. Streptavidin-peroxidase solution was then added and the slides were incubated for 45 minutes at room temperature. After each step, the slides were washed with phosphate-buffered saline.
Staining was completed with diaminobenzidine, and counterstaining was performed with Mayer's haematoxylin (HMM999; Scytek Laboratories, Logan, Utah, USA). The in situ apoptosis detection kit (S7100; Invitrogen, New York, USA) was used for this assay. After mounting with Entellan® medium, the slides were examined independently by two histologists; 100 cells which were brown-stained were accepted as terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling positive in each organ of Corti, spiral limbus and ganglion on randomly chosen fields per case. The percentage of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling positive cells which were stained among 100 cells was determined.Reference Vatansever, Sorkun, Guran, Ozdal-Kurt, Turkoz and Gencay 18
Immunohistochemistry
The paraffin-embedded sections were washed with phosphate-buffered saline for 15 minutes. Endogenous peroxidase activity was quenched by incubation with 3 per cent hydrogen peroxide (K31355100; Merck, Darmstadt, Germany) for 5 minutes at room temperature. Cells were then washed with phosphate-buffered saline, and incubated in blocking serum (ready to use according to the manufacturer's instruction) (85-9043; Invitrogen, Camarillo, California, USA) for 1 hour.
The primary antibodies anti-Bax (DB005; Delta Biolabs, Gilroy, California, USA), anti-caspase 3 (ab2302; Abcam, Cambridge, UK), anti-cytochrome c (sc-13156; Santa Cruz Biotechnology, Dallas, Texas, USA) and anti-Fas ligand (FasL-DB042; Delta Biolabs) were applied at 4 °C overnight in a dilution of 1:100. After removal of the primary antibodies, the slides were washed with phosphate-buffered saline. The secondary antibodies biotinylated goat immunoglobulin G (IgG) anti-rabbit/mouse IgG and peroxidase-conjugated streptavidin (D01-110; Life Science Research, Merck, Darmstadt, Germany) were applied, and the cells were incubated for 30 minutes for each step.
Between the secondary antibody incubation steps and after peroxidase-conjugated streptavidin, the cells were washed with phosphate-buffered saline three times. The samples were then incubated with diaminobenzidine/hydrogen peroxide (00-2020; Invitrogen, Camarillo, California, USA) for 5 minutes. Cells were counterstained with Mayer's haematoxylin (HMM999; Scytek Laboratories).
After washing with distilled water, the cells were mounted with mounting medium (AML060; Scytek Laboratories) and covered with coverslips before evaluation under a light microscope (Olympus BX40; Olympus, Tokyo, Japan).
The negative control samples were processed in an identical manner, but instead of primary antibodies the same type of IgGs were used.Reference Vatansever, Sorkun, Guran, Ozdal-Kurt, Turkoz and Gencay 18
Evaluation of sections
Immunolabelling intensity was graded (independently, by two observers who were blinded to the experimental conditions) as follows: weak, moderate and strong.
Statistical analysis
Statistical analysis was performed with SPSS software, version 20.0 for Windows (IBM, Armonk, New York, USA). The study data are expressed as mean values. For the immunohistochemical analysis, statistical comparisons of continuous variables among the groups were performed using a Kruskal–Wallis test based on their abnormal distribution. A paired samples t-test was performed for the analysis of TEOAE responses and apoptotic cells. A p-value of less than 0.05 was considered to be statistically significant.
Results
One guinea pig in the therapeutic group died before sacrifice. After amikacin administration, the signal-to-noise ratio from the TEOAE recordings of both the study and positive control groups in the therapeutic group (group A) were significantly decreased for all frequencies (p < 0.05). The signal-to-noise ratio at 1, 1.4 and 2.8 Hz were found to be slightly increased after autologous serum application, but without significance (p > 0.05). The TEOAE responses for all frequencies decreased after the intramuscular administration of amikacin in the protective group (group B). A significantly protective effect of autologous serum was observed only at 4 kHz (p < 0.05) (Table I).
Table I Signal-to-noise ratios of TEOAEs in therapeutic, protective and positive control groups
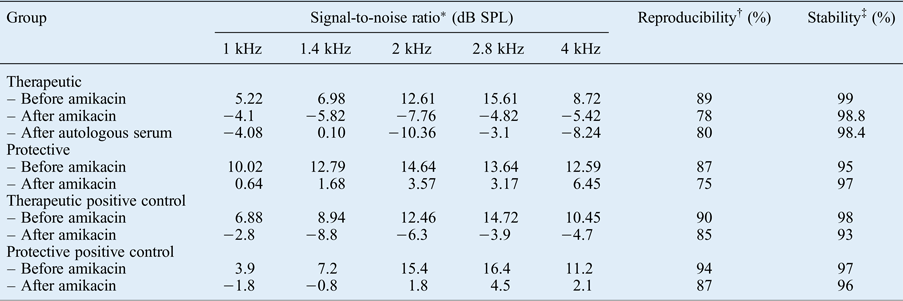
* Indicates the amplitude of otoacoustic emissions above the noise floor.
† Indicates the percentage correlation between two averages during data collection for otoacoustic emission measurement.
‡ Stability of the stimulus peak level during the otoacoustic emission recording, reflecting the stability of the probe fit during measurement. TEOAEs = transient evoked otoacoustic emissions
No therapeutic or protective effects of autologous serum were observed in the organ of Corti according to our immunohistochemical analysis following autologous serum administration (p > 0.05). Both the therapeutic and protective groups’ apoptotic cells in the spiral limbus were significantly reduced compared to the positive control group (p < 0.05). Apoptosis was reduced in the spiral ganglion after autologous serum application compared to the positive controls in groups A and B, but without significance (p > 0.05) (Table II and Figure 1).

Fig. 1 Immunohistochemical examination of (a) therapeutic group (group A) and (b) protective group (group B), for both study and control groups. (H&E; ×200)
Table II Comparison between mean apoptotic cell counts of study and control groups
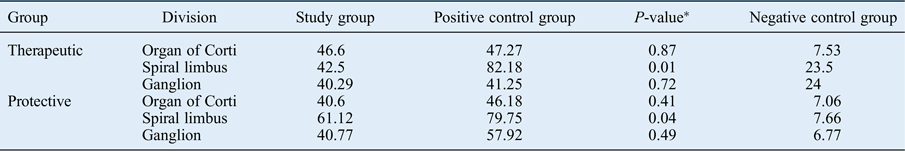
Data represent mean apoptotic cell count, unless indicated otherwise.
* P-values concern the differences between the study group and the positive control group.
When the mean number of apoptotic cells of the guinea pigs in both study groups were compared with the mean value of the positive control groups, a significantly protective effect was observed in four spiral limbus and in three spiral ganglion samples from the animals in group B (Table III).
Table III Comparison between apoptotic cell count of each individual animal and mean value of positive control group animals
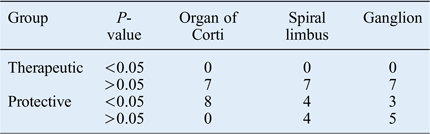
Data represent mean apoptotic cell count, unless indicated otherwise.
The immunoreactivity of Bax and Casp3 was greater than that of sit-C and Fas ligand, suggesting that the intrinsic mitochondrial pathway was primarily activated. The weak immune reaction of Fas ligand indicates that the extrinsic pathway was not triggered as much in this case. All apoptotic pathways, and particularly the intrinsic pathway, were more affected in the positive control groups than in the study and negative control groups (Table IV).
Table IV Immunohistochemical analysis of therapeutic and protective groups
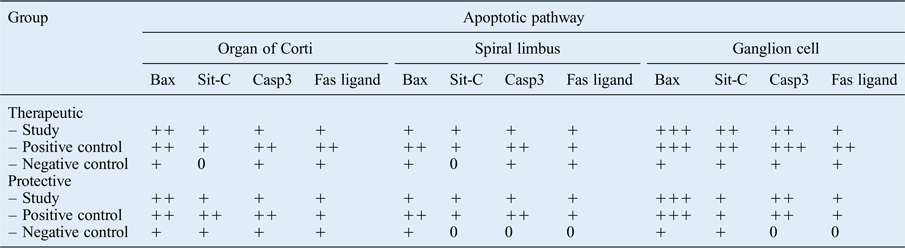
Immunolabelling intensity: ‘0’ = absent, ‘+’ = weak, ‘++’ = moderate and ‘+++’ = strong
Discussion
The protection of both hair cells and neurons is a highly attractive strategy for combating ototoxicity. In order to prevent ototoxic cell death, researchers have developed various strategies, including the inhibition of apoptosis, the neutralisation of reactive oxygen species and the administration of neurotrophic factors. Anti-apoptotic agents confer significant protection against hair cell damage from aminoglycosides, preserving hair cell morphology and function in vitro and in vivo.Reference Forge and Li 19 – Reference Nakamagoe, Tabuchi, Uemaetomari, Nishimura and Hara 21 Unfortunately, long-term treatment with anti-apoptotic agents is associated with a potential carcinogenic risk because apoptosis plays a crucial primary role in preventing uncontrolled cell proliferation.Reference Olsson and Zhivotovsky 22
A large number of studies have investigated the otoprotective effects of antioxidants (e.g. aspirin, N-acetylcysteine, dihydroxybenzoate and melatonin) on aminoglycoside ototoxicity. Overall, antioxidants attenuate ototoxic damage from aminoglycosides. However, the majority of antioxidants have not demonstrated complete protection against aminoglycoside ototoxicity.Reference Campbell, Meech, Klemens, Gerberi, Dyrstad and Larsen 23 , Reference Erdem, Ozturan, Iraz, Miman and Olmez 24 The contribution of neurotrophic growth factors in preventing aminoglycoside ototoxicity suggests involvement of the auditory nerve and sensorineural cells. However, evidence exists which indicates that the effects of neurotrophic growth factors alone are not beneficial.Reference Gillespie, Clark, Bartlett and Marzella 25
Autologous serum can be considered a good candidate for ototoxicity prevention. Because autologous serum incorporates anti-apoptotic activity, it is able to increase the antioxidant capacity of tissues and it contains growth factors.Reference Quinto, Campos and Behrens 26 – Reference Poon, Geerling, Dart, Fraenkel and Daniels 28 Autologous serum has been used over the last two decades to promote the healing of epithelial and cartilaginous damage of both known and unknown aetiologies.Reference Kakehata, Hirose, Kitani, Futai, Maruya and Ishii 13 , Reference Kamal, Kumar and Goel 29 – Reference Nimura, Muneta, Koga, Mochizuki, Suzuki and Makino 31 At the same time, it includes various immunoglobulins (e.g. immunoglobulin G and immunoglobulin A) and lysozyme.Reference Fortier, Barker, Strauss, McCarrel and Cole 8 , Reference Geerling, Maclennan and Hartwig 32
Apoptosis is a distinct form of cell death controlled by an internally encoded suicide programme that includes independent extrinsic and intrinsic pathways. Amikacin, which is not metabolised and remains in hair cells for months, causes the formation of reactive oxygen species, leading to apoptosis. When the formation of reactive oxygen species overwhelms the capacity of the intrinsic protective and repair systems, the cell undergoes apoptotic cell death, resulting in ototoxicity and deafness.Reference Berkiten, Slturk, Topaloglu and Ugras 3 , Reference Huth, Ricci and Cheng 33 , Reference Alharazneh, Luk, Huth, Monfared, Steyger and Cheng 34
The present study evaluated the possible therapeutic and protective effects of autologous serum on amikacin-induced apoptosis in an animal model, by both TEOAE and histopathological examination. After amikacin application, TEOAE assessments revealed a statistically significant decrease in signal-to-noise ratio in group A. Autologous serum had no therapeutic effect on hearing function according to the TEOAE responses, but its signifıcantly protective effects appeared at 4.0 kHz. However, intratympanic amikacin may have reached a higher concentration in the inner ear. Thus, intratympanic amikacin is more destructive than when it is administered intramuscularly. In addition to this, autologous serum might be effective in partial or minor cell destruction according to the TEOAE responses. The number of apoptotic cells was significantly higher in the negative control guinea pigs that received intratympanic rather than intramuscular administration of 0.9 per cent saline (p < 0.05).
The main purpose of autologous serum application is to induce healing via the complex autologous serum mixture, which harbours growth factors, vitamins and immunoglobulins. Recently, the local application of growth factors to the inner ear has been presented as an effective method for protecting the cochlea from insertional trauma during cochlear implantation.Reference Kikkawa, Nakagawa, Ying, Tabata, Tsubouchi and Ido 35 Autologous serum may inhibit apoptotic cascades or activate healing factors via multiple endocytic mechanisms in neuro-sensitive, mesenchymal and neural cells. On the other hand, serum components such as lysozyme may have a detrimental effect on inner-ear cells. Inflammation and lysozyme may trigger apoptosis in the rather sensitive cells of the organ of Corti.Reference Fortier, Barker, Strauss, McCarrel and Cole 8 The decreased therapeutic and protective effects of autologous serum in the organ of Corti compared to the spiral limbus and ganglion cells may be explained by lysozyme activity. Autologous serum lacking lysozyme, which has a destructive effect on cells, may improve the healing and protective effects of autologous serum.
The histopathological results of aminoglycoside ototoxicity revealed that nerve fibres, spiral ganglion neurons and supporting cells were damaged much later than hair cells. Strial degeneration has been reported previously, but the authors were unsure whether it was a secondary or primary effect of aminoglycoside treatment.Reference Guthrie 4 , Reference Huth, Ricci and Cheng 33 The connective tissue cells of the spiral limbus are highly susceptible to ototoxicity. At the same time, the spiral limbus may serve as one of the most sensitive indicators of changes in the chemical composition of perilymph.Reference Kimura, Nye and Southard 36 The highest apoptotic cell count was encountered in the spiral limbus of both positive control groups when compared with ganglion cells and the organ of Corti. According to the present study, the spiral limbus is more susceptible to amikacin-induced ototoxicity than ganglion cells and the organ of Corti.
It is thought that mammalian cochlear hair cells and spiral ganglion neurons are terminally differentiated, and once completely damaged they do not regenerate.Reference Guthrie 4 Recently, the beneficial effect of platelet-rich plasma and neural-induced human mesenchymal stem cells was demonstrated on axonal regeneration from a facial nerve axotomy injury in a guinea pig model.Reference Cho, Jang, Lee, Jeong, Park and Han 37 Although a significantly protective effect was observed in three guinea pig ganglion cells, according to immunohistochemical examination, the current study did not show therapeutic or protective effects of autologous serum in ganglion cells. However, significant therapeutic and protective effects of autologous serum were detected in the spiral limbus. The current study suggests that autologous serum is beneficial in connective tissue cells like the spiral limbus, rather than in sensorineural and neural cells. Our immunohistochemical examination revealed that the intrinsic mitochondrial pathway, particularly Bax and Casp3, was activated to a greater extent than the extrinsic pathway.
-
• Autologous serum is a new treatment option which harbours growth factors, vitamins and immunoglobulins
-
• Ototoxic cell death can be induced by apoptosis inhibition, reactive oxygen species neutralisation and neurotrophic factor administration
-
• Autologous serum may inhibit apoptosis or activate healing via endocytic mechanisms in neuro-sensitive, mesenchymal and neural cells
-
• This study indicates that autologous serum may be protective against ototoxicity-induced hearing loss
This study has a number of limitations, including a lack of low- and high-frequency thresholds in the TEOAE assessment. The lack of quality control prevented assessment of autologous serum levels among the animals. This study explored uncontrolled autologous serum levels after application. In addition, the levels of growth factors and lytic enzymes were not determined. Future research should focus on the enhancement of autologous serum with growth factors and antioxidant agents, to create a greater anti-apoptotic effect. The anti-inflammatory properties of cytokines in autologous serum should be useful in treating autoimmune inner-ear diseases.
Conclusion
Autologous serum may offer protection against ototoxicity-induced hearing loss, but it cannot restore hearing. Autologous serum conferred therapeutic and protective effects on mesenchymal cells as opposed to neurosensory or neural cells in amikacin-induced ototoxicity. However, the question of how much damage will be reversed or what amount of cell damage can be prevented remains unanswered. Further study is needed on the use of autologous serum for diseased or damaged inner-ear cells and nerves.







