Introduction
Lewy body dementia is a common neurodegenerative disease in older people (Walker et al. Reference Walker, Possin, Boeve and Aarsland2015). It is responsible for 5–25% of diagnosed cases of dementia (Vann Jones & O'Brien, Reference Vann Jones and O'Brien2014), giving it a likely prevalence of around 1% in people over 65 years old (Ballard et al. Reference Ballard, Aarsland, Francis and Corbett2013). The disease is characterised by fluctuations in attention and alertness, recurrent visual hallucinations, and parkinsonian motor features (McKeith et al. Reference McKeith, Dickson, Lowe, Emre, O'Brien, Feldman, Cummings, Duda, Lippa, Perry, Aarsland, Arai, Ballard, Boeve, Burn, Costa, Del Ser, Dubois, Galasko, Gauthier, Goetz, Gomez-Tortosa, Halliday, Hansen, Hardy, Iwatsubo, Kalaria, Kaufer, Kenny, Korczyn, Kosaka, Lee, Lees, Litvan, Londos, Lopez, Minoshima, Mizuno, Molina, Mukaetova-Ladinska, Pasquier, Perry, Schulz, Trojanowski and Yamada2005). It is broadly considered to consist of two related disorders – dementia with Lewy bodies and Parkinson's disease dementia – that are distinguished by the relative timing of when cognitive and motor symptoms appear. Dementia with Lewy bodies is diagnosed if dementia develops either prior to parkinsonian motor symptoms or within 1 year of their onset. By contrast, Parkinson's disease dementia is diagnosed if dementia develops after 1 year of parkinsonian motor symptoms. The two disorders share a common underlying pathophysiology, but likely vary in the sequence by which brain areas underpinning cognition and motor function are affected (Aarsland et al. Reference Aarsland, Londos and Ballard2009; McKeith, Reference McKeith2009).
Lewy body dementia poses a number of challenges for clinical management that differ from those of other dementias. People with Lewy body dementia typically experience visual hallucinations and may be more likely to have delusions than people with other types of dementia (Ballard et al. Reference Ballard, Holmes, McKeith, Neill, O'Brien, Cairns, Lantos, Perry, Ince and Perry1999; Aarsland et al. Reference Aarsland, Brønnick, Ehrt, De Deyn, Tekin, Emre and Cummings2007; Brodaty et al. Reference Brodaty, Connors, Xu, Woodward and Ames2015). These types of symptoms can be very distressing to both patients and their carers, and are a risk factor for early institutionalisation (Black & Almeida, Reference Black and Almeida2004; Brodaty et al. Reference Brodaty, Connors, Xu, Woodward and Ames2014). Pharmacological management, however, is limited because of the condition's adverse sensitivity to neuroleptics (antipsychotic drugs) (McKeith et al. Reference McKeith, Fairbairn, Perry, Thompson and Perry1992; Aarsland et al. Reference Aarsland, Perry, Larsen, McKeith, O'Brien, Perry, Burn and Ballard2005; Ballard et al. Reference Ballard, Aarsland, Francis and Corbett2013). Antidepressants also appear to be poorly tolerated (Culo et al. Reference Culo, Mulsant, Rosen, Mazumdar, Blakesley, Houck and Pollock2010) with no clear evidence that they are effective for treating mood disturbances in this population (Ballard et al. Reference Ballard, Aarsland, Francis and Corbett2013; Stinton et al. Reference Stinton, McKeith, Taylor, Lafortune, Mioshi, Mak, Cambridge, Mason, Thomas and O'Brien2015), though further research is needed. At the same time, patients typically have parkinsonian motor disturbances and treatment responses are limited by the fact that some antiparkinson medications can exacerbate psychotic symptoms (McKeith et al. Reference McKeith, Dickson, Lowe, Emre, O'Brien, Feldman, Cummings, Duda, Lippa, Perry, Aarsland, Arai, Ballard, Boeve, Burn, Costa, Del Ser, Dubois, Galasko, Gauthier, Goetz, Gomez-Tortosa, Halliday, Hansen, Hardy, Iwatsubo, Kalaria, Kaufer, Kenny, Korczyn, Kosaka, Lee, Lees, Litvan, Londos, Lopez, Minoshima, Mizuno, Molina, Mukaetova-Ladinska, Pasquier, Perry, Schulz, Trojanowski and Yamada2005). People with Lewy body dementia are also prone to falls, which further increases the risks of using medications that exacerbate orthostatic hypotension, impair cognition, or otherwise predispose to falls. Finally, people with Lewy body dementia may undergo faster cognitive decline (Rongve et al. Reference Rongve, Soennesyn, Skogseth, Oesterhus, Hortobágyi, Ballard, Auestad and Aarsland2016), require higher costs of care (Murman et al. Reference Murman, Kuo, Powell and Colenda2003; Boström et al. Reference Boström, Jönsson, Minthon and Londos2007; Vossius et al. Reference Vossius, Rongve, Testad, Wimo and Aarsland2014), result in greater caregiver burden (Svendsboe et al. Reference Svendsboe, Terum, Testad, Aarsland, Ulstein, Corbett and Rongve2016), and progress more quickly to death (Oesterhus et al. Reference Oesterhus, Soennesyn, Rongve, Ballard, Aarsland and Vossius2014) than people with Alzheimer's disease and other dementias.
Despite these challenges and the relatively high prevalence of the disease, the evidence base for intervention strategies remains unclear. A previous systematic review and meta-analysis of pharmacological treatments for Lewy body dementia identified a lack of high-quality evidence for commonly used drugs in this population, though found evidence for efficacy of cholinesterase inhibitors (Stinton et al. Reference Stinton, McKeith, Taylor, Lafortune, Mioshi, Mak, Cambridge, Mason, Thomas and O'Brien2015). Another systematic review evaluated the benefits of exercise in Lewy body dementia and likewise found little high-quality research (Inskip et al. Reference Inskip, Mavros, Sachdev and Fiatarone Singh2016). There is, however, no synthesis of evidence for non-pharmacological options more generally for people with Lewy body dementia. Such options have been shown to be helpful in other types of dementia (Brodaty & Arasaratnam, Reference Brodaty and Arasaratnam2012; Livingston et al. Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris, Patel, Omar, Katona and Cooper2014; Orgeta et al. Reference Orgeta, Qazi, Spector and Orrell2015), but are likely to be especially important in Lewy body dementia given the risks of medications in this population. In this paper, we addressed this issue and reviewed studies that assessed non-pharmacological interventions for patients with Lewy body dementia.
Method
The protocol for this systematic review was registered at PROSPERO (registration number CRD42016050492).
Eligibility criteria
The review focused on primary research that assessed a non-pharmacological intervention for either patients with Lewy body dementia or carers of such patients. The review considered studies evaluating non-pharmacological interventions in the broader categories of dementia or Parkinson's disease if the results of a Lewy body dementia subgroup were available. Studies that were confounded by a concurrent pharmaceutical intervention (i.e. medication initiated at the same time as a non-pharmacological intervention or given to one group in a study but not the other) were excluded. There were no other restrictions on study design and no requirements for a comparator group. There were also no restrictions on language, time period, or clinical context in which the study was conducted.
Search strategy
The search identified studies through bibliographic databases, trial registers, and the grey literature. Bibliographic databases and trial registers included the following: Medline (1946–present); PreMedline, PubMed; EMBASE (1974–present), Scopus, Web of Science (1900–current); PsychInfo (1806–present); CINAHL (1981–present); Cochrane libraries: Cochrane database of systematic reviews (2005–October 2016), Cochrane central register of controlled trials (August 2016), Cochrane Methodology register (3rd–Quarter 2012); other EBM databases: ACP journal club (1991–September 2016), Database of Abstracts of Reviews of Effects (1st-quarter 2015), Health technology assessment (3rd-quarter 2016), and NHS economic evaluation (1st-quarter 2015); Ageline (1978–present); ALOIS; AMED (Allied and Complementary Medicine; 1985–present); PEDro (Physiotherapy Evidence Database; 1929–present); Social work Abstracts (1968–present); and the National Association of Social Workers (NASW) clinical register (14th edition). The grey literature was searched using such resources as SIGLE (System for Information on Grey Literature in Europe), NTIS (National Technical Information Service) database, and PsychEXTRA (1908–present).
The search strategy used only population and intervention terms to maximise the likelihood of identifying relevant studies (comparator and outcome terms were not used). The population was people with Lewy body dementia or their carers. This was identified using the search terms: [(Lewy OR Park*) and Dementia]. Interventions were any non-pharmacological treatment and identified using a wide range of terms: (activit*, acupuncture, alternative, animal, aromatherapy, art therapy, assisted, balance, behav*, bicycle, calisthenics, carer intervention, caregiver intervention, CBT, Chi gong, cognit*, cognitive behavioral therapy, cognitive behavioural therapy, counsel*, creative arts, dance, dancing, diet, direct current stimulation, drama, ECT, educat*, electroconvulsive therapy, enhanc*, environmental intervention, environmental modification, exercise, flexibility, humor therapy, humour therapy, hydrotherapy, intervention*, leisure, light therapy, management, martial arts, massage, meditation, Montessori, multisensory, music, non-pharm*, nonpharm*, nutrition, occupational therapy, pet therapy, physical activity, physical therapy, physiotherapy, pilates, psychoeducation, psychol*, psychosocial, psychotherapy, Qi gong, reality orientation, recreation*, reminiscence, resistance training, run*, sensory, simulated presence, stimulation, Snoezelen, support*, support group*, swim*, tai chi, therap*, therapeutic activity, TMS, training, training carers, training caregivers, transcranial magnetic stimulation, treatment*, validation, weight training, yoga). Searches were conducted on 30 October 2016.
In addition to bibliographic database searches, the reference lists of papers included in the review and previous systematic reviews on both Lewy body dementia and non-pharmacological interventions were checked for relevant papers. Advice was also sought from experts in the field.
Study selection
Two reviewers (MHC and LQ) independently assessed search results for inclusion by title and abstract. All articles deemed relevant by either reviewer were obtained in full. Both reviewers then independently evaluated full-text articles for inclusion. Any disagreements were resolved through discussion or, if necessary, with a third reviewer (JTO).
Data extraction
Two reviewers independently extracted relevant data from publications using a standardised form. This included participant details (e.g. demographics, number, recruitment, clinical context, dementia severity), intervention type, study design, measures, and results. Qualitative data were also collated.
The primary outcomes were measures of cognition, function, neuropsychiatric symptoms, and motor symptoms. The secondary outcomes were measures of any other clinically relevant outcomes, such as quality of life, carer burden, financial costs, other symptoms (sleep or autonomic disturbances), and objective endpoints (e.g. falls, hospitalisation, institutionalisation, mortality). Secondary outcomes also included the perceived acceptability of treatments, reported side effects, and dropout rates (a measure of treatment acceptability).
Quality assessment
Two reviewers independently assessed study quality and risk of bias using standardised tools. These included the Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies (Effective Public Health Practice Project, 2007; Armijo-Olivo et al. Reference Armijo-Olivo, Stiles, Hagen, Biondo and Cummings2012) and the NICE Methodology Checklist: Qualitative Studies (NICE, 2016). Any disagreements were resolved through discussion.
Data synthesis
Although a quantitative synthesis of findings using meta-analytic techniques was originally intended, this was not possible due to the small sample sizes and poor quality of the included studies. As a result, only a descriptive synthesis was provided.
Results
Search results
The search identified 73 093 publications, of which 30 419 were unique and 42 674 were duplicates. Of these, 76 were considered by one or both reviewers to be potentially relevant and obtained in full. In turn, 21 studies were found eligible for inclusion (a flow diagram in PRISMA format is shown in Fig. 1). The included studies consisted of 12 case studies (Kung & O'Connor, Reference Kung and O'Connor2002; Loher et al. Reference Loher, Krauss, Wielepp, Weber and Burgunder2002; Graff et al. Reference Graff, Vernooij-Dassen, Zajec, Olde-Rikkert, Hoefnagels and Dekker2006; Cheston et al. Reference Cheston, Thorne, Whitby and Peak2007; Huh et al. Reference Huh, Areán, Bornfeld and Elite-Marcandonatou2008; Freund et al. Reference Freund, Kuhn, Lenartz, Mai, Schnell, Klosterkoetter and Sturm2009; Ciro et al. Reference Ciro, Hershey and Garrison2013; Gil-Ruiz et al. Reference Gil-Ruiz, Osorio, Cruz, Agüera-Ortiz, Olazarán, Sacks, Álvarez-Linera and Martínez-Martín2013; Tabak et al. Reference Tabak, Aquije and Fisher2013; Dawley, Reference Dawley2015; Hsu et al. Reference Hsu, Flowerdew, Parker, Fachner and Odell-Miller2015; Ricciardi et al. Reference Ricciardi, Piano, Bentivoglio and Fasano2015), seven case series (Rasmussen et al. Reference Rasmussen, Russell, Kung, Rummans, Rae-Stuart and Kevin O'Connor2003; Rochester et al. Reference Rochester, Burn, Woods, Godwin and Nieuwboer2009; Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009; Ota et al. Reference Ota, Iseki, Murayama, Fujishiro, Arai and Sato2012; Elder et al. Reference Elder, Firbank, Kumar, Chatterjee, Chakraborty, Dutt and Taylor2016; Yamaguchi et al. Reference Yamaguchi, Matsuoka, Ueda, Takada, Takahashi, Kiuchi, Hashimoto, Kosaka, Yasuno and Kishimoto2016) – two of which were reported in the same paper (Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009) – and two randomised controlled trials (Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008; Telenius et al. Reference Telenius, Engedal and Bergland2015). Both randomised trials focused on the broader conditions of dementia and/or Parkinson's disease but had separate results for a Lewy body dementia subgroup available. One case report was reported in two separate papers (Freund et al. Reference Freund, Kuhn, Lenartz, Mai, Schnell, Klosterkoetter and Sturm2009; Barnikol et al. Reference Barnikol, Pawelczyk, Barnikol, Kuhn, Lenartz, Sturm, Tass and Freund2010); our descriptive synthesis focused on the more detailed of these papers (Freund et al. Reference Freund, Kuhn, Lenartz, Mai, Schnell, Klosterkoetter and Sturm2009). A further case report on electroconvulsive therapy was excluded (Fujiwara et al. Reference Fujiwara, Honda, Ito and Koyama2004) because of a concurrent pharmacological intervention. Twelve studies focused on dementia with Lewy bodies (Kung & O'Connor, Reference Kung and O'Connor2002; Rasmussen et al. Reference Rasmussen, Russell, Kung, Rummans, Rae-Stuart and Kevin O'Connor2003; Cheston et al. Reference Cheston, Thorne, Whitby and Peak2007; Huh et al. Reference Huh, Areán, Bornfeld and Elite-Marcandonatou2008; Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009; Ota et al. Reference Ota, Iseki, Murayama, Fujishiro, Arai and Sato2012; Ciro et al. Reference Ciro, Hershey and Garrison2013; Gil-Ruiz et al. Reference Gil-Ruiz, Osorio, Cruz, Agüera-Ortiz, Olazarán, Sacks, Álvarez-Linera and Martínez-Martín2013; Hsu et al. Reference Hsu, Flowerdew, Parker, Fachner and Odell-Miller2015; Telenius et al. Reference Telenius, Engedal and Bergland2015; Yamaguchi et al. Reference Yamaguchi, Matsuoka, Ueda, Takada, Takahashi, Kiuchi, Hashimoto, Kosaka, Yasuno and Kishimoto2016), eight focused on Parkinson's disease dementia (Loher et al. Reference Loher, Krauss, Wielepp, Weber and Burgunder2002; Graff et al. Reference Graff, Vernooij-Dassen, Zajec, Olde-Rikkert, Hoefnagels and Dekker2006; Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008; Freund et al. Reference Freund, Kuhn, Lenartz, Mai, Schnell, Klosterkoetter and Sturm2009; Rochester et al. Reference Rochester, Burn, Woods, Godwin and Nieuwboer2009; Tabak et al. Reference Tabak, Aquije and Fisher2013; Dawley, Reference Dawley2015; Ricciardi et al. Reference Ricciardi, Piano, Bentivoglio and Fasano2015), and one included patients from both groups (Elder et al. Reference Elder, Firbank, Kumar, Chatterjee, Chakraborty, Dutt and Taylor2016). A summary of studies is shown in Table 1.
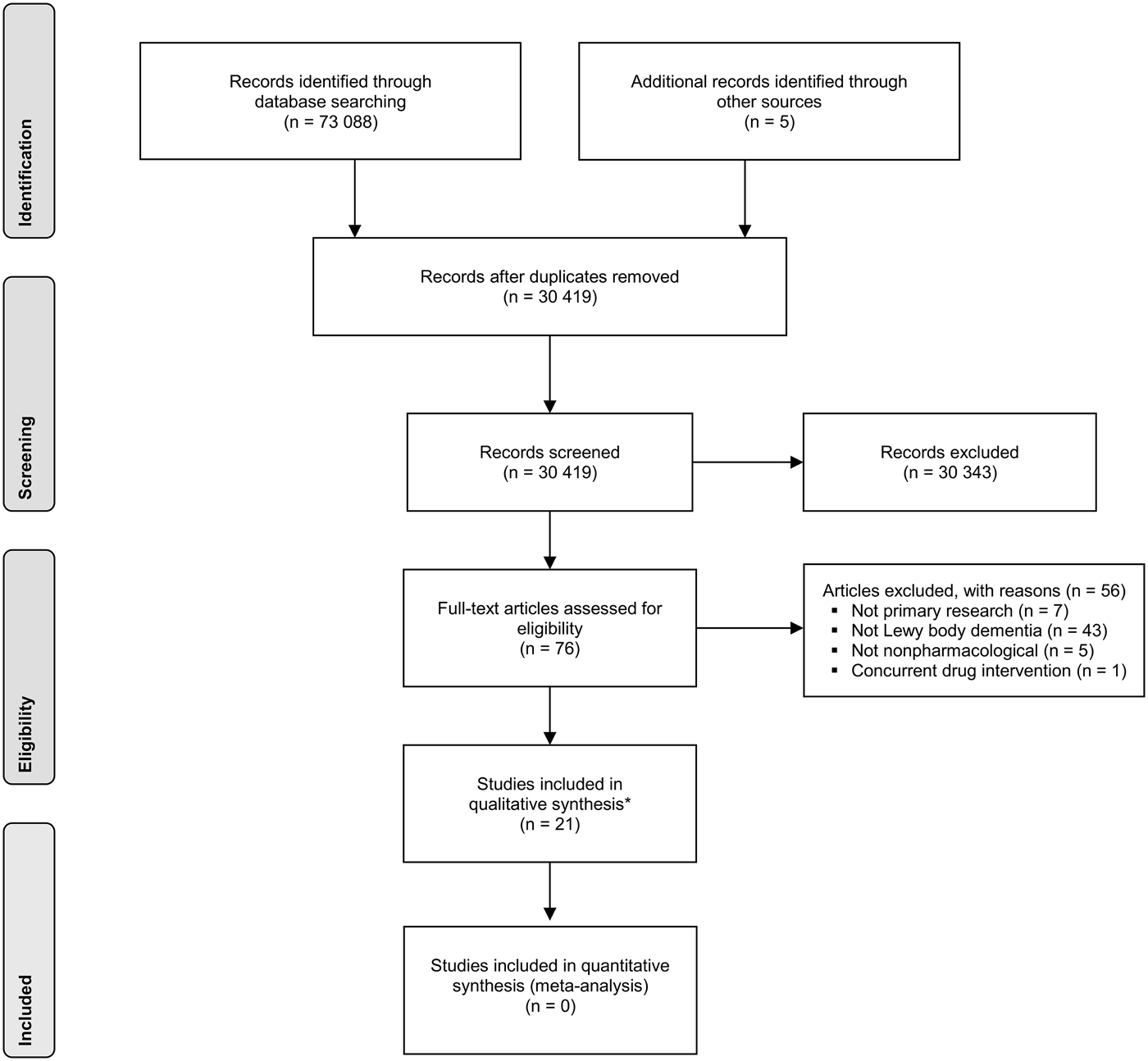
Fig. 1. PRISMA flow chart for study selection. *Two of the studies were reported in the same article.
Table 1. Studies assessing a non-pharmacological intervention for Lewy body dementia
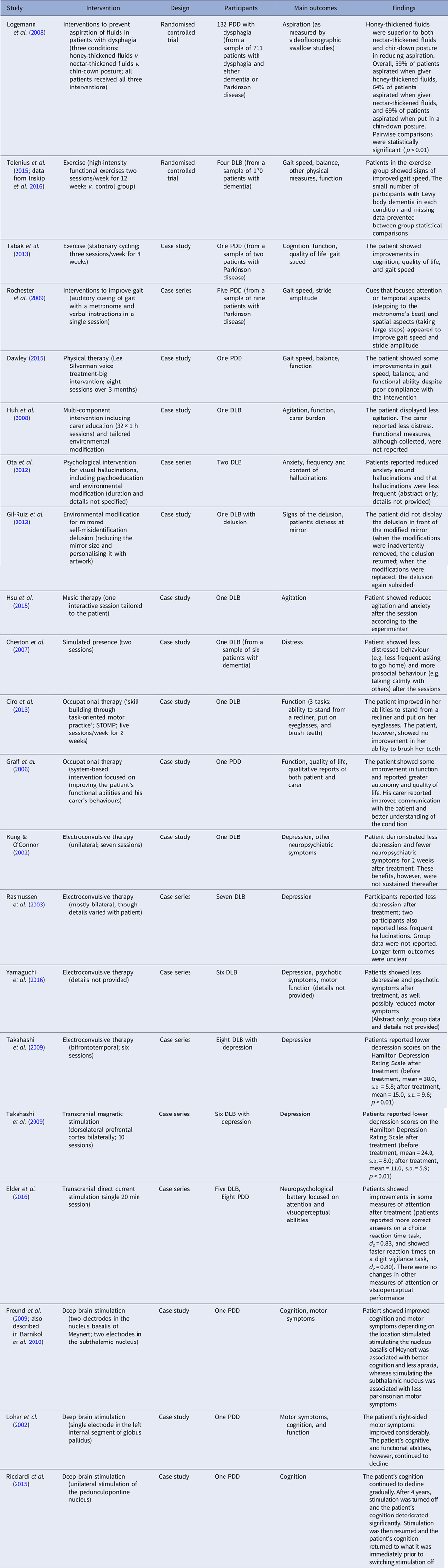
DLB, dementia with Lewy bodies; PDD, Parkinson's disease dementia.
Quality assessment
Of the 21 studies, 19 were rated as poor quality due to their small sample size, uncertainties about recruitment, and the lack of a control group. One randomised controlled trial, which focused on exercise in nursing homes for patients with dementia (Telenius et al. Reference Telenius, Engedal and Bergland2015), was evaluated as poor quality with respect to Lewy body dementia specifically (it only included four participants with the condition and these participants did not complete all tasks) (Inskip et al. Reference Inskip, Mavros, Sachdev and Fiatarone Singh2016). The other randomised controlled trial (Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008), which investigated dietary fluid and postural interventions for dysphagia, was evaluated as moderate quality. This study, however, was limited by its lack of blinding and the fact that it relied on measures of immediate efficacy in a research setting, without any longer term follow-up or measures of real-world effectiveness. It was also limited by the absence of a control group that did not receive a treatment, making it difficult to establish the absolute effectiveness of the interventions.
Participants
The total number of participants across the included studies was 195. This comprised 44 patients with dementia with Lewy bodies and 151 patients with Parkinson's disease dementia (of whom, 132 were from one study; Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008). Demographic information was not consistently reported. It was not provided for the subgroup of 132 patients with Parkinson's disease dementia in the largest study (Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008) and for two case series (Ota et al. Reference Ota, Iseki, Murayama, Fujishiro, Arai and Sato2012; Yamaguchi et al. Reference Yamaguchi, Matsuoka, Ueda, Takada, Takahashi, Kiuchi, Hashimoto, Kosaka, Yasuno and Kishimoto2016) – altogether 140 participants. Of the data available (n = 55), the mean age of participants was 70.6 years (s.d. = 8.2) and included 27 males and 28 females. Measures of patients’ cognition and dementia severity were lacking from the majority of reports. Of the 21 studies, nine recruited participants from nursing homes or hospitals, five recruited participants from the community, and seven did not report participants’ residential status. One of the studies that recruited participants from nursing homes examined the effect of an intervention on both a patient with dementia with Lewy bodies and her carers (Huh et al. Reference Huh, Areán, Bornfeld and Elite-Marcandonatou2008).
Interventions
Interventions included carer education (Huh et al. Reference Huh, Areán, Bornfeld and Elite-Marcandonatou2008), psychological interventions (for visual hallucinations) (Ota et al. Reference Ota, Iseki, Murayama, Fujishiro, Arai and Sato2012), physical exercise (Tabak et al. Reference Tabak, Aquije and Fisher2013; Telenius et al. Reference Telenius, Engedal and Bergland2015), gait cueing (Rochester et al. Reference Rochester, Burn, Woods, Godwin and Nieuwboer2009), environmental modification (Gil-Ruiz et al. Reference Gil-Ruiz, Osorio, Cruz, Agüera-Ortiz, Olazarán, Sacks, Álvarez-Linera and Martínez-Martín2013) (for mirrored self-misidentification, a delusion that usually occurs in the context of dementia; Connors & Coltheart, Reference Connors and Coltheart2011; Connors et al. Reference Connors, Langdon, Coltheart, Bhugra and Malhi2015), music (Hsu et al. Reference Hsu, Flowerdew, Parker, Fachner and Odell-Miller2015), simulated presence (Cheston et al. Reference Cheston, Thorne, Whitby and Peak2007), occupational therapy (Graff et al. Reference Graff, Vernooij-Dassen, Zajec, Olde-Rikkert, Hoefnagels and Dekker2006; Ciro et al. Reference Ciro, Hershey and Garrison2013), physical therapy (Dawley, Reference Dawley2015), dietary fluid and postural interventions to prevent aspiration (Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008), electroconvulsive therapy (Kung & O'Connor, Reference Kung and O'Connor2002; Rasmussen et al. Reference Rasmussen, Russell, Kung, Rummans, Rae-Stuart and Kevin O'Connor2003; Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009; Yamaguchi et al. Reference Yamaguchi, Matsuoka, Ueda, Takada, Takahashi, Kiuchi, Hashimoto, Kosaka, Yasuno and Kishimoto2016), transcranial magnetic stimulation (Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009), transcranial direct current stimulation (Elder et al. Reference Elder, Firbank, Kumar, Chatterjee, Chakraborty, Dutt and Taylor2016), and deep brain stimulation (Loher et al. Reference Loher, Krauss, Wielepp, Weber and Burgunder2002; Freund et al. Reference Freund, Kuhn, Lenartz, Mai, Schnell, Klosterkoetter and Sturm2009; Ricciardi et al. Reference Ricciardi, Piano, Bentivoglio and Fasano2015). The most studied interventions were electroconvulsive therapy (four studies) and deep brain stimulation (three studies).
Effectiveness of interventions
Given the heterogeneity of interventions and the poor quality of the research evidence, no quantitative synthesis was possible. A descriptive summary of individual studies is provided in Table 1.
All studies reported some effectiveness of their respective intervention. The strongest evidence came from the randomised control trial that assessed interventions to prevent fluid aspiration in participants with dysphagia (Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008). This trial compared honey-thickened fluids, nectar-thickened fluids, and a chin-down posture in 132 patients with Parkinson's disease dementia (see Table 1). It found that the use of honey-thickened fluids was superior to the other two methods in preventing fluid aspiration as measured by videofluorographic swallow studies.
Four small uncontrolled studies (Kung & O'Connor, Reference Kung and O'Connor2002; Rasmussen et al. Reference Rasmussen, Russell, Kung, Rummans, Rae-Stuart and Kevin O'Connor2003; Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009; Yamaguchi et al. Reference Yamaguchi, Matsuoka, Ueda, Takada, Takahashi, Kiuchi, Hashimoto, Kosaka, Yasuno and Kishimoto2016) – involving 22 participants in total – found that electroconvulsive therapy had some effectiveness in treating depression in dementia with Lewy bodies. In one of these studies, two patients exhibited confusion immediately after electroconvulsive therapy (Rasmussen et al. Reference Rasmussen, Russell, Kung, Rummans, Rae-Stuart and Kevin O'Connor2003); in another study, an unspecified number displayed signs of autonomic dysfunction, though without any lasting effects (Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009). No other adverse events were reported. Another three case studies of deep brain stimulation (Loher et al. Reference Loher, Krauss, Wielepp, Weber and Burgunder2002; Freund et al. Reference Freund, Kuhn, Lenartz, Mai, Schnell, Klosterkoetter and Sturm2009; Ricciardi et al. Reference Ricciardi, Piano, Bentivoglio and Fasano2015) identified benefits in either cognition or motor symptoms depending on the location of stimulation in patients with Parkinson's disease dementia. All three studies used different brain locations for stimulation. None of these studies reported adverse effects.
Other individual studies variously reported benefits of carer education for reducing agitation (Huh et al. Reference Huh, Areán, Bornfeld and Elite-Marcandonatou2008); psychological interventions for reducing distress around visual hallucinations (Ota et al. Reference Ota, Iseki, Murayama, Fujishiro, Arai and Sato2012); physical exercise to improve gait and function (Tabak et al. Reference Tabak, Aquije and Fisher2013; Telenius et al. Reference Telenius, Engedal and Bergland2015); gait cueing to improve gait speed (Rochester et al. Reference Rochester, Burn, Woods, Godwin and Nieuwboer2009); environmental modification to reduce the distress associated with mirrored self-misidentification delusion (Gil-Ruiz et al. Reference Gil-Ruiz, Osorio, Cruz, Agüera-Ortiz, Olazarán, Sacks, Álvarez-Linera and Martínez-Martín2013); music therapy to reduce agitation (Hsu et al. Reference Hsu, Flowerdew, Parker, Fachner and Odell-Miller2015); simulated presence to reduce distress (Cheston et al. Reference Cheston, Thorne, Whitby and Peak2007); occupational therapy interventions to improve functional ability (Graff et al. Reference Graff, Vernooij-Dassen, Zajec, Olde-Rikkert, Hoefnagels and Dekker2006; Ciro et al. Reference Ciro, Hershey and Garrison2013); physical therapy to improve gait (Dawley, Reference Dawley2015); dietary fluid and postural interventions to prevent aspiration (Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008); transcranial magnetic stimulation to reduce depression (Takahashi et al. Reference Takahashi, Mizukami, Yasuno and Asada2009); and transcranial direct current stimulation to improve attention (Elder et al. Reference Elder, Firbank, Kumar, Chatterjee, Chakraborty, Dutt and Taylor2016) (see Table 1). For all these studies, however, limitations in their design, including small sample sizes, lack of blinding, and lack of adequate controls, mean that it is not possible to rule out confounding, selection bias, experimenter expectancy, publication bias, and other causes for the reported effects.
Discussion
The systematic review identified very little research on non-pharmacological interventions in Lewy body dementia. No randomised control trials focusing specifically on Lewy body dementia were identified and the majority of research consisted of case studies and case series. Of the studies identified, the best quality evidence came from a randomised control trial that focused on preventing fluid aspiration in Parkinson's disease patients with dysphagia (Logemann et al. Reference Logemann, Gensler, Robbins, Lindblad, Brandt, Hind, Kosek, Dikeman, Kazandjian, Gramigna, Lundy, McGarvey-Toler and Gardner2008). As already noted, however, even this study was limited by the fact that it only examined the immediate effects of interventions in an experimental context and did not assess longer term effectiveness in more naturalistic settings. Overall, given the heterogeneity of interventions, small sample sizes, and poor quality of research, no treatment recommendations can be offered.
The lack of research in this area is in contrast to the large amount of research on non-pharmacological interventions in other types of dementia (Brodaty & Arasaratnam, Reference Brodaty and Arasaratnam2012; Livingston et al. Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris, Patel, Omar, Katona and Cooper2014; Orgeta et al. Reference Orgeta, Qazi, Spector and Orrell2015; Livingston & Cooper, Reference Livingston, Cooper, Ames, O'Brien and Burns2017; Seeher & Brodaty, Reference Seeher, Brodaty, Ames, O'Brien and Burns2017). It is consistent, though, with what has been found in other systematic reviews on Lewy body dementia (Hindle et al. Reference Hindle, Petrelli, Clare and Kalbe2013; Stinton et al. Reference Stinton, McKeith, Taylor, Lafortune, Mioshi, Mak, Cambridge, Mason, Thomas and O'Brien2015; Inskip et al. Reference Inskip, Mavros, Sachdev and Fiatarone Singh2016). Despite the lack of research, however, non-pharmacological interventions are likely to be important in the management of Lewy body dementia. As already noted, pharmacological treatment of psychotic symptoms and movement disturbances in this condition is limited by the difficulties patients have tolerating first-line medications.
At the same time, there is evidence for the effectiveness of non-pharmacological interventions for similar symptoms in other conditions. In the case of psychosis, for example, multi-factorial interventions, including physical activity, occupational therapy, and music therapy, have some evidence of effectiveness in other types of dementia (Brodaty & Arasaratnam, Reference Brodaty and Arasaratnam2012; Chen et al. Reference Chen, Liu, Lin, Peng, Chen, Liu and Chen2014). In the case of motor disturbances, interventions such as exercise and gait training have been found to be effective in Parkinson's disease (Bloem et al. Reference Bloem, de Vries and Ebersbach2015). There is also strong evidence that non-pharmacological interventions are effective at ameliorating symptoms that are common across different dementias (Brodaty & Arasaratnam, Reference Brodaty and Arasaratnam2012; Livingston et al. Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris, Patel, Omar, Katona and Cooper2014; Orgeta et al. Reference Orgeta, Qazi, Spector and Orrell2015; Seeher & Brodaty, Reference Seeher, Brodaty, Ames, O'Brien and Burns2017). Caregiver education, training, and support, for example, have been shown to reduce both carers’ distress and patients’ neuropsychiatric symptoms (Brodaty & Arasaratnam, Reference Brodaty and Arasaratnam2012; Seeher & Brodaty, Reference Seeher, Brodaty, Ames, O'Brien and Burns2017). Likewise, psychological interventions, such as cognitive behavioural therapy, have been shown to reduce patients’ depression (Orgeta et al. Reference Orgeta, Qazi, Spector and Orrell2015). Finally, organised activities, music therapy, and sensory stimulation have been shown to reduce patients’ agitation (Livingston et al. Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris, Patel, Omar, Katona and Cooper2014). All of these interventions could be investigated more systematically in Lewy body dementia.
Altogether, the review highlights the clear and urgent need for research in this area. Possible barriers to this include challenges establishing the diagnosis and distinguishing it from other dementias (McKeith et al. Reference McKeith, Dickson, Lowe, Emre, O'Brien, Feldman, Cummings, Duda, Lippa, Perry, Aarsland, Arai, Ballard, Boeve, Burn, Costa, Del Ser, Dubois, Galasko, Gauthier, Goetz, Gomez-Tortosa, Halliday, Hansen, Hardy, Iwatsubo, Kalaria, Kaufer, Kenny, Korczyn, Kosaka, Lee, Lees, Litvan, Londos, Lopez, Minoshima, Mizuno, Molina, Mukaetova-Ladinska, Pasquier, Perry, Schulz, Trojanowski and Yamada2005; Vann Jones & O'Brien, Reference Vann Jones and O'Brien2014); the limited research resources devoted to Lewy body dementia as opposed to other neurodegenerative conditions; and the general lack of funding for non-pharmacological interventions. Each of these, however, is not insurmountable. The use of multi-centre registries or networks of centres with expertise in Lewy body dementia, for example, could help to address the problem of recruitment. Given the prevalence of the disease, its disproportionately high social and economic burden (Murman et al. Reference Murman, Kuo, Powell and Colenda2003; Boström et al. Reference Boström, Jönsson, Minthon and Londos2007; Vossius et al. Reference Vossius, Rongve, Testad, Wimo and Aarsland2014; Svendsboe et al. Reference Svendsboe, Terum, Testad, Aarsland, Ulstein, Corbett and Rongve2016), and the limited suitability of medications, future research into non-pharmacological interventions is important to improving management at both individual and population levels.
Acknowledgements
This review presents independent research from the DIAMOND-Lewy study funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (DTC-RP-PG-0311-12001). The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health.
Conflicts of interest
JOB has acted as a consultant or received grant income from GE Healthcare, Avid/Lilly, TauRx, Axon, and Axovant. HB has worked on drug trials for patients with Alzheimer's disease sponsored by major pharmaceutical companies including Eisai Pharmaceuticals, Eli Lilly and Company, GlaxoSmithKline, H Lundbeck A/S, Janssen-Cilag Pty Limited, Medivation Inc., Novartis Pharmaceuticals, Nutricia, Pfizer Inc., Prana Biotechnology Limited, Sanofi-Aventis, Voyager Pharmaceutical Corporation, and Wyeth Limited. HB has also been a consultant, advisory board member, and sponsored speaker for Baxter, H Lundbeck A/S, Janssen-Cilag Pty Limited, Medivation Inc., Novartis Pharmaceuticals, Pfizer Inc., Prana Biotechnology Limited, Voyager Pharmaceutical Corporation, and Wyeth Limited. J-PT has acted as a consultant and been paid speaker fees by GE Healthcare, Axovant, and Shire Pharmaceutical.
MHC, LQ, IM, LA, CB, and AT have no conflicts of interest to declare.
Author contributions
MHC conducted the search, reviewed papers for inclusion and quality, extracted data, and wrote the paper. LQ reviewed papers for inclusion and quality and extracted data. JTO supervised the project, which all authors were involved in conceptualising. All authors revised the manuscript for critically meaningful content and approved the final version.




