Isoflavones are micronutrients of non-steroidal structure derived mainly from soyabeans that mimic oestrogen in structure and action and are therefore classified as bioactive phyto-oestrogens( Reference Setchell 1 ). Unlike oestrogen, isoflavones preferentially bind to oestrogen receptor β (ER-β), expressed in the vasculature, rather than to the ER-α ( Reference Setchell 1 ). The predominant isoflavones are genistein and daidzein( Reference Setchell 1 ). Isoflavones are proposed to be protective against CHD( Reference Clarkson and Anthony 2 ). The beneficial effect of isoflavones on CHD may be derived from their atheroprotective properties mediated via: (a) reductions in blood lipids( Reference Taku, Umegaki and Sato 3 ) and blood pressure( Reference Taku, Lin and Cai 4 ), (b) improved endothelial function( Reference Teragawa, Higashi and Kihara 5 , Reference Walker, Dean and Sanders 6 ), (c) antioxidant activity that may prevent oxidative damage to LDL-cholesterol( Reference Wiseman, O’Reilly and Adlercreutz 7 , Reference Jenkins, Kendall and Vidgen 8 ) and other properties( Reference Nestel, Yamashita and Sasahara 9 – Reference Wilcox and Blumenthal 11 ).
Randomised controlled trials of dietary isoflavones on coronary and carotid arteries in monkeys clearly demonstrate the atheroprotective properties of isoflavones( Reference Anthony, Clarkson and Bullock 12 , Reference Wagner, Cefalu and Anthony 13 ). Furthermore, observational studies in Asian countries, where dietary intake of isoflavones is common have documented significant inverse associations between isoflavones and incident CHD( Reference Zhang, Shu and Gao 14 – Reference Zhang, Gao and Yang 16 ). However, a recent randomised controlled trial of dietary isoflavones on atherosclerosis in the USA, where dietary intake of isoflavone is miniscule compared with Asian countries, failed to show a benefit( Reference Hodis, Mack and Kono 17 ). An equol hypothesis has been proposed to explain the discrepancy in cardiovascular benefits among animals, Asian and Western countries( Reference Setchell and Clerici 18 ). Equol is a metabolite of the dietary isoflavone daidzein that is produced by the action of gut microflora( Reference Setchell and Clerici 18 ). Equol has a stronger affinity for the ER-β than its precursor daidzein and similar to genistein, has a longer half-life and bio-availability than genistein and daidzein, and the highest antioxidant properties of the isoflavones, in vitro ( Reference Setchell and Clerici 18 , Reference Setchell and Clerici 19 ). Thus, equol may have greater atheroprotective properties than other isoflavones. According to the ‘equol hypothesis’, individuals with the ability to produce equol are termed ‘equol-producers’. They derive greater clinical benefits from equol than individuals who are unable to produce equol termed ‘equol non-producers’( Reference Setchell and Clerici 18 ). All monkeys can produce equol( Reference Rafii, Sutherland and Bridges 20 ). Although the prevalence of equol-producers is reported to be 50–60 % in Asian countries, it is reported to be much lower in Western (25–30 %) than Asian countries( Reference Setchell and Cole 21 ).
The atheroprotective properties of dietary isoflavones have been demonstrated in both male and female monkeys( Reference Anthony, Clarkson and Bullock 12 , Reference Wagner, Cefalu and Anthony 13 ) unlike humans where the atheroprotective effects are primarily investigated in women( Reference Zhang, Shu and Gao 14 – Reference Zhang, Gao and Yang 16 ). No previous study has examined the association between dietary isoflavones or equol with the presence of coronary artery calcification (CAC). CAC is a well-established biomarker of atherosclerosis that is positively associated with the future risk of CHD independent of traditional risk factors( Reference Greenland, Bonow and Brundage 22 ). We aimed to examine the association of dietary isoflavone and equol-producer status with the presence of CAC in Japanese men in the Electron-Beam Computed Tomography and Risk Factor Assessment among Japanese and U.S. Men in the Post World War II Birth Cohort study. We hypothesise that the atheroprotective effect of isoflavones are mediated through equol therefore equol but not isoflavones would be associated with reduced CAC.
Methods
Study population
The methods for selecting the participants have been described in detail previously( Reference Sekikawa, Curb and Ueshima 23 ). In brief, from 2002 to 2007, 303 men aged 40–49 years were randomly selected from Kusatsu, Shiga, Japan. Participants were without clinical CVD, type 1 diabetes or other severe diseases. All participants provided informed consent. The study was approved by the Institutional Review Boards of the Shiga University of Medical Science, Otsu, Japan and the University of Pittsburgh, Pittsburgh, USA.
Measurement of covariates
A questionnaire for lifestyle habits, physical examination and laboratory assessment were conducted on all participants( Reference Sekikawa, Curb and Ueshima 23 ). Alcohol intake was categorised as nil, 1–23, 24–46, 46–69 and >69 g/d( Reference Okamura, Kadowaki and Sekikawa 24 ). Heavy drinking was defined as >69 g/d of alcohol intake. Meat eaters were defined as participants consuming meat ≥2–3 times/week. Exercise was defined as at least 1 h of exercise/week. Education was ascertained as years of schooling. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or use of antihypertensive medications( Reference Weber, Schiffrin and White 25 ). Diabetes was defined as fasting glucose ≥7mmol/l or use of medications for diabetes( Reference American Diabetes 26 ). Use of blood pressure-lowering, diabetes and lipid-lowering medications were ascertained by questionnaire.
Blood samples were stored at −80°C and shipped on dry ice to the University of Pittsburgh. Serum/plasma samples were assayed for lipids including total cholesterol, LDL-cholesterol and HDL-cholesterol, glucose, and C-reactive protein (CRP) at the University of Pittsburgh as described previously( Reference Sekikawa, Curb and Ueshima 23 ). Serum n-3 fatty acids were analysed by capillary GLC( Reference Sekikawa, Curb and Ueshima 23 ). n-3 Fatty acids were defined as the sum of EPA and DHA.
Measurements of serum isoflavones
Dietary isoflavones were assessed as the sum of the serum levels of daidzein and genistein. Daidzein, genistein and equol concentrations were measured using a modified method of Pumford et al.( Reference Pumford, Morton and Turkes 27 ). Daidzein-d4 and genistein-d4 (Cambridge Isotope Laboratories) and equol-d4 (Medical Isotopes Inc.) were added and the samples incubated with β-glucuronidase. The samples were extracted with diethyl ether, dried under N2 and silylated. The samples were analysed by GC-MS in the selected ion monitoring mode. Ions monitored (m/z) were: 425/482, 234/470 and 555 for daidzein, equol and genistein, respectively; 428/485, 236/474 and 559 for daidzein-d4, equol-d4 and genistein-d4, respectively. The CV were 9·5, 15·2 and 5·4 % for daidzein, genistein and equol, respectively.
Measurement of coronary artery calcification
CAC scanning was performed with a GE-Imatron C150 EBT scanner (GE Medical Systems) as described elsewhere in detail( Reference Sekikawa, Curb and Ueshima 23 ). In brief, a standardised protocol was used to perform CAC scanning; 30–40 contiguous, 3-mm-thick transverse images from the level of the aortic root to the apex of the heart. Images were obtained during maximal breath holding by using electrocardiogram triggering. Images were recorded onto a disc in Japan and shipped to the University of Pittsburgh to be read by a trained reader using Digital Imaging and Communications in Medicine workstation and software by AccuImage (AccuImage Diagnostic Corp.). The software program implements the widely accepted Agatston scoring method( Reference Agatston, Janowitz and Hildner 28 ). CAC was considered to be present when 3 contiguous pixels (area=1 mm2) >130 Hounsfield Unit were detected overlying the vessels of interest. The reader was blinded to the participants’ characteristics. Intra-class correlation for re-examination of non-zero coronary calcium score (CCS) was 0·98.
Statistical analyses
The associations of dietary isoflavones and equol-producer status with CAC were examined in 274 participants after excluding very heavy alcohol drinkers (n 31). In a previous report for this population, we reported that heavy alcohol drinkers, those consuming ≥69 g/d of alcohol, had more than ten times higher risk for CAC than moderate drinkers (23–45 g/d of alcohol) and about four times higher risk for CAC than never drinkers independent of traditional risk factors( Reference Okamura, Kadowaki and Sekikawa 24 ), which is unlikely to be completely attenuated by anti-atherosclerotic properties of any single known dietary component such as isoflavones or equol. The cut-off point of CCS ≥10 Agatston unit was used to define the presence of CAC, as previously reported( Reference Sekikawa, Miura and Lee 29 ). CCS between 1 and 9 is likely to be noise.
Tertiles of dietary isoflavones were used as the independent variables in the model because distribution of isoflavones was highly skewed to the right. Baseline characteristics of participants were expressed as means (standard deviation) or medians (interquartile range) for continuous variables and as percentages for categorical variables according to tertiles of dietary isoflavones. To examine the trend for the association among tertiles of dietary isoflavones with cardiovascular risk and other factors, general linear model, Jonckheere–Terpstra trend test and Cochran–Armitage trend test for continuous variables distributed normally, continuous variables distributed non-normally and binary variables were used, respectively.
To examine the association of equol-producer status with the presence of CAC, serum levels of >83 nm was used as the cut-off point for defining equol-producer status as recommended by Setchell et al.( Reference Setchell, Brown and Lydeking-Olsen 30 ). The baseline characteristics of the participants were expressed as means (standard deviation) or medians (interquartile range) and as percentages for continuous and categorical variables according to equol producing status. t Tests or Kruskal–Wallis tests and χ 2 tests were used to test for differences in continuous variables and categorical variable between equol-producers and equol non-producers.
To analyse the multivariable association of dietary isoflavones and equol-producer status with CAC, multivariable logistic regression was used in the following models: (i) unadjusted; (ii) adjusted for traditional cardiovascular risk factors, age, pack-years of smoking, BMI, LDL-cholesterol, diabetes (yes/no) and hypertension (yes/no) (model I); (iii) model I+further adjusted for other cardiovascular risk factors, alcohol consumption, CRP and lipid medication (yes/no) (model II); (iv) model II+further adjusted for lifestyle risk factors, serum levels of n-3 fatty acids and meat consumption (≥2–3 times/week) (model III); and (v) only for association of equol-producer status with CAC, model III+further adjusted for total serum isoflavones (model IV).
The primary aim of our study was to examine differences in subclinical atherosclerosis among different races. Therefore, in this study, we performed an exploratory analysis. A logistic regression of CCS ≥10 on equol-producer status with a sample size of 567 observations (of which 84 % are equol non-producers and 16 % are equol-producers as in our study) achieves 80 % power at a 0·05 significance level to detect a change in probability of CCS ≥10 from the baseline value of 0·11 (proportion of equol non-producers in our study) to 0·02 (proportion of equol-producers in our study). This change corresponds to an OR of 0·17. An adjustment was made since a multiple regression of equol-producer status on the other independent variables in the logistic regression obtained an R 2 of 0·13.
In sensitivity analyses, the association of dietary isoflavones and equol-producer status with CAC was examined without excluding heavy drinkers. The definition of equol-producers using serum equol concentrations is not firmly established. Therefore, alternate definitions of 20 and 40 nm, utilised by previous studies were also used for defining equol-producer status( Reference Morton, Arisaka and Miyake 31 ). A two-sided P value<0·05 was considered significant. All analyses were performed using SAS software version 9.3 of the SAS System.
Results
Table 1 describes characteristics of the study population by tertiles of dietary isoflavones. The ranges of serum levels of isoflavones in tertile 1, tertile 2 and tertile 3 were 0·0–274·7, 274·8–843·8, and 843·9–7639·5, respectively. With increasing tertiles of dietary isoflavones, no significant trend existed for BMI, hypertension, LDL-cholesterol, diabetes, pack-years of smoking or CAC. Table 2 presents the descriptive characteristics of the study population by equol producing status. About 16 % of the individuals (n 43) were equol-producers. There were no significant differences between the two groups in age, BMI, hypertension, LDL-cholesterol, diabetes and pack-years of smoking. The levels of serum n-3 fatty acids were significantly lower but the concentrations of dietary isoflavones were significantly higher in equol-producers than among equol non-producers. The prevalence of CAC was lower among equol-producers (2·3 %) than non-producers (10·9 %) but the difference was not statistically significant (P=0·09).
Table 1 Characteristicsof 40–49-year-old Japanese men according to tertiles of serum isoflavones (n 274) in the Electron-Beam Computed Tomography and Risk Factor Assessment among Japanese and U.S. Men in the Post World War II Birth Cohort study from 2002 to 2007 (Mean values and standard deviations; medians and interquartile ranges (IQR); percentages are presented for continuous and categorical variables; 50th and 75th percentiles)

BP, blood pressure; CCS; coronary calcium scores.
* n-3 Fatty acids were estimated as the sum of EPA and DPA.
Table 2 Characteristics of 40–49-year-old Japanese men by equol producing status (>83 nm) (n 274) in the Electron-Beam Computed Tomography and Risk Factor Assessment among Japanese and U.S. Men in the Post World War II Birth Cohort study from 2002 to 2007 (Mean values and standard deviations; medians and interquartile ranges (IQR); percentages are presented for continuous and categorical variables; 50th and 75th percentiles)

BP, blood pressure; CCS, coronary calcium scores.
* Significant at <0·0.
† n-3 Fatty acids were estimated as the sum of serum levels of EPA and DHA.
‡ Dietary isoflavones were estimated as the sum of serum levels of daidzein and genistein.
Table 3 describes the results of multiple logistic regression analyses between tertiles of isoflavones and the presence of CAC. In the unadjusted model, the isoflavones were not significantly associated with the presence of CAC. The non-significant association between dietary isoflavones and the presence of CAC persisted after adjusting for covariates. The results did not materially change after including heavy alcohol drinkers (online Supplementary Table S1).
Table 3 Association of tertiles of serum isoflavones with the presence of coronary calcium score ≥10 among men in Japan (n 272) (Odds ratio and 95 % confidence intervals)

* Model I: adjusted for age, pack-years of smoking, BMI, LDL-cholesterol, hypertension and diabetes.
† Model II: model I+further adjusted for alcohol consumption, C-reactive protein and lipid medication.
‡ Model III: model II+further adjusted for serum levels of n-3 fatty acids and meat consumption.
Table 4 presents the results for the multivariable logistic regression association of equol-producers with CAC. In the unadjusted analysis, the OR for the presence of CAC in equol-producers compared with equol non-producers was 0·19 (95 % CI 0·03, 1·47, P=0·11). After multivariable adjustment, OR for the presence of CAC was 0·09 (95 % CI 0·01, 0·89, P=0·04) with 90 % lower odds of CAC in equol-producers compared with equol non-producers. In sensitivity analysis, when heavy drinkers were included, multivariable adjusted OR was 0·49 (95 % CI 0·15, 1·58, P=0·23) that did not reach statistical significance (online Supplementary Table S2).
Table 4 Association of equol-producer status (>83 nM) with the presence of coronary calcium score ≥10 among men in Japan (n 272) (Odds ratio and 95 % confidence intervals)
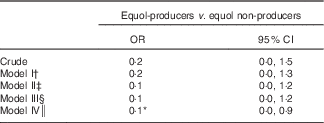
* Significant at <0·05.
† Model I: adjusted for age, pack-years of smoking, BMI, LDL-cholesterol, hypertension and diabetes.
‡ Model II: model I+further adjusted for alcohol consumption, C-reactive protein and lipid medication.
§ Model III: model II+further adjusted for serum levels of n-3 fatty acids and meat consumption.
║ Model IV: model III+further adjusted for serum levels of isoflavones.
The percentage of participants with equol ≥40 nm was 22·4 % and with equol ≥20 nm was 40·3 %. There were no statistically significant associations between equol-producer status and the presence of CAC using these alternate definitions for equol-producers (online Supplementary Table S3), although the odds for the presence of CAC were 50 % lower among equol-producers than among equol non-producers when using a cut-off value of 40 nm.
Discussion
In this population based of healthy middle-aged Japanese men in Japan, dietary isoflavones were not significantly associated with CAC. However, equol-producers had significantly less CAC than equol non-producers. These results suggest that equol may be an important factor for the anti-atherosclerotic effects of dietary isoflavones. This is the first study to report a significant inverse association of equol with CAC among men.
In accordance with our study, in a case–control study of Chinese adults, urinary levels of equol but not of isoflavones were significantly and inversely associated with incident CHD in women( Reference Zhang, Gao and Yang 16 ). In women, the OR of CHD was 0·46 (95 % CI 0·24, 0·89, P=0·02) in the highest compared with the lowest quartile of equol. The men had 34 % lower odds of CHD in the highest compared with the lowest quartile of equol but the OR was not significant 0·76 (95 % CI 0·39, 1·49). This may be due to a shorter duration of follow-up for the men than the women, 5 years compared with 10 years, respectively.
Three clinical trials among post-menopausal women have examined the effect of dietary isoflavone on progression of intima-media thickness of the carotid artery (CIMT), another biomarker of atherosclerosis( Reference Hodis, Mack and Kono 17 , Reference Curtis, Potter and Kroon 32 , Reference Liu, Ho and Chen 33 ). These trials generally reported negative results. However, in the Women’s Isoflavone Soy Health (WISH) trial, a positive association between dietary isoflavone and progression of CIMT was observed in women who were in their initial 5 years of menopause. The WISH trial examined the association of equol-producers with progression of CIMT; no significant association was found. Nevertheless, the WISH trial was not statistically powered to examine this hypothesis. Moreover, the WISH trial defined equol-producers as plasma levels of equol≥20 nm. This cut-off point for defining equol-producers may have classified several equol non-producers as equol-producers that may have led to a non-significant association between equol-producers and progression of CIMT.
It is notable that dietary isoflavones have been associated with reduced incidence of CHD in China and Japan( Reference Zhang, Shu and Gao 14 – Reference Zhang, Gao and Yang 16 ), but not in Western countries( Reference van der Schouw, Kreijkamp-Kaspers and Peeters 34 ), in observational epidemiological studies. This variable effect of isoflavones between Asian and Western countries may be attributed to differences in the amount of intake of isoflavones and the prevalence of equol-producers. The daily intake of isoflavones is much higher in China and Japan than in Western countries, 25–50 mg compared with 1–2 mg, respectively( Reference Klein, Nahin and Messina 35 ). In line with the higher dietary intake of isoflavones in Japan than Western countries, more than ten times higher levels of serum isoflavones have been reported in Japan than in Western countries( Reference Morton, Arisaka and Miyake 31 , Reference Adlercreutz, Markkanen and Watanabe 36 , Reference Heald, Bolton-Smith and Ritchie 37 ). In our study, the high levels of serum isoflavones with a median of 512 nmol/l are in accordance with the high levels previously reported in the Japanese. Moreover, the prevalence of equol-producers is reported to be almost double in Japan than Western countries( Reference Setchell and Cole 21 ). We are unable to compare the percentage of equol-producers in our study with previous studies because different serums cut-off values were used to define equol-producers in Japanese men( Reference Morton, Arisaka and Miyake 31 ).
It is argued that the discrepancy between the results of observational studies in Asian countries showing a favourable effect of isoflavones on the risk of CHD and clinical trials in the Western countries showing a null effect may be attributed to: (1) ‘healthy user effect’, a form of selection bias, and/or (2) the differences in the form of intake of isoflavones and the timing and duration of the exposure of isoflavones( Reference Messina 38 , Reference Messina 39 ).
First, ‘healthy user effect’ in observational studies among Asians is less likely to play a role because the intake of isoflavones in Asian countries is almost universal( Reference Messina, Nagata and Wu 40 ). Therefore, in these studies, the possibility of selection of participants consuming higher amount of isoflavones with underlying healthy behaviours, leading to a significant inverse association between isoflavone intake and CHD is less likely. Second, the favourable results in Asian countries are based on intake of traditional soya foods such as tofu, miso and soya milk, etc. in contrast to isoflavone supplements utilised in randomised clinical trials in the Western countries. There is a possibility of interaction of isoflavones with other components of soya in populations consuming traditional soya foods such that this interaction may lead to more beneficial effects than supplementation of individual soya components in clinical trials. Interaction of isoflavones with other components of soya in traditional soya food might potentially affect the percentage of individuals converting isoflavones to equol that needs investigation in the future. Furthermore, the exposure of isoflavones in Asian begins at an early age compared with the Western countries. In fact, a body of evidence suggests that to derive benefits of isoflavones in case of breast cancer, the exposure to isoflavones must begin in childhood/adolescence( Reference Messina 39 ). Although, currently there is no such evidence in context of CHD; the possibility of an early exposure of soya foods leading to a greater percentage of equol-producers than the exposure late in life is again a theme to be investigated.
Our study has several strengths. This is the first study to report lower coronary atherosclerosis among equol-producers than equol non-producers. Previous studies reporting the association of dietary isoflavones with atherosclerosis and CHD are primarily among women yet we have shown a significant inverse association among men. The population-based sample of our study minimises the opportunity for selection bias in explaining the association between equol and CAC. Our study population had a large variation in serum levels of isoflavones; association between exposure and outcome can be more easily assessed when level of exposure is variable.
The results of this study should be interpreted in light of certain limitations. The sample size of our study is small and therefore there is a probability of type 1 error. Men were the only participants in our study and therefore generalisability cannot be inferred. We used a single measure of serum levels of isoflavones as an indicator of long-term intake. Serum levels of isoflavones provide an estimate of short-term dietary exposure to isoflavones( Reference Fraser, Franke and Jaceldo-Siegl 41 ). However, serum levels of isoflavones are a good indicator of dietary intake in the Japanese because Japanese consume soya products daily( 42 ). In the Japanese, the correlation coefficients of serum and 28-d dietary records for daidzein and genistein is reported to be 0·39 (P<0·05) and 0·42 (P<0·05)( Reference Yamamoto, Sobue and Sasaki 43 ), respectively, considered high for dietary components( Reference Jenab, Slimani and Bictash 44 ). Moreover, the seasonal variation in isoflavone intake assessed by serum and dietary questionnaire is small in Japan( Reference Iwasaki, Inoue and Otani 45 ).
Serum levels of equol are influenced by variations in the intake of dietary isoflavones, absorption and bioavailability of isoflavones, and in the methodologies used to measure equol( Reference Setchell and Cole 21 ). However, because the variation in the serum levels of equol occurs randomly, the actual association of equol-producers with coronary atherosclerosis would be stronger than was observed in this study. Debate exists in the scientific community whether it is equol per se or the ability to produce equol, ‘equol phenotype’ that is responsible for clinical effectiveness of soya isoflavones( Reference Lampe 46 ). Genetic and microbiome studies on the equol phenotype would illuminate this debate in the future.
We defined the presence of coronary atherosclerosis as CCS ≥10 that may not be strongly related to cardiovascular outcome. Yet, a meta-analysis show that individuals with CCS ≥0 compared with individuals with CCS=0 have a significant higher total mortality( Reference Blaha, Budoff and Shaw 47 ). Although we adjusted for various potential confounders (e.g. meat, n-3, CRP); we cannot exclude the possibility of residual or unmeasured confounding.
To conclude, among Japanese men, equol-producers had lower CAC than equol non-producers independent of cardiovascular risk factors. The inverse association of CAC was not observed with dietary isoflavones, thus indicating that equol may be a key factor for the atheroprotective properties of dietary isoflavones. Not all human adults are able to convert isoflavone daidzein to equol. The availability of equol as a nutraceutical or pharmaceutical agent( Reference Uchiyama, Kumemura and Imaizumi 48 ) may extend the benefit of preventing CHD to everyone. Prospective studies and clinical trials are warranted to expand on these findings.
Acknowledgements
This research was funded by the National Institute 324 of Health (RO1 HL68200), from the Japanese Ministry of Education, Culture, Sports, Science and Technology (B16790335 and A13307016), and small grant from the Departmental of Epidemiology, University of Pittsburgh, PA, USA.
V. A., K. M., A. V., A. F., R. E., M. Z., N. M., T. H., A. K., T. O., H. U. and A. S. contributed to conception and design. T. O., A. K., H. U. and R. E. collected the data. V. A., A. V., K. M. and A. S. analysed and interpreted the data. V. A. and A. V. drafted the article. All authors critically revised the article for intellectual content. All authors approved the final version. V. A., A. V. and A. S. are guarantors of this work.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451600458X






