1. Introduction
The WTO's India–Agricultural Products disputeFootnote 1 involves a US challenge to the Indian government's regulatory policies for addressing avian influenza (AI) – otherwise known as ‘bird flu’. While India had not been a major market for US poultry exports, the decision by India to ban imports of poultry meat, eggs, feathers, and seven other products from any country that reported an AI outbreak in its territory raises a number of important legal, economic, and public policy issues.
Our analysis of the dispute explores the relationship between WTO Members, the WTO Agreements, and international standards setting organizations, including the World Organisation for Animal Health (OIE).Footnote 2 The dispute calls into question the appropriate design for international institutions that establish health and safety standards, how such institutions interact with domestic regulators, and the indirect constraints that such institutions place on domestic policymaking via membership in the WTO.
While this dispute also joins a growing list at the intersection of the WTO and domestic regulatory policy over human, animal or plant health,Footnote 3 its central concerns are also of both immediate and long-term policy relevance. Given continued outbreaks of AI globally – including major new outbreaks in the United States in 2014‒15 – our assessment of the Panel Report and the OIE underscores the need to find the appropriate balance between a country's right to regulate for the health and safety of its people, animals, and plants, while at the same time participating in a rules-based trading system that relies on science-based assessments of the risks of continuing to trade in products from countries found to harbor infectious diseases or contagious viruses.
2. Economic and policy background
2.1 Avian influenza and the OIE
Avian Influenza is an infectious viral disease that arises in birds, and, in particular, wild water fowl such as ducks and geese. While AI is mainly carried in wild birds, it can be transmitted to domestic poultry. Scientists have developed criterion by which to classify the many different (and mutating) strains of the AI virus so that they fall into one of two categories: high pathogenic notifiable avian influenza (HPNAI) and low pathogenic notifiable avian influenza (LPNAI).Footnote 4
Thus far, the evidence linking most forms of AI to humans is weak, though certain strains of AI have resulted in human infections – including H5N1 and H7N9 – these strains hold the potential to cause a global public health crisis. The transmission of the virus to humans is thought to have occurred based on direct or indirect contact with live or dead infected poultry.Footnote 5 There is no evidence thus far that the virus can be transmitted through poultry meat that has been cooked according to established guidelines.
The OIE is the international organization that establishes the ‘standards’ defining what it means for poultry to potentially be infected with AI and thus for one country to be able to implement trade barriers on poultry-related products arising from AI-infected trading partners. The OIE does this through a process by which it develops and maintains Scientific Commissions composed of outside subject experts, and it then publishes (and updates) the recommendations of such experts as standards via a legal document referred to as its Terrestrial Code.
Chapter 10.4 of the OIE's Terrestrial Code provides detail on the latest standards relating to the detection of Avian Influenza and commercial poultry products, such as live birds, eggs and feathers. In particular, paragraph 1 of Chapter 10.4.1 of the Terrestrial Code clearly delineates the distinction between HPNAI and LPNAI by establishing a definition for when a particular mutation of an AI virus is sufficiently deadly to be classified as HPNAI; any forms of AI that do not meet that threshold are then classified as LPNAI.
The Terrestrial Code develops additional policy guidelines for countries in the face of their own and their trading partners' AI status – i.e., whether countries or sub-regions (zones) within a country are ‘NAI-free’ (no evidence of HPNAI or LPNAI) or at least ‘HPNAI-free’ (while evidence of LPNAI may exist, no evidence of HPNAI exists). This includes discussion of surveillance regimes, stamping out procedures in light of NAI infections and outbreaks, and defining the length of incubation periods (e.g., 21 days). There are also different procedures for different types of products; e.g., live birds other than poultry, eggs (for hatching or human consumption), feathers, and other poultry products. Importantly, the Code explains how a country can regain NAI-free status after an NAI outbreak, and the extremely sharp distinctions between HPNAI versus LPNAI infections. The Code makes explicit the conditions under which trading partners are (and are not) justified in imposing import bans in light of NAI outbreaks, and it has an established procedure permitting importing countries the right to ‘escape’ from obligations and deviate from OIE standards, so long as they provide their own risk assessment and scientific evidence.
2.2 The policies under dispute
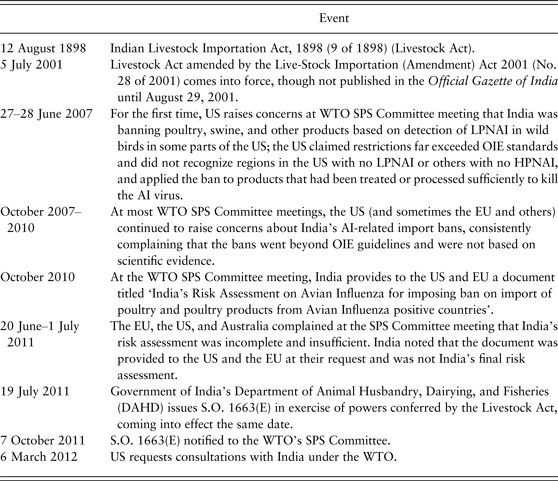
Table 1 provides a timeline of the Indian policies related to Avian Influenza and poultry that form the crux of the WTO dispute.
Table 1. Timeline and critical dates of interest

Source: Compiled by the authors from Panel Report and minutes of the SPS Committee.
On 5 July 2001, the Indian government amended the 1898 Livestock Act with the Live-Stock Importation (Amendment) Act of 2001. The US interest in the extent to which the Livestock Amendment Act applied to poultry products in light of avian influenza dates at least to 2007 according to minutes from the WTO SPS Committee meetings held that summer. The Government of India's Department of Animal Husbandry, Dairying, and Fisheries (DAHD) issued S.O. 1663(E) on 19 July 2011, an order imposing an import ban (and other trade-restricting measures) on a number of poultry-related products from countries reporting NAI, including both HPNAI and (importantly) LPNAI. On 7 October 2011, India notified S.O. 1663(E) to the WTO's SPS Committee, and on 6 March 2012, the US requested consultations with India under the WTO.
2.3 Economic markets for poultry and related products
The commerce at issue in this dispute consisted of a number of poultry and livestock-related products that could potentially be affected by avian influenza.Footnote 6 To analyze the potential impact of the policies under dispute, we focus below mainly on poultry, the most economically vibrant of these product markets globally and representative of a number of the salient political-economy issues in the dispute.
The United States is the world's largest producer of poultry and home to household brands such as Tyson Foods and Perdue. These and other large agribusinesses have historically been able to organize politically so as to overcome the free-rider problem and engage with the office of the US Trade Representative to work on their behalf on market-opening issues related to trade policy. Indeed, commercial poultry is no stranger to trade disputes. Between 1995 and 2014, at least seven antidumping investigations over poultry were initiated globally involving ten different countries (Bown, Reference Bown2015), and no fewer than 12 formal WTO disputes over poultry were initiated under the DSU involving nine different WTO members.
The US is the second largest exporter of poultry meat behind Brazil (USDA, 2015a); between 2005 and 2010, for example, the US exported an average of 15% to 20% of its total domestic broiler production each year. By 2012, US export sales to the world were over $5 billion, and the largest US export markets for poultry were Mexico, Canada, China, Hong Kong, Taiwan, Angola and Kazakhstan.
Despite the potential market access interests for the highly populated Indian market, US exports of poultry products to India have historically been small, at an estimated $2 million per year or less.Footnote 7 While Bown and Reynolds (Reference Bown and Reynolds2015) note that this is not unusual, as there are a substantial number of formal WTO disputes over similarly small amounts of realized bilateral trade flows,Footnote 8 the potential that India's expanding middle class might turn from vegetarian-based proteins to chicken suggested a potentially significant growth market for US poultry exporters.
Indian commercial poultry production is quite different from the structure of the US industry (USDA, 2011). Because of minimal availability of cold storage infrastructure, the Indian poultry processing and retail sectors are much smaller than production; as such, poultry commerce is less likely to involve meat and more likely to involve live birds. To the extent that AI is more transmissible via live birds, Indian wariness toward foreign-introduced AI is perhaps more easily understood relative to countries in which commerce is more likely to occur in frozen meat.
Nevertheless, even in the absence of the potential import restrictions related to AI, India maintained an MFN applied import tariff on a number of poultry products that could severely limit trade, ranging from 30.9% to 111.2% (USDA, 2011).
3. The legal claims and issues raised
Fourteen out of the fifteen claims that the US raised stem from alleged violations of the WTO's Agreement on the Application of Sanitary and Phytosanitary Measures (‘SPS Agreement’),Footnote 9 one of the new disciplines added as part of the Uruguay Round. The SPS Agreement was intended to promote the harmonization of SPS standards around international standards developed by the Codex Alimentarius Commission (food safety), the OIE (animal health and safety), and the International Plant Protection Convention (plant health), and to ensure that WTO members' SPS measures are based on sound science and are not more trade restrictive than necessary to protect human, animal, or plant health.
The US challenged India's SPS regime for controlling AI,Footnote 10 which banned imports of ten (mostly poultry and egg) products if they were exported from countries reporting certain types of AI. Because the US had reported incidences of LPNAI outbreaks, its exports were subject to the ban. The challenge included alleged violations of 14 different SPS provisions, with the heart of the US claims centering on:
1. Conformity with international standards: a violation of the SPS Agreement's requirements that India's measure be ‘based on’ (Article 3.1) or ‘conform to’ (Article 3.2) international standards, in this case the OIE's Terrestrial Code, Chapter 10.4.
2. Non-discrimination: a violation of the SPS Agreement's prohibition in Article 2.3 on arbitrary or unjustifiable discrimination, given that India's measure banned domestic trade in poultry products within a 10 km radius of an AI outbreak in India, while banning imports from the entire US for a breakout anywhere in the country;
3. Scientific basis: a violation of the SPS Agreement's Article 2.2 requirement that measures must be based on scientific principles and sufficient scientific evidence, given the absence of any risk assessment (as required by Article 5.1) performed by India to demonstrate the scientific basis for its measures.
4. Necessity test: India's AI measures, in imposing a ban on all imports of poultry products from the US, are more trade restrictive than necessary to achieve India's desired level of protection (ALOP) against AI.
5. Adaptation to regional condition: India is required under Article 6 to recognize that the US had a system of regions or areas that were certified AI-free and therefore India was not justified in banning imports from the entire country.
6. Transparency: alleged violations of the specific notice and transparency provisions found in Article 7 of the SPS Agreement.
India, for its part, rested very heavily on the SPS Agreement's references to international standards, asserting that its measures ‘conform to’ the OIE's relevant standards. India insisted that if that were the case, India's compliance with the other provisions of the SPS Agreement must be presumed, and that it was under no further obligation to provide a scientific risk assessment or other proof that its AI measures were based on scientific principles and evidence. For India, conformity with an international standard was the only real obligation imposed on it by the SPS Agreement.
In the end, as can be seen in Table 2, the Panel decided virtually all of the claims in favor of the United States.Footnote 11 However, the way in which the Panel arrived at its decision raises a number of interesting and significant legal issues. First is the significant number of consequential findings made by the Panel, resulting in the building of a ‘house of cards’, with virtually the entire foundation of the Panel's decisions resting on its first finding that India's measures did not ‘conform to’ the OIE standard. A second important area involved the Panel's engagement with the OIE, including its experts, its written standards and its experts' participation in meetings of the WTO's SPS Committee. Third, the Panel developed new jurisprudence with respect to Article 6's requirement that SPS measures be adapted to the area from which the imports originated and take into account that certain areas (whether entire countries or just part of a country) may be disease-free or have low levels of disease prevalence.Footnote 12
Table 2. Legal elements of India – Agricultural Products

3.1 It all comes down to the international standards
The SPS Agreement's Article 3 is at the heart of this case, as it contains the core provisions to promote the harmonization of measures around international standards, guidelines, or recommendations. It starts with the requirement that Members base measures on international standards where they exist (Article 3.1), and follows by giving Members an incentive to do so in that it provides a ‘safe harbor’ for those measures which ‘conform to’ international standards (Article 3.2). It then permits Members to deviate from international standards, provided their deviation is to achieve a higher level of sanitary or phytosanitary protection and that there is a scientific justification for that deviation (Article 3.3).
One key premise underlying the Panel's analysis was its reliance on the Appellate Body's EC‒Hormones distinction between the interpretive standard applied to Article 3.1, which requires that members base their SPS measures on international standards, guidelines, or recommendations, and Article 3.2, which provides that if a measure conforms to international standards, it is presumed to be consistent with the SPS Agreement.Footnote 13 In EC–Hormones, the Appellate Body set forth (and the India Panel adopted) a very strict definition of Article 3.2's ‘conform to’ phrase: it requires that an SPS measure ‘embody the standard completely’ or ‘match it exactly’. Article 3.1, on the other hand, is a less rigorous threshold, permitting a measure to incorporate only some elements of a standard in order to pass the ‘based on’ test. Therefore, failure to meet the ‘based on’ threshold in Article 3.1 would also result in a failure to meet the more rigorous ‘conform to’ threshold in Article 3.2.
3.2 United States only had to show a discrepancy to prove a violation of Article 3.2
Once the Panel adopted the definition of the ‘match exactly’ standard under Article 3.2, the US could prevail by simply proving the existence of any discrepancy ‒ any failure to match exactly ‒ between the OIE standard and India's AI regime. Demonstrating a violation of Article 3.2 would then deprive India of its principle defense ‒ its claim that if its measures conform to the OIE standard, then its entire regime is presumed to be based on sufficient science and to be consistent with the SPS Agreement.
The US chose to challenge three discrepancies:
1. Product coverage
India's AI regime included two products (live pigs and pathological material and biological products from birds) that were not mentioned or regulated in the OIE's Terrestrial Code.Footnote 14 As such, the Panel found that India could not rely on Articles 3.1 or 3.2 to justify its import ban on those two products. Thus, inclusion of them on India's banned list was a clear failure to ‘match exactly’ the international standard.
2. NAI versus HPNAI versus LPNAI
The Terrestrial Code set recommendations for specific poultry products in three categories: (1) those from NAI-free countries, zones, or compartments; (2) those from HPNAI-free countries, zones, or compartments; and (3) those regardless of the NAI status of the country, zone, or compartment. India's AI regime, on the other hand, was pitched at NAI with no distinction for high pathogenicity versus low pathogenicity AI. India contended that because the Terrestrial Code provided for three different recommendations, India was entitled to apply one but not necessarily all of them. The question therefore became whether India, in order to demonstrate that its measures ‘conformed to’ the Terrestrial Code, was required to make a distinction between high pathogenicity and other forms of AI. The Panel answered that question in the affirmative, establishing another discrepancy between India's measures and the OIE standard.
3. Country-wide versus zone or compartment requirements
The US also challenged India's countrywide application of its AI-based import ban, given the Terrestrial Code's recommendations that trade restrictions be applied at the zone or compartment level when appropriate surveillance controls and biosecurity measures are in place to prevent the spread of disease. India argued that it could choose whether to extend its requirements to an entire exporting country or only to its zones or compartments, particularly because the Terrestrial Code consistently referred to the three terms in the alternative: country or zone or compartment. The Panel found that the standard was: if the exporting country does not apply zoning to reduce the size of the affected population, then the measure recommended in the Terrestrial Code should be applied to the entire country. It went on to infer that: if the opposite situation were the case – i.e., if an exporting country does engage in compartmentalization – then an importing country is required to take those zones or compartments into account when developing trade restrictions. Because India's AI regime did not provide for such disparate treatment, its measure was found not to ‘conform to’ the OIE standards.
3.3 The house of cards
The consequences of the Panel's finding that India's measures did not conform to the OIE standard were enormous and led directly to most of the other findings. First, India also violated Article 3.1's requirement that measures be ‘based on’ international standards, as the same three discrepancies noted above were also evidence that India's measure and the OIE standard contradict each other, with prior Appellate Body rulings noting that measures which contradict an international standard cannot be understood as having been based on that international standard. Second, India violated Article 2.2's requirement that its measures be based on scientific principles. Third, India violated Article 5.1 and 5.2's requirement for a risk assessment, given that India declared that the risk assessment document submitted to the SPS Committee was not an official risk assessment, and its reference to but ultimate refusal to claim that its AI measures were based on Australia's risk assessment meant that India had no risk assessment. Fourth, India violated the non-discrimination provisions of Article 2.3, because the measures treated imports less favourably than domestic poultry by banning imports from anywhere in an exporting country with AI while only banning domestic poultry found within a 10 km radius of AI outbreak and by banning imports from countries with LPNAI but failing to monitor for LPNAI in India. Fifth, India violated Article 5.6 by imposing measures that were more trade restrictive than necessary, as the Panel found that the very same international standard, the OIE's Terrestrial Code, that India had claimed it was conforming to was instead an alternative measure that India could have reasonably adopted and that would have been less trade restrictive than India's import ban.
Each finding flows directly from the Panel's adoption of the strict ‘match exactly’ test of what it means to ‘conform to’ an international standard, with the discrepancies highlighting India's failure to match exactly the OIE standard. Had India's measure been found to conform to the OIE standard, then this entire house of cards would likely have come tumbling down.
3.4 Engagement with the OIE and the SPS Committee
The Panel relied heavily on the testimony of experts, on a written exchange with the OIE, and on statements and evidence presented at meetings of the WTO's SPS Committee. Furthermore, the experts and the OIE statements played a central role in the Panel's interpretation of the risk assessment issues, of the interpretation of the intent of the OIE standards with respect to import bans, the presence of LPNAI in India, and the conclusion that India was not willing to recognize areas within another country that might be disease-free. As such, this case shines a spotlight on the delicate issues raised by the WTO's deferral of authority to the OIE and of the difficult position faced by the Panel, which was obligated to interpret OIE standards in order to come to its conclusion that India had violated WTO standards. The SPS Agreement leaves the very difficult interpretation of technical standards to the work of a Panel made up of trade experts, while leaving the fate of WTO Members' trade restrictions in the hands of outside technical experts and institutions.
For example, India made one key argument that it was not discriminating against poultry imports from countries that reported LPNAI outbreaks because India did not have LPNAI in its territory. Because LPNAI was ‘exotic’ to India, the policy was not favoring Indian poultry relative to poultry from countries where LPNAI had been detected. The Panel asked the experts whether India had LPNAI. In a largely backhanded way, the experts answered by noting that LPNAI is virtually ubiquitous in several wild water bird families, particularly ducks, and that India has substantial populations of these wild water birds and ducks. On that basis, the Panel ruled that LPNAI is not ‘exotic’ to India and therefore India was discriminating by banning imports from countries with LPNAI.
Similarly, there was great controversy over the significance and substance of a document India provided at an SPS Committee meeting entitled ‘India's Risk Assessment on Avian Influenza for imposing ban on import of poultry and poultry products from Avian Influenza positive countries (Summary Document)’. India subsequently indicated that the Summary Document was not its final risk assessment, which would take some additional time to prepare. Nearly a year later, the US, apparently unbeknownst to India, sent the Summary Document to the OIE, asking the OIE for its assessment of whether it qualified as a risk assessment. At a subsequent SPS Committee meeting, the US asked that the OIE be given the floor to present its quite critical analysis of India's Summary Document. India, for its part, appears to have taken great umbrage at the presence of the OIE representatives and their criticism of its Summary Document, with India asserting a ‘breach of trust reposed by India in the United States’ and its view that the OIE acted in disregard of its mandate and overstepped its position as an observer at SPS Committee meetings, since ‘the OIE does not have a separate mandate to assess, judge or comment on the existence or content of a Member's risk assessment’.Footnote 15
3.5 New jurisprudence on the required recognition of areas with low-prevalence or no disease
A third noteworthy development is the Panel's first interpretation of Article 6 of the SPS agreement, which sets forth the requirement that Members ensure that their SPS measures are adapted to the SPS characteristics of the area ‒ whether all of a country, part of a country, or all or parts of several countries. Article 6.2 states that Members shall ‘recognize the concepts of pest- or disease-free areas and areas of low pest or disease prevalence’, with the determination that such areas be based on factors such as geography, ecosystems, epidemiological surveillance, and the effectiveness of sanitary or phytosanitary controls. The US's basic claim was that India's measures explicitly ban poultry from all parts of the country, thereby ignoring disease-free areas, areas of low disease prevalence, the existence of eradication or control programs, and the relevant OIE guidelines. India countered that the ‘recognition of the concept’ language does not require any particular form of ‘recognition’ and that India's form was to accept and evaluate proposals and evidence of pest-free zones, which India claimed the US had never submitted.
The Panel agreed with India that the format of the recognition of the concept of pest- or disease-free areas was not set forth in the SPS Agreement. Nevertheless, it went on to establish the following test: to comply with Article 6.2, ‘SPS measures adopted by WTO Members must at a minimum not deny or contradict the recognition of the concepts of such areas when these concepts are relevant with respect to the disease at issue’ (Panel Report at 7.698).
The Panel then examined both India's Livestock Act and the regulations imposing the ban on imports from countries reporting NAI (S.O 1663(E)). It found that the Act gives the Indian government wide discretion with no evidence that the discretion has ever been used to recognize, deny, or contradict the recognition of such areas, but it found nothing in S.O. 1663 that allows for the recognition of disease-free areas within a country that reports NAI. The Panel concluded that S.O. 1663 violates Article 6.2 by imposing a prohibition on a countrywide basis, contradicting the requirement to recognize the concept of disease-free areas.
India contended that it had the legal authority to recognize regions or zones, but that the US never presented a proposal highlighting zones that had been kept free of AI. Here again the Panel relied on SPS Committee statements as evidence in favor of the US's claim that India would not recognize regional zones, as India had stated before the SPS Committee (and to the US Foreign Agricultural Service) that its (country-wide) conditions for imports were ‘uniform’ for all countries and could not be changed for some and not others.Footnote 16 Within Article 6, the Panel also established another, albeit smaller, house of cards, by first deciding that because India's S.O. 1663 contradicts the requirement to recognize the concept of a disease-free area, India had violated the first sentence of 6.2 and that such a violation also automatically resulted in a finding that India violated the first sentence of Article 6.1, which requires Members to ensure that their SPS measures are adapted to the SPS characteristics of the zone. The finding of the violation of the first sentence of Article 6.1 led directly to the violation of its second sentence, which sets out the factors Members must take into account in their adaptation to the SPS conditions of a particular zone. The final ‘card’ in the house was an additional resultant finding that India violated the second sentence of Article 6.2 by failing to examine the specific, listed factors on which the determination of disease- or pest-free areas or areas of low pest or disease prevalence was to have been based.
4. Legal-economic analysis and implications of the panel ruling
This section introduces economic models that incorporate the potential spread of AI as a negative externality into the commercial market for poultry products, beginning with the simplest model.
4.1 Some simple economics of avian influenza, standards, and policy intervention
Assume a partial equilibrium (supply and demand), closed economy model of the poultry market and that AI arises as a negative externality only through poultry production. Poultry production leads to increased incidences of AI, and this disease can spill over beyond the farm and cause other damage to the local economy and society – e.g., to other non-poultry commercial products, into the wild, and potentially to humans.
The first-principles result is that, in the presence of the possibility of AI, if such a poultry market is left ‘untreated’ by government policy, the market equilibrium will entail too much production and consumption of poultry relative to the social optimum, as poultry producers do not internalize the entire social costs of their production. The resulting economic inefficiency identifies a welfare-enhancing role for government intervention and national regulation.
The well-known first-best policy response is for the government to impose a Pigouvian tax on poultry production equivalent to the size of the negative externality, i.e., the damage the AI causes to the rest of the economy. Relative to no policy intervention, the imposition of such a tax incentivizes producers to face the full (private plus externality) social costs of their production. The standard result holds that some amount of AI (the externality) is nonetheless likely to arise in equilibrium; i.e., complete eradication of AI may be too costly relative to its social benefit.
While this simple description focuses on Pigouvian taxes, in reality, the optimal government policy intervention to address the AI externality is more complicated and nuanced. For example, in lieu of a tax, the government may implement inspection procedures and mandatory stamping out regimes designed to identify and contain AI outbreaks. More sophisticated regulatory regimes may seek to address the problem of asymmetric information – i.e., that farmers, firms, and potentially even governments could have a disincentive to reveal private information that they learn about local AI outbreaks.
While such policies are clearly relevant for the real world, for ease of exposition, our formal economic analysis will remain limited to a discussion of an optimal tax policy. In broad terms this tax should be interpreted as merely an indicator for a directed government policy intervention. Despite this admittedly simplistic approach, the framework is sufficiently rich to identify important implications of the impact of international trade and international institutions such as the OIE.
4.2 The OIE, global public goods, and standard-setting in the face of externalities
A collection of member governments and other interested parties, including foundations (e.g., Gates Foundation) and other inter-governmental organizations (e.g., the WTO and FAO), fund the OIE. Our baseline assessment is that the institutional design features of the OIE are broadly consistent with insights arising from a basic economic model. Three specific examples include its provision of public goods, its assistance to the global community in overcoming free-rider problems, and, in the case of AI, that its standards differentiate between HPNAI and LPNAI.
The OIE provides to the world trading system important public goods, such as knowledge and information that is non-rival and non-excludable. It contributes knowledge about animal disease by regularly convening scientific panels of experts and establishing standards for conditions under which certain diseases are sufficiently problematic as to require policy intervention. Second, it collects and disseminates information about disease outbreaks to the world.
The OIE also helps countries overcome what would otherwise likely result in a number of free rider problems. First, the globally optimal underlying level of scientific research on diseases like AI is less likely to arise without OIE coordination due to under-investment at the national level – i.e., scientific knowledge discovered by one country could not be kept from spilling over to other countries.Footnote 17 Second, the OIE serves as a centralized clearing house of information to assist monitoring and information dissemination about disease outbreaks. A world without the OIE would likely result in an additional free rider problem in which bilateral or regional surveillance initiatives would arise that provided a more geographically limited (sub-global) public good.
The OIE differentiates its standards for goods produced in countries infected with HPNAI, for which it advocates relatively severe and costly policy interventions, versus for goods produced in countries that have ‘only’ been infected with LPNAI, for which the OIE advocates less costly policy interventions limited to surveillance. This is qualitatively consistent with the basic Pigouvian argument introduced above that high levels of regulation should target the high damage externality (HPNAI) and low levels of regulation should attack the low damage externality (LPNAI).
4.3 Linking international standards organizations and trade agreements
Here we examine the trade policy and WTO issues involved by sequentially introducing additional complexities to the model analyzed above.
4.3.1 Baseline analysis – small countries and local bird flu externalities
Consider a static (one shot) model with two producing countries – country U (which also exports) and country I (which also imports) – that are assumed to be ‘small’ enough that their trade does not affect foreign prices. Continue to assume the AI externality is strictly local.
Introducing trade into the model leads to the standard result that each country gains relative to autarky; nevertheless, there will be winners and losers within each country. The importing country's consumers gain through lower prices and additional quantities of consumption, and import-competing domestic producers of poultry lose. In the exporting country, producers of poultry gain and domestic consumers lose through higher prices and lower quantities consumed at home.Footnote 18 On the other hand, if country I imposed a new import restriction, the identities of these winners and losers would simply be reversed.
With small countries and a negative local production externality, the policy implications noted above continue to hold: the first best policy outcome involves governments in both countries allowing free trade in poultry products, but each government should be permitted the domestic policy flexibility to attack AI with a domestic production tax equal to the size of the local externality. Any alternative policy that does not address the externality at its source would be inefficient in that it would introduce what is known as a by-product distortion (Bhagwati and Ramaswami, Reference Bhagwati and Ramaswami1963).
At this point, there is no efficiency-enhancing role for an interventionist trade policy – such as tariffs or, as we further elucidate below, import bans – in this setting. In the absence of a trans-boundary component to the AI externality, if first best policies like domestic taxes and inspections are available, there is no efficiency motive for introducing a trade policy to address the externality.Footnote 19 This particular result is consistent with at least one aspect of the OIE's general standards and its treatment of LPNAI. Because there is no trans-boundary externality associated with products produced in countries with LPNAI outbreaks, there is no role for restrictive trade policy to target imports from those countries.
4.3.2 Small countries and trans-boundary bird flu externalities
Next we consider a critical modification to our assumptions by supposing that some forms of AI have externalities that are also trans-boundary. Here the key distinction is between outbreaks of high-damage HPNAI versus low-damage LPNAI. To clarify for modeling purposes, it is not the production of the poultry in the exporting country U that gives rise to an additional (non-local) externality in country I. Instead, we assume the poultry produced in country U must be traded from U to I for the additional, trans-boundary externality to arise in country I.Footnote 20
In a series of papers, Costello and McAusland (Reference Costello and McAusland2003), McAusland and Costello (Reference McAusland and Costello2004), and McAusland (Reference McAusland2008) consider implications on optimal policymaking of these types of trans-boundary externalities in the presence of international trade.Footnote 21 McAusland and Costello (Reference McAusland and Costello2004) allow for countries to address such trans-boundary externalities with two policy instruments – an import tariff and port inspections. They find that the optimal import tariff is positive and set at its Pigouvian level, which in this case is the sum of the port inspection costs and the anticipated economic damage associated with those imported goods not rejected at the port.Footnote 22
The intuition for optimal government policymaking follows the same targeting principle (Bhagwati and Ramaswami, Reference Bhagwati and Ramaswami1963) – attack the externality at its source. In this case, because the trans-boundary component of the externality arises only through trade, the optimal targeted intervention to address that component of the externality would be a Pigouvian tax on only the trade. This leads to a different first-best policy outcome relative to Section 4.3.1, in which the AI externality was purely ‘local’. With both local and trans-boundary externality components, the optimal policy outcome would also involve two components: (1) each country of production (I and U) imposes a Pigouvian production tax that is equivalent to the size of the local production externality, and (2) the country of import (I) imposes a separate and additional Pigouvian import tax that is equivalent to the size of the trans-boundary component of the externality.
We interpret the OIE's basic framework for treating trade in poultry and other potentially AI-impacted products as establishing a system for governments to follow this logic for policymaking. In specific instances in which there is a sufficiently high probability of trans-boundary transmission of a costly externality – e.g., in countries of production where HPNAI has been detected – the OIE allows for heightened regulations such as additional trade restrictions and increases in inspections. On the other hand, in instances where there is no trans-boundary externality – e.g., because there is no scientific evidence of possibly economically harmful transmission of AI in countries with ‘only’ LPNAI outbreaks – the OIE standards do not justify a government policy intervention that would impede trade flows.
When viewed through this framework, the economic argument against India's complete import ban on poultry from a country where any form of AI is present – whether HPNAI or LPNAI – is that such a policy is too costly. The import ban on LPNAI goods raises the price to Indian consumers above the socially optimal level, it decreases consumption below the socially optimal level, and it also increases domestic production of poultry above the socially optimal level.Footnote 23
One additional question is raised by the results of McAusland and Costello (Reference McAusland and Costello2004) regarding the implications for optimal policy design when country I has multiple trading partners that differ in the characteristics of their trans-boundary externalities, such as their infection rates, the expected damages (externality costs) to I associated with such infections, as well as differences in their production costs. These considerations may be particularly relevant in light of general WTO/GATT requirements for non-discrimination, particularly MFN treatment. If countries are constrained legally with respect to their tariffs, the model indicates that it may be optimal for them to compensate by discriminating in their application of other complementary policies, such as the rigor with which they conduct inspections of trading partners based on their AI-infection rates. Indeed, the intuition behind the issue of discrimination for trans-boundary externalities would also apply if the AI can be contained within regions of the same country (e.g., US poultry produced in Georgia versus Iowa). The policy implication would be a less-than-country-wide ban even in the presence of HPNAI; if regions in a country can be split into HPNAI-affected and HPNAI-unaffected regions, the optimal policy would distinguish between the two. This is roughly consistent with the Terrestrial Code's principle of ‘zoning’ or ‘regionalization’.
4.3.3 Large countries and bird flu externalities
Finally, the analysis is likely to change if countries are ‘large’ and can shift some of the costs of their policy choices onto trading partners through a reduction in foreign exporter-received prices for their poultry.Footnote 24 While we are not aware of a specific model in the literature that has focused on our exact scenario – i.e., local production externalities and additional, trans-boundary externalities – we found relevant a model with negative consumption externalities where the consumption good can be produced locally or imported (Staiger and Sykes, Reference Staiger and Sykes2011). The Staiger‒Sykes approach is motivated by disputes such as EC–Hormones and EC–Asbestos, where Panels were asked to consider standards that were alleged to be too high (relative to the social optimum), and whether there were economic incentives for such standards to arise in practice when countries are involved in trade agreements such as the WTO.Footnote 25
Staiger and Sykes (Reference Staiger and Sykes2011) show that after a trade agreement constrains the country's import tariff through tariff bindings, if the importing country is further constrained by a national treatment requirement to impose the same, non-discriminatory standard on the domestically produced and imported good, the government will have an incentive to ‘over-regulate’. The government raises its standard to a level that is higher than optimal because it is ‘large’ and can shift some of the costs of those higher standards onto the trading partner through the reduction in foreign exporter-received prices. Staiger and Sykes conclude that in such a scenario, in order to achieve the first best outcome, the agreement also requires a rule to prevent excessively stringent regulation and domestic taxes; without such a rule, governments have a unilateral incentive to make such policies excessive so as to shift some of the costs onto trading partners through reductions in exporters' received prices (terms of trade).
To the extent that such a result were to also arise in a model with trans-boundary externalities, this might identify another potential role for the OIE – it could help to establish the maximum levels of acceptable standards to prevent such international cost-shifting from taking place. Furthermore, the ‘exceptions’ to the OIE framework may also be consistent with such an interpretation – countries like I can be permitted to impose higher-than-OIE standards, but in order to do so, they must bear the cost of conducting additional risk assessments and providing scientific evidence that such regulations are warranted.
5. Conclusion: public policy implications
While our legal-economic analysis is broadly supportive of the India–Agricultural Products Panel Report, the institutional design of the OIE, and elements of its Terrestrial Code that establish and apply ‘standards’ for Avian Influenza, we conclude by highlighting four important public policy issues.Footnote 26
First, the OIE's standard setting role is one that balances long-run and short-run tradeoffs between transparency and policy flexibility. We interpret the rather sharp line that the OIE draws between permissible interventions toward products from HPNAI-affected versus LPNAI-affected countries – and, in particular, OIE's rather forceful reaction against India's position in the dispute – as stemming from the OIE's concern that permitting import restrictions in the case of LPNAI could result in even less transparency regarding AI outbreaks over the long run. Under a strict interpretation of current OIE standards for LPNAI, countries have no disincentive to report LPNAI outbreaks because such outbreaks cannot trigger import restrictions by trading partners in the absence of additional scientific evidence generated through costly independent risk assessments. Put differently, if the OIE allowed a country to ‘get away’ with imposing import restrictions after outbreaks of only LPNAI, trading partners would be discouraged from reporting LPNAI outbreaks in the first place, leading to more uncertainty and furthering the severity of already existing information asymmetries. This could change the nature of the ‘repeated’ game between countries and make cooperation over trade and regulatory agreements less likely to be sustainable.Footnote 27
Indeed, evidence from the Panel Report suggests an already substantial asymmetry in the reporting of LPNAI across countries. Whether due to technical capacity, resource constraints, or something else, the testimony from the three experts brought in by the Panel indicated that India did not have a comprehensive surveillance regime in effect that would systematically allow it to detect LPNAI. From this asymmetric information starting point, the OIE would surely prefer the outcome whereby informational ‘symmetry’ across countries is restored through a process by which India increased its monitoring and domestic surveillance relative to an outcome whereby the US and other countries (that are currently monitoring and reporting) decreased their surveillance activities.
A second potential concern is how this dispute threatens, over the longer-term, the OIE's role in the trading system. On the one hand, the deference that the WTO Panel showed to the OIE legitimizes it as a provider of global public goods and an independent scientific expert on sound levels of standards. The efficiency-enhancing properties of the OIE described in Section 4.2 also help put into perspective any potential political ‘costs’ to national governments associated with loss of sovereignty due to forced harmonization of standards and policy.
The public policy concern is that the WTO put the OIE in the middle of a politically sensitive commercial dispute between its members that may have unintentionally weakened the source of the OIE's strength that derives from its political independence and ability to base its standards and policy advice on sound scientific evidence. Similar political pressure has already confronted other standards-setting international organizations such as Codex Alimentarius in light of the EC–Hormones and EC‒Approval and Marketing of Biotech Products disputes.Footnote 28
A third concern is the long-run impact of this dispute on the SPS Committee. India was certainly put off by the fact that materials that it had shared informally with other WTO members in the SPS committee were brought into formal litigation. Will this make countries less likely to utilize the SPS committee to share, discuss, and potentially resolve their differences without having to turn things over to a Panel?
Fourth, is it feasible for countries to employ less-than-country-wide SPS measures to deal with HPNAI and other similar disease outbreaks? Recent events suggest that such regulatory cooperation across countries can be achieved, even after the occurrence of HPNAI outbreaks. Specifically in 2014‒2015, after the issuance of the Panel Report, the US experienced a number of HPNAI outbreaks that began in wild birds and eventually were found in commercial poultry establishments. US farmers had to ‘stamp out’ upwards of 50 million chickens and turkeys, and the US Department of Agriculture notified the public and the global community through the OIE.
While a number of trading partners announced immediate bans on all US poultry exports, regardless of whether the source was in a location deemed as ‘HPNAI-free’ by US veterinary scientists after testing (USDA, 2015b), the European Union treatment of US exported poultry provides an important exception to this response and potential guidance for how countries can implement in practice the regulatory cooperation envisaged by the OIE.Footnote 29 In late March 2015, the European Commission (2015) announced Implementing Regulation 2015/526, in which it did not implement an import ban on US poultry products; instead, the regulation can be interpreted as following the OIE ‘regionalization’ guidelines that distinguish between zones within an exporting country regarding HPNAI-free status.