Antidepressant medications are at the forefront of the management of depressive disorders in adults. Reference Gelenberg, Freeman, Markowitz, Rosenbaum, Thase and Trivedi1 Their use reduces the severity of symptoms and promotes the remission of depressive episodes, Reference Wilson, Mottram, Sivanranthan and Nightingale2-Reference Cipriani, Furukawa, Salanti, Geddes, Higgins and Churchill4 but as many as 50% of patients fail to respond satisfactorily to first-line antidepressant treatment. Reference Rush, Trivedi, Wisniewski, Nierenberg, Stewart and Warden5 Attempts to augment response to antidepressants with other agents have produced inconsistent results, and findings have been difficult to generalise because of the characteristics of study samples (predominantly participants with treatment-resistant illness) and diversity of the approaches tested. Reference Fleurence, Williamson, Jing, Kim, Tran and Pikalov6-Reference Rush, Trivedi, Stewart, Nierenberg, Fava and Kurian8 This lack of success in enhancing the efficacy of antidepressant treatment is, in part, because of our fragmented understanding of the physiological pathways that lead to the development and maintenance of depressive symptoms. Nonetheless, advances have been made. A handful of clinical investigations in the 1960s reported that mental disorders, including depression, were associated with folate deficiency, Reference Carney9 a finding that was later confirmed by Reynolds and colleagues in a convenience sample of 101 British adults with depression. Reference Reynolds, Preece, Bailey and Coppen10 Subsequently, it became apparent that a large proportion of people with megaloblastic anaemia showed evidence of clinically significant depressive symptoms, either in association with deficiency of folate (vitamin B9) or vitamin B12, Reference Shorvon, Carney, Chanarin and Reynolds11 leading to suggestions that these B vitamins could potentially lie on the causal pathway that leads to depression. Reference Fava, Borus, Alpert, Nierenberg, Rosenbaum and Bottiglieri12 Folate is a co-substrate of various cell reactions involved in methylation and synthesis of nucleic acid and neurotransmitters, such as serotonin, dopamine and noradrenaline. Reference Smith, Kim and Refsum13 The intracellular concentrations of folate and vitamin B12 can be inferred, indirectly, through the total plasma concentration of homocysteine (tHcy), which can be converted to methionine through an enzymatic reaction that uses 5-methyletetrahydrofolate as the methyl donor group. Reference Smith, Kim and Refsum13 Consequently, the plasma concentration of homocysteine falls as the intracellular concentration of various folates and vitamin B12 rises. Numerous observational studies have since confirmed that depression, particularly in later life, is associated with relatively low concentrations of folate and vitamin B12, as well as with high tHcy, Reference Kim, Stewart, Kim, Yang, Shin and Yoon14-Reference Beydoun, Shroff, Beydoun and Zonderman17 although vitamin supplementation has negligible effects on the mood of adults and older adults free of clinically significant depressive symptoms. Reference Andreeva, Galan, Torres, Julia, Hercberg and Kesse-Guyot18-Reference Ford, Flicker, Thomas, Norman, Jamrozik and Almeida20
A meta-analysis of genetic association studies showed that a common polymorphism of the methylenetetrahydrofolate reductase (MTHFR) enzyme that increases the basal concentration of tHcy also increases the risk of depression, Reference Almeida, McCaul, Hankey, Norman, Jamrozik and Flicker21 suggesting that high tHcy lies on the causal pathway that leads to depression in later life. The findings of four small double-blind clinical trials published in the mid 1980s and 1990s were generally consistent with a causal relationship between high tHcy and depression: they reported that folate supplementation improves response to antidepressant treatment, albeit inconsistently. Reference Coppen, Chaudhry and Swade22-Reference Coppen and Bailey25 More recently, a secondary analysis of a randomised, placebo-controlled, double-blind trial of folate, vitamin B12 and vitamin B6 to prevent cardiovascular events among stroke survivors showed that participants assigned vitamins had lower risk of experiencing a depressive episode over 7 years than those treated with placebo. Reference Almeida, Marsh, Alfonso, Flicker, Davis and Hankey26 Data from a randomised, double-blind, placebo-controlled augmentation trial of 75 adults with major depression resistant to treatment with a selective serotonin reuptake inhibitor (SSRI) showed that 15 mg/day of l-methylfolate decreased the severity of symptoms and increased remission over 60 days. Reference Papakostas, Shelton, Zajecka, Etemad, Rickels and Clain27 These results suggest that the use of folate and vitamin B12 may increase the efficacy of standard antidepressant treatment in an unselected sample of adults with major depression and, potentially, prevent relapse over time. Reference Papakostas, Petersen, Mischoulon, Green, Nierenberg and Bottiglieri28 However, supportive trial evidence is not currently available. B-VITAGE was a randomised, double-blind, placebo-controlled trial designed to assess the short- and long-term efficacy of antidepressant treatment associated with B vitamins or placebo for 1 year (registered with the Australian and New Zealand Clinical Trials Registry (ANZCTR): 12609000256279). We hypothesised that a greater proportion of adults aged 50 years or over with major depression assigned treatment with citalopram and vitamins B6, B12 and folate would experience remission of symptoms after 12 and 52 weeks than would controls treated with citalopram and placebo. We also hypothesised that relapse after remission of symptoms by week 12 would be more frequent among participants treated with placebo than with vitamins.
Method
Trial design
B-VITAGE was a 1-year parallel, randomised, double-blind, controlled trial of citalopram and adjunctive treatment with vitamins B6, B12 and folate or placebo. The allocation ratio was 1:1. Three weeks after the trial commenced, the lower age limit of participants was decreased from 60 to 50 years to facilitate recruitment and increase the generalisability of findings. The Human Ethics Committee of the Royal Perth Hospital approved the study protocol Reference Ford, Flicker, McCaul, van Bockxmeer, Hegarty and Hirani29 and all participants provided written informed consent.
Participants
Eligibility criteria included: (a) age 50 years or over, (b) major depressive episode in the context of a major depressive disorder (single episode or recurrent) according to DSM-IV-TR criteria; 30 (c) a Montgomery-Åsberg Depression Rating Scale (MADRS) score ⩾20; Reference Montgomery and Asberg31 (d) fluency in written and spoken English; (e) Alcohol Use Disorders Identification Test (AUDIT) ⩽15; Reference Bohn, Babor and Kranzler32 (f) Mini-Mental State Examination (MMSE) score ⩾24; Reference Folstein, Folstein and McHugh33 (g) negative clinical history for stroke or neurodegenerative diseases (such as Parkinson’s disease); (h) negative clinical history of allergic reactions to citalopram or escitalopram; (i) negative clinical history of life-threatening illness likely to compromise 1-year survival (such as metastatic cancer); (j) no evidence of prominent psychotic symptoms or suicidal intent; (k) negative clinical history for schizophrenia, schizoaffective disorder or bipolar disorder; and (l) not undergoing electroconvulsive therapy or using antidepressants at the baseline assessment.
Participants were community-dwelling adults living in the Perth metropolitan region. We used the electoral roll (voting is compulsory in Australia) to send information about the study and a brief screening questionnaire that included the Patient Health Questionnaire (PHQ-9). Reference Kroenke, Spitzer and Williams34 Thirty-five participants were referred by local general practitioners (GPs). Consenting adults interested in the study posted the screening questionnaire back to the research office and those with PHQ-9 ⩾10 and AUDIT ⩽15 were invited for a face-to-face assessment at the Royal Perth Hospital that included assessment with the Mini-International Neuropsychiatric Interview (MINI) structured clinical interview, which produces diagnostic groupings consistent with DSM-IV-TR criteria. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller35
Interventions
Eligible participants were randomly assigned to treatment with citalopram plus 0.5 mg of vitamin B12, 2 mg of folic acid and 25 mg of vitamin B6, or citalopram plus placebo. The prescribed dosages of vitamins are effective in reducing tHcy by about 20%. Reference Flicker, Vasikaran, Thomas, Acres, Norman and Jamrozik36 Citalopram was introduced at a daily dosage of 10 mg and, 2 weeks later, it was increased to 20 mg. Flexible adjustment of the daily dosage of citalopram occurred at 4 and 8 weeks up to a maximum of 40 mg. These adjustments were guided by the persistence of depressive symptoms and side-effects of treatment, as determined by a certified psychiatrist. After 12 weeks, the management of antidepressant treatment was devolved to the GP of participants, who could choose to maintain the citalopram or change antidepressants. We advised GPs to maintain treatment with citalopram for an additional 9 months for patients whose depressive episode had remitted after 3 months. Participants were actively encouraged to maintain treatment with vitamins or placebo for 52 weeks. The Royal Perth Hospital Pharmacy dispensed the vitamins/placebo at baseline, 4, 8, 12 and 26 weeks. Vitamins and placebo were administered in the form of one daily capsule consumed after breakfast.
Participants of this trial were explicitly instructed not to use vitamin supplements for the duration of the study.
Outcomes
The primary outcome of interest of this study was remission of DSM-IV-TR major depressive episode after 12, 26 and 52 weeks of treatment, as assessed by the MINI. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller35 A reduction of 50% or more in the MADRS scores over the same period was another outcome of interest. Reference Montgomery and Asberg31 Secondary outcomes included reduction of MADRS scores over time (4, 8, 12, 26 and 52 weeks), relapse of major depressive symptoms following remission by 12 weeks (26 and 52 weeks) and change from citalopram to another antidepressant.
We collected data on age (in years), gender, place of birth, marital status, past self-reported clinical history of cardiovascular diseases (such as coronary heart disease), chronic respiratory diseases (such as asthma or emphysema), diabetes, hearing impairment and history of past depression. More specifically, we asked: ‘Have you ever been told by a doctor that you had depression?’ Possible answers to this question were ‘yes’ or ‘no’. Participants maintained a medication diary for the duration of the study, where they recorded all medications consumed each day of the year (including changes of antidepressants). In addition, bottles containing the study tablets (citalopram for 12 weeks and vitamins/placebo for 52 weeks) were returned to the pharmacy at each assessment (4, 8, 12, 26 and 52 weeks) and the number of pills dispensed, consumed and returned were recorded. Individuals who had consumed at least 75% of the study tablets prescribed were considered to have adhered to treatment.
During the face-to-face assessments (weeks 4, 8, 12, 26 and 52) participants were asked about adverse effects experienced since their last visit, and rated their presence as ‘not at all or a little’ or ‘quite a bit or a lot’ for: tremor of the hands, muscle stiffness, involuntary muscle contractions, muscle cramps, pins and needles in the body, difficulty concentrating, agitation or restlessness, irritability, dizzy of faint, headache, other pain, arthritis or pain in the joints, nausea, diarrhoea, constipation, vomiting, anorexia, weight loss, weight gain, skin rash, nightmares, excessive somnolence, poor sleep, palpitations, dry mouth, other such as delayed ejaculation or anorgasmia. Complaints that were present at baseline (i.e. before the start of treatment) and that persisted at the same level of intensity during follow-up were not attributed to treatment.
Fasting blood samples were collected at 08.00 h on the day of the baseline assessment, and again after 12, 26 and 52 weeks. We used chemiluminescent microparticle immunoassay (CMIA) technology to measure tHcy on an Architect i2000SR Analyser (Abbott Ireland Diagnostics Division, Lisnamuck, Longford Co., Longford, Ireland). The coefficient of variation of the assay ranges from 2.3 to 2.8%. Red cell folate and serum B12 were assayed with the Immulite 200 XPi (Siemens Healthcare Diagnostic Ltd. Llanberis, Gwynedd) and the Architect i2000SR Analyser. Coefficients of variations were 3.5 and 4.5-8.6%, respectively.
Sample size
We based our sample size calculations on published data from a trial that used folic acid together with fluoxetine for treatment of adults aged 18 years and older with depression. Reference Coppen and Bailey25 They reported that 64.7% of participants treated with fluoxetine and folic acid were free of symptoms after 10 weeks compared with 48.3% of those who had been treated with fluoxetine and placebo. We calculated that a study with 310 participants (155 in each treatment arm) would have 80% power to declare such a difference between the groups as significant (α: 5%, two-tailed). We estimated that 15% of participants would have been lost by the end of week 12, and another 10% by week 52, resulting in a target sample of 388 people (194 per group). Reference Ford, Flicker, McCaul, van Bockxmeer, Hegarty and Hirani29 We screened 2150 people, interviewed 478 and randomised 153 (Fig. 1). The addition of two panels of data at 26 and 52 weeks was expected to circumvent the possible loss of power caused by the lower than planned number of participants recruited. Reference Jonsson37

Fig. 1 Flow of trial participants from the time of screening to analysis.
PHQ-9, Patient Health Questionnaire; AUDIT, Alcohol Use Disorders Identification Test; MADRS, Montgomery-Åsberg Depression Rating Scale; MDE, Major Depressive Episode according to DSM-IV criteria.
Randomisation and masking
The pharmacy of the Royal Perth Hospital carried out the independent randomisation of participants according to a list of random numbers generated by computer in random permuted blocks of 6 to 16 (1:1 allocation). Vitamins and placebo were dispensed in the form of daily capsules that had the same size, shape, colour, texture, smell and taste. Blackmores Australia manufactured all capsules. Investigators and participants remained masked to treatment assignment until all participants had completed all assessments. In addition, both investigators and study participants remained masked to the results of biochemical analyses until the final collection of end-points in September 2013.
Statistical analyses
The data were managed and analysed using the statistical software package Stata version 13.0 for Mac. We used descriptive statistics to describe the sociodemographic and clinical characteristics of participants, and Pearson’s chi-squared tests, Mann-Whitney ranked sum tests and Student’s t-tests to compare their distribution among those assigned to placebo and vitamins. Outcomes were analysed as panel data and made use of all data available at each time point. (That is, all data, including available information for participants lost to follow-up, were entered in these models and contributed to its final intention-to-treat results. In this case, no assumptions were made about missing data that could potentially bias the study findings). We used xtlogit to analyse binary outcomes such as remission of depressive episode (yes/no) (effect estimate expressed as odds ratio (OR) and respective 95% confidence intervals) and multilevel mixed-effects linear regression (xtmixed) to analyse changes of MADRS scores over time (effect estimate expressed as mean change of score). These analyses were adjusted for gender and baseline tHcy imbalances. Finally, we used contingency tables to calculate the number needed to treat (NNT) for one person to benefit from vitamin treatment, taking into account the number of participants lost to follow-up. The NNT is the reverse of the absolute risk reduction.
Results
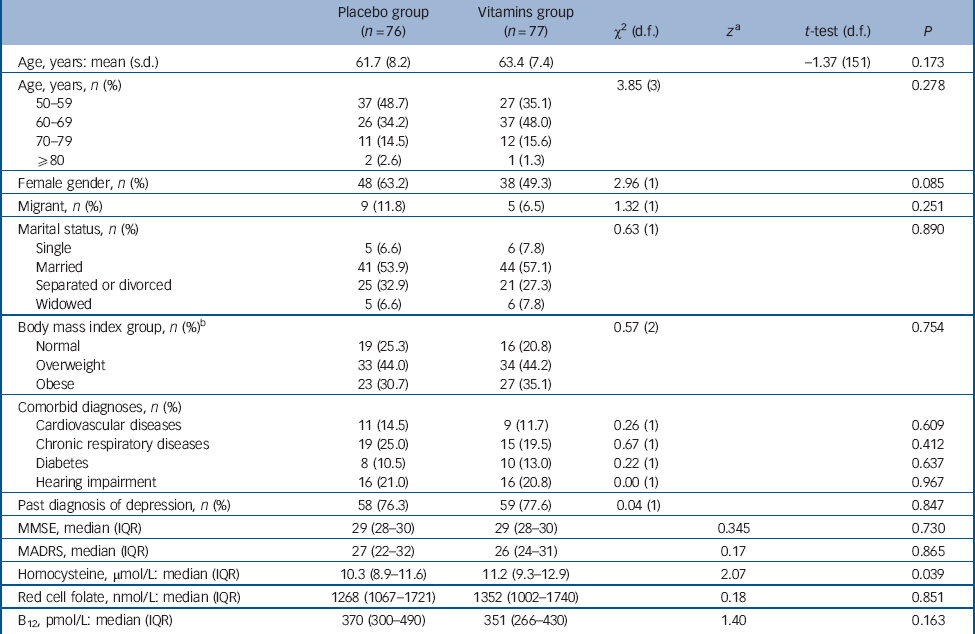
Figure 1 shows the flow of participants from screening to analysis. The first participant entered the study on the 31 March 2009 and the last exited the study on the 11 September 2013. The age of the 153 participants ranged from 50 to 85 years and 86 of them (56.2%) were women. Table 1 shows the characteristics of participants randomly allocated to treatment with placebo and vitamins at their baseline assessment. Treatment groups were well balanced for most measured factors, except for a non-significant excess of women in the placebo group and significantly higher tHcy among those assigned vitamins. None of the participants showed evidence of low concentration of red cell folate (<260 nmol/L) or of serum vitamin B12 (<140 pmol/L).
Intention-to-treat analysis using xtlogit showed that more people treated with vitamins than placebo experienced remission over 52 weeks (OR = 2.49, 95% CI 1.12-5.51, after adjustment for gender and baseline tHcy, Table 2). The interaction between time and intervention grouping was not statistically significant. Treatment with vitamins was associated with 6.5% absolute risk reduction of major depression by 52 weeks (taking into account loss to follow-up), indicating that 16 people would require treatment for one to benefit. These calculations assumed that loss to follow-up had occurred at random. If we assume that all participants lost to follow-up had negative outcomes (worst possible scenario), then the number needed to treat would have been 33. We also assessed the effect of treatment without adjusting the analyses for the gender and tHcy imbalance. The non-adjusted analyses showed a diminished effect of the intervention (OR = 1.76, 95% CI 0.81-3.82), mostly because women had significantly lower tHcy at baseline than men (mean difference 1.4, 95% CI 1.0-1.8, P<0.001). When we limited the unadjusted analyses to those in the highest 50 percentile of tHcy, the effect of the intervention was statistically significant (OR = 2.71, 95% CI 1.03-7.10) and consistent with the main results of the trial.
Table 1 Characteristics of participants with major depression at study entry according to their random assignment to treatment with citalopram + placebo or citalopram + vitamins.
| Placebo group (n = 76) |
Vitamins group (n = 77) |
χ2 (d.f.) | z Footnote a | t-test (d.f.) | P | |
|---|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 61.7 (8.2) | 63.4 (7.4) | -1.37 (151) | 0.173 | ||
| Age, years, n (%) | 3.85 (3) | 0.278 | ||||
| 50-59 | 37 (48.7) | 27 (35.1) | ||||
| 60-69 | 26 (34.2) | 37 (48.0) | ||||
| 70-79 | 11 (14.5) | 12 (15.6) | ||||
| ⩾80 | 2 (2.6) | 1 (1.3) | ||||
| Female gender, n (%) | 48 (63.2) | 38 (49.3) | 2.96 (1) | 0.085 | ||
| Migrant, n (%) | 9 (11.8) | 5 (6.5) | 1.32 (1) | 0.251 | ||
| Marital status, n (%) | 0.63 (1) | 0.890 | ||||
| Single | 5 (6.6) | 6 (7.8) | ||||
| Married | 41 (53.9) | 44 (57.1) | ||||
| Separated or divorced | 25 (32.9) | 21 (27.3) | ||||
| Widowed | 5 (6.6) | 6 (7.8) | ||||
| Body mass index group, n (%)Footnote b | 0.57 (2) | 0.754 | ||||
| Normal | 19 (25.3) | 16 (20.8) | ||||
| Overweight | 33 (44.0) | 34 (44.2) | ||||
| Obese | 23 (30.7) | 27 (35.1) | ||||
| Comorbid diagnoses, n (%) | ||||||
| Cardiovascular diseases | 11 (14.5) | 9 (11.7) | 0.26 (1) | 0.609 | ||
| Chronic respiratory diseases | 19 (25.0) | 15 (19.5) | 0.67 (1) | 0.412 | ||
| Diabetes | 8 (10.5) | 10 (13.0) | 0.22 (1) | 0.637 | ||
| Hearing impairment | 16 (21.0) | 16 (20.8) | 0.00 (1) | 0.967 | ||
| Past diagnosis of depression, n (%) | 58 (76.3) | 59 (77.6) | 0.04 (1) | 0.847 | ||
| MMSE, median (IQR) | 29 (28-30) | 29 (28-30) | 0.345 | 0.730 | ||
| MADRS, median (IQR) | 27 (22-32) | 26 (24-31) | 0.17 | 0.865 | ||
| Homocysteine, μmol/L: median (IQR) | 10.3 (8.9-11.6) | 11.2 (9.3-12.9) | 2.07 | 0.039 | ||
| Red cell folate, nmol/L: median (IQR) | 1268 (1067-1721) | 1352 (1002-1740) | 0.18 | 0.851 | ||
| B12, pmol/L: median (IQR) | 370 (300-490) | 351 (266-430) | 1.40 | 0.163 |
MMSE: Mini-Mental State Examination score; MADRS, Montgomery-Åsberg Depression Rating Scale score.
a. z-statistic derived from Mann-Whitney test adjusted for ties.
b. There was missing data for one person in the placebo group.
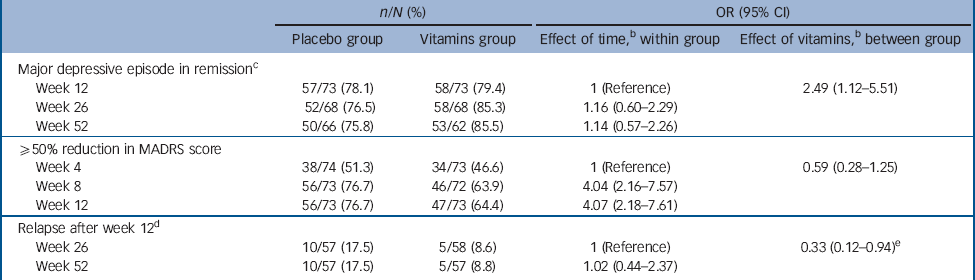
After 12 weeks of treatment, 78.1 and 79.4% of participants treated with citalopram plus placebo and citalopram plus vitamins no longer fulfilled criteria for a major depressive episode (P = 0.840, Table 2). The odds of remission in the vitamin group relative to the placebo group was 1.55 (95% CI 0.64-3.72, after adjustment for gender and baseline tHcy). By week 26 the adjusted odds of remission associated with vitamin treatment was 2.32 (95% CI 0.91-5.97), and by the end of 1 year 2.54 (95% CI 0.94-6.80). There was no evidence that adjunctive treatment with vitamins was associated with faster or more pronounced reduction of MADRS scores over 12 (Table 2) and 52 weeks (Fig. 2). However, among those who were no longer depressed by week 12, relapse of symptoms at 26 and 52 weeks was less frequent among participants assigned vitamins than placebo (OR = 0.33, 95% CI 0.12-0.94).
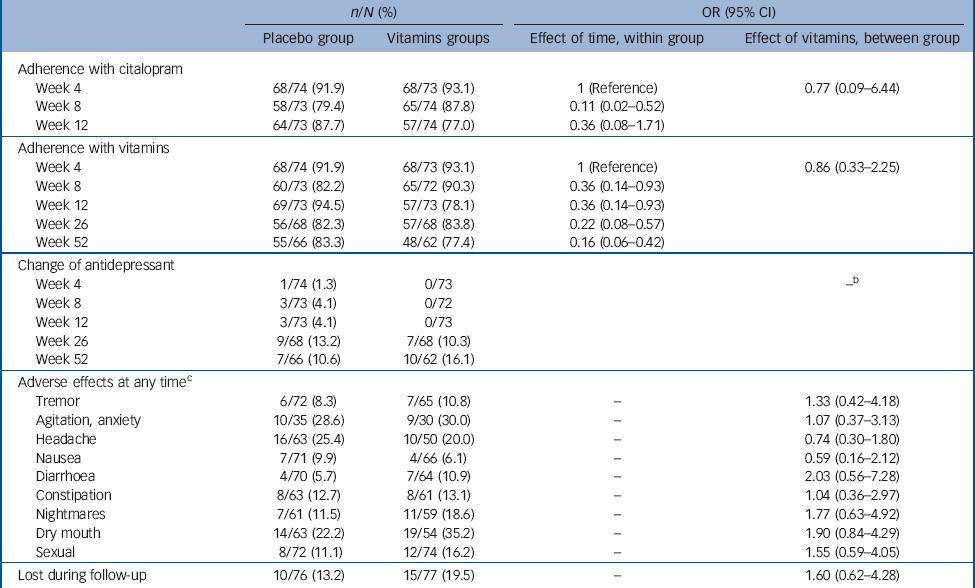
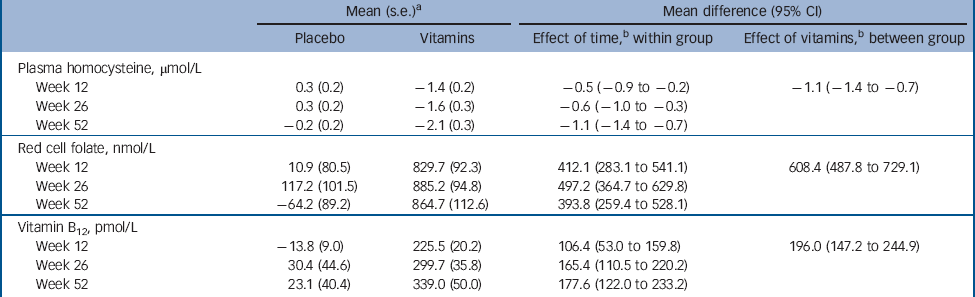
Table 3 summarises data on adherence to treatment with citalopram and vitamins/placebo, as well changes of anti-depressants and adverse experiences of participants assigned treatment with placebo and vitamins. There were no differences between the study groups in any of these measures. Moreover, there were no breaches of protocol or unmasking during the study. Finally, treatment with vitamins was associated with a significant reduction in tHcy (–1.1 μmol/L, 95% CI –1.4 to –0.7 μmol/L; adjusted for gender and baseline tHcy), and increased red cell folate (608.4 nmol/L, 95% CI 487.8 to 729.1 nmol/L; adjusted for gender and baseline red cell folate) and serum B12 (196.0 pmol/L, 95% CI 147.2 to 244.9 pmol/L; adjusted for gender and baseline serum B12), indicating that treatment had the expected effect on these measures (Table 4).
We also completed post hoc analyses to ascertain whether the benefits of treatment varied according to basal tHcy. The medium basal tHcy for the sample was 10.4 μmol/L (IQR 9.1-12.3 μmol/L). Restriction of the analyses to the subgroup of 76 participants (32 placebo, 44 vitamins) with tHcy >10.4 μmol/L confirmed that the use of vitamins was associated with greater odds of remission of symptoms over 52 weeks compared with placebo (OR = 3.47, 95% CI 1.22-9.84). Conversely, those with tHcy ⩽10.4 μmol/L at baseline showed no evidence of benefiting from treatment with vitamins (OR = 1.09, 95% CI 0.32-3.75).
Discussion
Main findings
This placebo-controlled randomised trial showed that the adjunctive use of folic acid, vitamin B6 and vitamin B12 is safe and more effective than placebo at enhancing response to antidepressant treatment over 52 weeks, but not over the initial 12 weeks. The effect of treatment with vitamins on symptom severity was not significant compared with placebo. In addition, relapse of major depression for participants who had experienced remission of symptoms by 12 weeks was less frequent among those treated with vitamins than with placebo. The beneficial effects of vitamins on mood was apparent among those in the highest 50th percentile of tHcy, but not among those in the bottom half of tHcy values.
Table 2 Clinical outcomes over time of participants with major depression treated with placebo or vitaminsFootnote a
| n/N (%) | OR (95% CI) | |||
|---|---|---|---|---|
| Placebo group | Vitamins group | Effect of time,Footnote b within group | Effect of vitamins,Footnote b between group | |
| Major depressive episode in remissionFootnote c | ||||
| Week 12 | 57/73 (78.1) | 58/73 (79.4) | 1 (Reference) | 2.49 (1.12-5.51) |
| Week 26 | 52/68 (76.5) | 58/68 (85.3) | 1.16 (0.60-2.29) | |
| Week 52 | 50/66 (75.8) | 53/62 (85.5) | 1.14 (0.57-2.26) | |
| ⩾50% reduction in MADRS score | ||||
| Week 4 | 38/74 (51.3) | 34/73 (46.6) | 1 (Reference) | 0.59 (0.28-1.25) |
| Week 8 | 56/73 (76.7) | 46/72 (63.9) | 4.04 (2.16-7.57) | |
| Week 12 | 56/73 (76.7) | 47/73 (64.4) | 4.07 (2.18-7.61) | |
| Relapse after week 12Footnote d | ||||
| Week 26 | 10/57 (17.5) | 5/58 (8.6) | 1 (Reference) | 0.33 (0.12-0.94)Footnote e |
| Week 52 | 10/57 (17.5) | 5/57 (8.8) | 1.02 (0.44-2.37) | |
MADRS, Montgomery-Åsberg Depression Rating Scale score.
a. n/N: number of participants in the cell/number of participants available in the group.
b. Analyses adjusted for gender and baseline total plasma homocysteine.
c. Major depressive episode according to DSM-IV-TR criteria. Number needed to treat (NNT) for 1 person to benefit by 52 weeks is 16. If all individuals lost to follow-up were still clinically depressed, then the NNT is 33.
d. A total of 57 and 58 participants treated with placebo and vitamins, respectively, were in remission by week 12 - this subgroup was followed up to investigate relapse of symptoms at weeks 26 and 52. e. Baseline total plasma homocysteine not included in these analyses, as relapse could only occur at or after week 26.
e. Baseline total plasma homocysteine not included in these analyses, as relapse could only occur at or after week 26.
Strengths and limitations
This single-site trial had a relatively small sample size and had to stop recruitment before the planned number of participants had been reached. The possible loss of power associated with decreased sample size was mitigated, in part, by the availability of repeated measures, Reference Jonsson37 which allowed us to analyse all available data and minimise the introduction of bias (intention-to-treat analyses). The randomisation of participants was centralised and independent of the investigators, and masking was maintained throughout the trial. The sample consisted of volunteers who consented to screening and who were subsequently diagnosed with a major depressive episode. The use of the electoral roll to approach participants living in the community might have yielded a sample that differs from samples recruited from specialised mental health services, and this might explain the relatively high proportion of participants who responded well to treatment. Reference Rush, Trivedi, Wisniewski, Nierenberg, Stewart and Warden5 In addition, the outcome measures that we used in this trial to establish the presence of major depression and to monitor changes in the severity of symptoms have well-established validity. Reference Montgomery and Asberg31,Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller35 This, together with the background characteristics of our sample, gives us confidence that our results are most likely applicable and generalisable to middle-aged and older adults with major depression living in the community.

Fig. 2 Mean change in Montgomery-Åsberg Depression Rating Scale (MADRS) scores from baseline over a period of 52 weeks for people treated with placebo and vitamins.
The whiskers represent the standard error of the mean change at each time point. All estimates were adjusted for baseline MADRS scores and gender. Treatment with vitamins was associated with a non-significant decline of 0.4 points over time compared with placebo (95% CI –2.6 to 1.8, z = –0.74, P = 0.739; analysis adjusted for gender and baseline total plasma homocysteine).
Contamination caused by the use of vitamins not prescribed by the study could represent another potential source of error in our trial, although participants were explicitly instructed not to use any other B vitamins. The consequence of such contamination would have been loss of power to declare as statistically significant differences between the groups (because some placebo-treated patients would have benefited from treatment with B vitamins). In that case, our findings could be considered conservative. However, we did have objective measures of adherence with the treatment protocol that also included blood tests. Total plasma homocysteine remained unchanged in the placebo group throughout the trial, but declined (as expected) among actively treated participants. Similarly, changes in the concentration of red cell folate and of vitamin B12 increased markedly among participants randomised to treatment with vitamins but not with placebo (Table 4). For these reasons, we are inclined to conclude that contamination is unlikely to have significantly affected the results of our trial.
Given the apparent beneficial results of the intervention, it might seem surprising that we did not find a significant difference in MADRS scores over time between the groups. We would suggest that this might have been as a result of the marked beneficial effect of the interventions on scores, which left little room to explore differences between the groups over time. We have also assumed that loss to follow-up in this trial occurred at random. Although our results are consistent with this interpretation, it is conceivable that those who did not return for assessment could have all done poorly. If that were the case, the absolute risk reduction associated with vitamins compared with placebo could be as low as 3%, and the NNT = 33. In this worst possible scenario, the use of vitamins would still enhance response to antidepressant treatment, but benefits would be small.
Table 3 Measures of adherence to treatment and change in the use of antidepressants over time, and adverse effects reported by participants treated with placebo and vitaminsFootnote a
| n/N (%) | OR (95% CI) | |||
|---|---|---|---|---|
| Placebo group | Vitamins groups | Effect of time, within group | Effect of vitamins, between group | |
| Adherence with citalopram | ||||
| Week 4 | 68/74 (91.9) | 68/73 (93.1) | 1 (Reference) | 0.77 (0.09-6.44) |
| Week 8 | 58/73 (79.4) | 65/74 (87.8) | 0.11 (0.02-0.52) | |
| Week 12 | 64/73 (87.7) | 57/74 (77.0) | 0.36 (0.08-1.71) | |
| Adherence with vitamins | ||||
| Week 4 | 68/74 (91.9) | 68/73 (93.1) | 1 (Reference) | 0.86 (0.33-2.25) |
| Week 8 | 60/73 (82.2) | 65/72 (90.3) | 0.36 (0.14-0.93) | |
| Week 12 | 69/73 (94.5) | 57/73 (78.1) | 0.36 (0.14-0.93) | |
| Week 26 | 56/68 (82.3) | 57/68 (83.8) | 0.22 (0.08-0.57) | |
| Week 52 | 55/66 (83.3) | 48/62 (77.4) | 0.16 (0.06-0.42) | |
| Change of antidepressant | ||||
| Week 4 | 1/74 (1.3) | 0/73 | -Footnote b | |
| Week 8 | 3/73 (4.1) | 0/72 | ||
| Week 12 | 3/73 (4.1) | 0/73 | ||
| Week 26 | 9/68 (13.2) | 7/68 (10.3) | ||
| Week 52 | 7/66 (10.6) | 10/62 (16.1) | ||
| Adverse effects at any timeFootnote c | ||||
| Tremor | 6/72 (8.3) | 7/65 (10.8) | - | 1.33 (0.42-4.18) |
| Agitation, anxiety | 10/35 (28.6) | 9/30 (30.0) | - | 1.07 (0.37-3.13) |
| Headache | 16/63 (25.4) | 10/50 (20.0) | - | 0.74 (0.30-1.80) |
| Nausea | 7/71 (9.9) | 4/66 (6.1) | - | 0.59 (0.16-2.12) |
| Diarrhoea | 4/70 (5.7) | 7/64 (10.9) | - | 2.03 (0.56-7.28) |
| Constipation | 8/63 (12.7) | 8/61 (13.1) | - | 1.04 (0.36-2.97) |
| Nightmares | 7/61 (11.5) | 11/59 (18.6) | - | 1.77 (0.63-4.92) |
| Dry mouth | 14/63 (22.2) | 19/54 (35.2) | - | 1.90 (0.84-4.29) |
| Sexual | 8/72 (11.1) | 12/74 (16.2) | - | 1.55 (0.59-4.05) |
| Lost during follow-up | 10/76 (13.2) | 15/77 (19.5) | - | 1.60 (0.62-4.28) |
a. Participants were considered adherent with treatment if they consumed 75% or more of prescribed tablets.
b. Data not fit for modelling.
c. List of complaints limited to those with prevalence >5%.
Table 4 Changes in total plasma homocysteine, red cell folate and serum B12 relative to baseline during the course of the trial
| Mean (s.e.)Footnote a | Mean difference (95% CI) | |||
|---|---|---|---|---|
| Placebo | Vitamins | Effect of time,Footnote b within group | Effect of vitamins,Footnote b between group | |
| Plasma homocysteine, μmol/L | ||||
| Week 12 | 0.3 (0.2) | –1.4 (0.2) | –0.5 (–0.9 to –0.2) | –1.1 (–1.4 to –0.7) |
| Week 26 | 0.3 (0.2) | –1.6 (0.3) | –0.6 (–1.0 to –0.3) | |
| Week 52 | –0.2 (0.2) | –2.1 (0.3) | –1.1 (–1.4 to –0.7) | |
| Red cell folate, nmol/L | ||||
| Week 12 | 10.9 (80.5) | 829.7 (92.3) | 412.1 (283.1 to 541.1) | 608.4 (487.8 to 729.1) |
| Week 26 | 117.2 (101.5) | 885.2 (94.8) | 497.2 (364.7 to 629.8) | |
| Week 52 | –64.2 (89.2) | 864.7 (112.6) | 393.8 (259.4 to 528.1) | |
| Vitamin B12, pmol/L | ||||
| Week 12 | –13.8 (9.0) | 225.5 (20.2) | 106.4 (53.0 to 159.8) | 196.0 (147.2 to 244.9) |
| Week 26 | 30.4 (44.6) | 299.7 (35.8) | 165.4 (110.5 to 220.2) | |
| Week 52 | 23.1 (40.4) | 339.0 (50.0) | 177.6 (122.0 to 233.2) | |
a. Standard error of the mean difference.
b. Effect estimates adjusted for the respective baseline levels and gender.
We also acknowledge that we cannot be certain about the effects of ongoing treatment with these vitamins beyond 52 weeks. Existing data suggest that extended treatment with these dosages of B vitamins is generally safe Reference Smith, Kim and Refsum13,Reference Group38 and that long-term consumption might contribute to preventing depressive episodes among those at risk. Reference Almeida, Marsh, Alfonso, Flicker, Davis and Hankey26 However, evidence from prolonged randomised clinical trials targeting people with or at risk of depression is not available. It is also important to note that none of our participants was B12 or folate deficient. Australia is a wealthy country where fortification of flour with folic acid is mandated and, as a result, folate deficiency is now rare. Reference Brown, Langshaw, Uhr, Gibson and Joshua39 This suggests that the potential antidepressant effects of these vitamins could be more pronounced in countries where deficiency of vitamins B12 and folate is common. A similar rationale would suggest that such an intervention might be particularly relevant for adults with depression aged 75 years or older, which is the age group with the highest tHcy. Reference Flicker, Vasikaran, Thomas, Acres, Norman and Jamrozik36
Implications
Existing observational data indicate that high tHcy and low folate increase the risk of depression and are associated with more frequent symptom relapse and treatment resistance. Reference Almeida, McCaul, Hankey, Norman, Jamrozik and Flicker21,Reference Papakostas, Petersen, Mischoulon, Green, Nierenberg and Bottiglieri28,Reference Papakostas, Petersen, Mischoulon, Ryan, Nierenberg and Bottiglieri40 A recent trial showed that folate supplementation increases response to antidepressant treatment in adults with treatment-resistant depression, Reference Papakostas, Shelton, Zajecka, Etemad, Rickels and Clain27 and our results now extend these findings to a community-derived unselected sample of middle-aged and older adults with major depression. In this trial, the use of vitamins B6, B12 and folate did not increase the 12-week efficacy of anti-depressant treatment, but enhanced and sustained antidepressant response over 1 year. Participants in the highest 50th percentile of plasma tHcy benefited the most from the use of these vitamins. Replication of these findings would mandate that treatment guidelines adopt the adjunctive use of B vitamins as a safe and inexpensive strategy to manage major depression in middle-aged and older adults.
Acknowledgements
We thank study participants for their generous contribution to this study, and Dr Stephen Fenner, Ms Jennifer Tasker and Ms Natalie DiRenzo for their assistance with recruitment and data collection.









eLetters
No eLetters have been published for this article.