In Table 1 of the above article, the protein sequence for pufM was published incorrectly. The correct sequence is GIHRWAIWMAVLVTLTGGIGIL (TM5) and this has been corrected in the table below. The authors would also like to draw attention to the original formatting of the table which is available in picture format below the table. The Publisher apologises for this error.
Table 1. Three chemically distinct types of α-helices: hydrophilic, hydrophobic and amphiphilic.
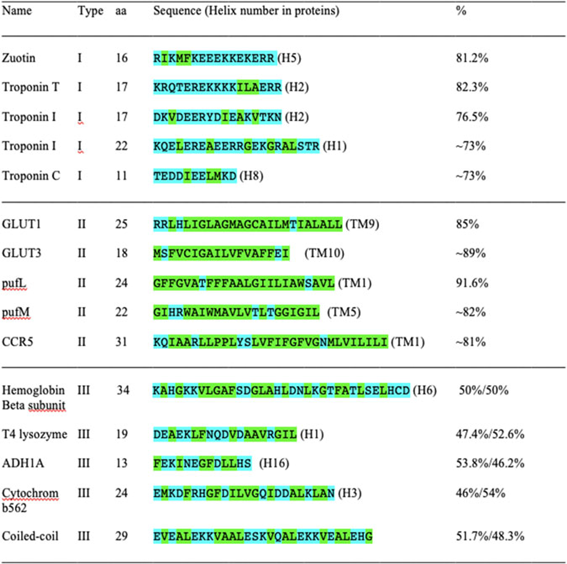
The α-helix can be classified into three chemically distinct Types. The Type I hydrophilic α-helix is mostly comprised of hydrophilic amino acids D, E, N, Q, K, R, S, T, Y; Type II hydrophobic α-helix is mostly comprised of hydrophobic amino acids L, I, V, F, M, P and A; Type III amphiphilic alpha-helix is comprised of both hydrophilic and hydrophobic amino acids, the hydrophobic face and the hydrophilic face. The Type III α-helix is sometimes attached to the surface of the membrane lipid bilayer, or partially buried in the hydrophobic core and partially exposed on the surface of water-soluble globular proteins. Glycine (G) is counted as hydrophobic because its side chain does not engage in H-bonding, although it is only very weakly hydrophobic. If G is not counted as hydrophobic, the percentages will be different.



