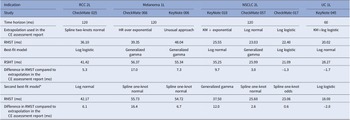
In the original publication of Grumberg et al. (Reference Grumberg, Roze, Chevalier, Borrill, Gaudin and Branchoux2022) a typesetting error occurred in Table 2. The correct Table 2 is reproduced below.
Table 2. Best-Fit and Second Best-Fit Models to OS Kaplan–Meier Curves with at least 18-Month Extended Follow-up

Abbreviations: HR: hazard ratio; KM: Kaplan-Meier; NSCLC: non-small cell lung cancer; RCC: renal cell carcinoma; RMST: restricted mean survival time; UC: urothelial cancer.
The publisher apologizes for this error.