INTRODUCTION
Lumpy skin disease (LSD) is one of the most serious poxvirus diseases of cattle caused by lumpy skin disease virus (LSDV) within the genus Capripoxvirus. The prototype strain is Neethling virus. It causes acute to subacute systemic disease characterized by mild to severe symptoms including fever, nodules on the skin, in the mucous membranes and in the internal organs, skin oedema, lymphadenitis and occasionally death [Reference Davies1, Reference Alexander, Plowright and Haig2]. The skin nodules are painful and could involve tissues up to the musculature. Where extensive generalization occurs, animals may become lame and reluctant to move mainly because of severe oedema in the brisket and around the legs. Superficial lymph nodes draining affected areas of skin become enlarged, up to 4–10 times normal size. Abortion may occur as a result of prolonged fever [Reference Prozesky and Barnard3, Reference Davies4]. The disease has high economic costs due to production losses and chronic debilitation. Milk production ceases and permanent damage can occur to the hides [Reference Ali5, 6].
Laboratory diagnosis to confirm the disease can be made either through the isolation and identification of the virus, or by using serological tests such as the virus neutralization test and indirect fluorescent antibody test (IFAT) [Reference Davies4, 6, Reference Gari7]. Under experimental infection the incubation period is 6–10 days. Clinically sick animals shed the virus through saliva, nasal and lachrymal discharges, blood and semen. Skin lesions have high viral concentration from days 7–18 post-infection. However, the virus can persist in skin plugs for about 42 days [Reference Babiuk8].
Morbidity and mortality of the disease vary considerably depending on the breed of cattle, the immunological status of the population, insect vectors involved in the transmission and isolates of the virus. In endemic areas morbidity is usually around 10% and mortality ranges between 1% and 3% [Reference Davies4, Reference Babiuk9]. The most effective method of transmission is mechanically through biting flies [Reference Chihota10–12]. The incidence of LSD occurrence is high during wet seasons when biting-fly populations are abundant and it decreases or ceases during the dry season [12]. LSD has been endemic in Africa for more than 70 years occurring in a wide range of ecotypes. The first outbreak in Egypt was reported in 1988 [Reference Ali5] but the disease has never reached northern African countries. Outside the African continent Israel has reported two outbreaks, the first in 1989 which was eliminated by the slaughter of all infected and contact animals [Reference Yeruham11], and the other more recently in 2006 [Reference Brenner13].
Ethiopia has the most abundant livestock population in Africa [14] and the cattle population is estimated to be 41·5 million [15]. The livestock subsector accounts for 40% of the agricultural gross domestic product (GDP) and 20% of the total GDP without considering other contributions, e.g. traction power, fertilization and transportation [Reference Aklilu, Irungu and Reda16]. In 2004 the livestock sector contributed around 12% of total foreign currency earnings [17]. About 99% of cattle populations are of local Zebu breed [15]. Genetically and geographically the main breed classifications in Ethiopia are Arsi, Fogera, Horo, Borana, Nuwer, Sheko and Afar breeds. The remaining 1% of exotic breeds are kept mainly for dairy production in and around urban areas. Traditional cattle management in the rural part of the country is extensive. Animals are free-ranging in communal grazing fields and different age groups are herded together. Natural grass, post-harvest crop residuals and straw are the main source of feed. Concentrate feeds and feed additives are seldom used [Reference Alemayehu18].
LSD was first observed in the western part of Ethiopia (southwest of Lake Tana) in 1983 [Reference Mebratu19]. It has now spread to almost all the regions and agro-ecological zones [20]. Vaccination is classically used to control outbreaks whenever they occur. Few epidemiological studies have been carried out since the disease has become established in the country, with limited scope in terms of the diverse agro-ecological and production systems. Studies based on clinical disease observation around Nekemt town have reported a prevalence of 7·02% [Reference Regassa21]. Another study based on seroprevalence in southern Ethiopia reported a prevalence of 6% [Reference Gari7]. Targeted sampling from outbreak areas around Southern Range land, Wolliso town and north Ethiopia reported prevalences of 11·6%, 27·9% and 28%, respectively [Reference Gari7, Reference Beshahwured22, Reference Asegid23]. Taking into account the countrywide distribution of the infection and the size and structure of the cattle population in Ethiopia it is likely that LSD is one of the most economically important livestock diseases in the country. This study aimed to address important knowledge gaps regarding the magnitude of LSD occurrence in different agro-climatic conditions and the associated risk factors. A cross-sectional study based on a questionnaire survey was conducted along with retrospective data investigation (the questionnaire is available online).
METHODS
Administrative and agro-climate structure in Ethiopia
The Ethiopian administrative structure encompasses 11 regions that include about 546 districts. Each district is composed of a different number of peasant associations (PA) [15]. Ethiopia's topography consists of a central high plateau bisected by the Great Rift Valley into northern and southern highlands and surrounded by lowlands. The lowland areas are more extensive on the east and southeast of the country than on the south and west (Fig. 1). The diverse topographic structure of the country forms the basis for several agro-climatic zones. The highlands range from 2300 to 3500 metres above sea level (m.a.s.l.) surrounded by a temperate transition zone between 1500 and 2300 m.a.s.l. Those areas having an altitude <1500 m.a.s.l. are classified as lowlands and include the Rift Valley, the southeast, southern and western border lands. The daytime temperature in the lowlands varies from 30°C to as high as 50°C in Denakil depression [24]. The rainfall has two major seasons: a long rainy season that occurs between mid-June to mid-September representing around 75% of the annual rainfall and a short rainy season from mid-February to end of April. In general, relative humidity and rainfall decrease from south to north and are always meagre in the eastern and south-eastern lowlands ranging from 50 to 300 mm per year [25]. The agricultural production system is mainly a sedentary crop–livestock production system in midland and highland altitudes whereas in most lowland parts semi-pastoral and pastoral production systems are dominant (herd-owners move their animals seasonally in search of feed and water, sometimes over long distances) [Reference Alemayehu18].

Fig. 1. Ethiopian topography and regional administrative divisions and lumpy skin disease (LSD) study sites.
Sampling technique and data collection
A cross-sectional study based on the administration of questionnaires to 330 herd-owners was conducted from April 2007 to July 2008 in 44 PAs distributed in 15 districts which were selected from 4/11 regions [Amhara, Oromia, Southern Nations, Nationalities and People region (SNNPR), Afar] as illustrated on Figure 1 and Table 1. The selection of the 44 PAs was performed using a multi-stage sampling strategy with four hierarchical stages. Regions followed by districts were purposively selected to include the main agro-climatic variations and different farming systems. At the third level, the selection of three PAs from each district was based on geographical representation and accessibility in consultation with district experts. The sample frame for PA was obtained from respective district agricultural offices. Finally individual herd-owners and their respective herds were selected for interview based on willingness to participate in the study. The questionnaire was administered by the first author during face-to-face interviews with the farmers using the local language.
Table 1. Administrative hierarchy of study locations, official reported and non-reported lumpy skin disease outbreaks in the selected districts and number of PA affected for years 2000–2007

H, Highland; L, Lowland; M, Midland; n.r., Non-reported outbreaks; PA, peasant association; SNNPR, Southern Nations, Nationalities and People region.
Source: MoARD, Veterinary Service Department and respective district agricultural offices.
In addition to the data produced from the questionnaires, data relating to LSD occurrence in the study area and countrywide for years 2000–2007 were obtained from district agricultural office documentation and from the national disease outbreak report database. Annual rainfall and temperature data for the same period were obtained from FAO Cropwat (CLIMWAT-database) [Reference Water26].
Questionnaire
The questionnaire was designed based on previous knowledge of the disease. Sixteen questions were structured under three main sections. The section on ‘The history of LSD occurrence’ included questions about year and month of LSD occurrence, number, sex and age of affected and/or animals that had subsequently died. The farmer's ability to identify LSD infection was cross-checked by enquiring about the clinical signs and local vernacular name for LSD. Sometimes the description of the disease was necessary in order to avoid confusion with other possible skin diseases such as bovine herpes mammilitis, dermatophilosis, demodecosis, and ringworm. Respondents describing the occurrence of a case with a clinical sign of generalized skin nodules, fever, peripheral lymph nodes swelling and discharge from eyes, nostrils and mouth were tentatively diagnosed as LSD by the authors. Moreover, epidemiological records from district public veterinary clinics were consulted to verify the occurrence of LSD in the time and place specified by the respondents. Recorded evidences of LSD occurrence both at district and central government levels were fully based on clinical disease observation reports.
The ‘Herd management’ section included questions about sedentary/transhumant farming system, herd size, vaccination against LSD, grazing/watering point management, contact with sheep and goats and introduction of new animals. Under the section entitled ‘Biting flies of cattle’, a question relating to the months during which the activity of biting flies was at its highest was recorded. In addition to the information obtained from the herd-owners, we recorded administrative hierarchies (region, districts, PAs) and their agro-climate classification for each questionnaire. ‘Herd’ was defined as a collective group of cattle from a single farmer ownership or family members. When at least one animal in a herd was reported as having had the infection in the past, the entire herd was considered infected. All the data was entered and stored electronically in Microsoft Excel XP 2003.
Data analysis
Data from the national disease outbreak report database and from district agricultural office documentation were compared to assess whether these two sources agreed about the occurrence of LSD in the selected districts. Data from both sources were then explored to investigate the disease distribution pattern in the country and LSD occurrence history in the selected study districts [20].
Descriptive statistics of the studied variables were obtained. Herd-level and animal-level prevalence, as well as the frequencies associated with four binomial variables (farming system, grazing and watering point management, introduction of new animals, and contact with sheep and goats) were estimated for each of the three agro-climate zones using back-transformed point estimates and confidence intervals from logistic regressions with agro-climate as a single explanatory variable. Statistical significance of the variation in agro-climate zones was assessed using homogeneity χ2 test for these prevalences and frequencies.
Spearman rank correlation tests were used to assess the temporal association between LSD occurrence and increase in the biting-fly population. For these correlation tests, the different months were considered as different statistical units. The number of respondents in each month for which the month was designated as a time when LSD occurred was considered as one variable. The other variable considered for the correlation test was the number of respondents for which this month was designated as a time when biting-fly density was increasing. One distinct test was performed for each type of agro-climatic zone.
Analysis to identify the risk factors of LSD considered the following potential risk factors: agro-ecological category, type of farming system, type of grazing and watering point management, introduction of new animals, herd size, and contact with sheep and goats. The age composition of the population at risk (i.e. the herd) at the time when LSD infected the herd could not be obtained from the farmers because the farmer could not recall this quite complex piece of information. It is therefore difficult to assess whether or not age is a risk factor for LSD from these data. Each of these factors was first tested for its association with LSD occurrence at herd level by means of χ2 association test for categorical variables and Kruskal–Wallis rank test for count variable. The factors that turned out to be associated with LSD occurrence with a P value <0·20 were shortlisted. All the factors shortlisted were included in a multiple logistic regression model. This model was then reduced step-wise by removing the factors with a P value >0·05. Finally, the effects of pair-wise interactions between all factors retained in the final model were tested [Reference East27–Reference Ellis-Iversen29]. Confounding was considered present if the coefficients of a variable in the final model changed by >50% compared to its value in univariate regression [Reference East27]. Odds ratio estimates were computed from the coefficients of the final multiple logistic regression model using an exponential transformation. The goodness-of-fit (GOF) test for all models was assessed using Pearson's χ2 GOF test for logistic regression.
Statistical analyses were conducted using Stata 8.0 (Stata Corp., USA, 1984–2003) and R 2.8.1 (R Development Core Team, Austria, 2008).
RESULTS
Description of LSD occurrence report data
Data investigations from the national disease outbreak report database and documentation of the respective district veterinary services showed that all selected districts had experienced at least one LSD outbreak during the period 2000–2007. A major epidemic outbreak of LSD occurred in 2000/2001 in the northern parts of the country in Amhara and West Oromia. Then it extended to the central and the southern parts of the country in 2003/2004 covering large parts of Oromia and SNNP regions. In 2006/2007 another extensive outbreak reappeared in Tigray, Amhara and Benishangul regions in the northern and north-western parts of the country. The frequency of occurrence of LSD was extremely variable across the study districts. Some districts reported outbreaks almost every year of the period considered (Yabello, Sebeta Awas), whereas others faced a limited number of outbreaks as shown in Table 1. In terms of the size and magnitude of its occurrence, an epidemic of LSD covering a number of PAs was reported to have occurred in some selected study districts (Adola and Yabello districts) in the years 2003–2005; other districts (Amuru and Bako-Tibe) experienced small foci of cases involving few animals. An outbreak record discrepancy between national disease outbreak data and district documentation report was noted in five districts (Bako-Tibe, Amuru, Chora, Sokoru, Ade'a) for which the cases had not been declared at national level although LSD had been reported at district level involving a different number of PAs.
Variation of LSD occurrence and its potential risk factors in agro-climatic zones
Of the 330 questionnaires administered in 44 PAs, 103 were collected from highlands, 165 from midlands and 62 from lowlands. Different vernacular names of LSD were recorded from different sampling locations, e.g. Suki, Kodhobo, Guribrib, Gifir.
Farmers in highland and midland agro-climate zones described their farming system as sedentary. In the lowland agro-climate, about 51·6% of herd-owners reported exercising a transhumant mode of life (Table 2). Communal grazing and watering point resource utilization was dominant in all highland, midland and lowland agro-climate zones (81·5%, 94·5% and 80·6%, respectively) and significantly higher in midland agro-climate zone.
Table 2. Descriptive statistics of potential risk factors assessed by the questionnaire survey

Values in parentheses are 95% confidence intervals.
*** P=0·001, ** P=0·01.
A noticeable proportion of farmers (32·1%) reported introducing new animals to their herd following purchase (for replacement, herd expansion, fattening), receiving cultural gifts or cattle exchange without any screening for the health status of the new animal. The frequency of introduction of new animals was higher in the midland agro-climate zone (40·6%) than in the highland and the lowland zones (25·2% and 21%, respectively).
Of the interviewees 42·8% reported occurrence of LSD in their herd. This proportion was higher in the midland zone (55·2%) than in the highland and the lowland zones (22·3% and 43·5%, respectively). Out of the total 4438 animals that farmers had owned, 8·1% were declared as having had LSD (observed prevalence) and 2·12% as having subsequently died (observed mortality). Observed prevalence and observed mortality were significantly higher in the midland zone (10·4% and 3·2%, respectively) than in lowland and highland zones (P<0·05) (Table 2).
The temporal association between LSD occurrence and increase in the biting-fly population was positive and significant (Spearman rank correlation coefficient was 0·88, 0·79 and 0·79 for highland, midland and lowland zones, respectively, and corresponding P values were 0·0001, 0·002 and 0·002). The time at which the biting-fly population begins to build up and reach its highest peak size was found to follow a similar pattern to the temporal pattern of LSD occurrence as shown on Figure 2. Both biting-fly activity and disease outbreak frequencies begin to increase from April reaching a maximum in September.

Fig. 2. Questionnaire survey results of seasonal increase in biting-fly activity vs. lumpy skin disease (LSD) occurrence. Data source for rainfall data: FAO Cropwat database. · · · ▴· · · , Average rainfall; - -◆- - , LSD outbreak; ![]() , fly population.
, fly population.
Logistic regression analysis
The final multiple regression model included effects of agro-climate, type of grazing/watering management and introduction of new animals as shown in Table 3. Communal grazing/watering point utilization increased the risk of LSD compared to privately owned resources [odds ratio (OR) 4·1]. Herds with new cattle introduced were also more likely to be infected by LSD than herds where no new animals had been added (OR 8·5). Agro-climate was significantly associated with LSD occurrence and the odds ratio in the final model for midland vs. highland and lowland vs. highland were 3·86 and 4·85, respectively. After having accounted for the effect of the two other variables included in the final model (grazing/watering management and introduction of new animals), the occurrence of LSD was much more likely in midland and lowland than in highland zones. Farming system, herd size and contact with sheep and goats were not significantly associated with LSD occurrence by both univariate tests and multivariable logistic regression model. No interaction between variables was observed. With a Pearson χ2 GOF test for logistic regression the P value was 0·55 which meant that the final model fitted the data adequately.
Table 3. Multivariable logistic regression model analysis of potential risk factors for lumpy skin disease occurrence at herd level
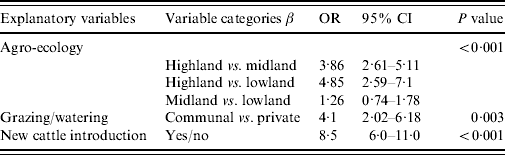
OR, Odds ratio; CI, confidence interval.
DISCUSSION
Immunity to LSD infection is predominantly driven by a cell-mediated response. Humoral immunity remains detectable only for 7 months [6, Reference Lefévre, Gourreau, Lefévre, Blancou and Chermette30]. Serological studies with vaccinated cattle have shown that many animals resist challenge with virulent LSDV while they have no detectable antibody [Reference Davies4]. Thus although LSD surveillance based on serological results would probably not have shown the long-standing infections and the true immunological status of the target population [Reference Gari7], the current questionnaire survey results have allowed us to evaluate at least 3 years of retrospective disease information in the studied areas. The retrospective questionnaires were limited to the 3-year period because of the possibility of memory loss regarding numerical estimates of reported facts by the farmers, thereby reducing potential recall bias beyond that time period.
The study represents the majority crop–livestock production system in the highland and midland agro-climates and has also included classical areas of semi-pastoral production system in the lowlands. Probabilistic sampling is a challenging task in a country with an infrastructure such as Ethiopia, since large areas have to be covered which are not easily accessible. Moreover, sampling frames of lower administrative units are not often available at central level. Under these circumstances, multi-stage sampling is the preferred technique to limit selection bias since the decision is then made with closer insight to the target population [Reference Waret-Szkuta31]. Nevertheless, our study might still have some degree of selection bias influencing the results.
The study was based on the symptomatic disease identification experience of the herd-owners complemented by veterinary office epidemiological records at different levels. Endemically occurring skin diseases of cattle such as bovine herpes mammilitis, dermatophilosis, demodecosis, and ringworm were taken into consideration in the differential diagnosis but we cannot exclude the possibility of some degree of misclassification. In the same way confusion with pseudo-LSD cases might have occurred although its presence has not yet been confirmed in Ethiopia. The discrepancy on LSD outbreak between the selected study districts and the national level reports was presumed to be the combination of errors committed at each level of reporting from grassroots to national level. The data obtained from the selected districts were considered to be a more credible source of information than from the national level [Reference Mork32].
The magnitude and frequency of LSD occurrence varied across districts during the study period. The observed LSD prevalence at animal level found in this study was 8·1% which was close to the 10% reported by Davies [Reference Davies4] and Babiuk et al. [Reference Babiuk9] in endemic areas of Africa. Prevalence of LSD in southern Ethiopia was less than the estimate reported here. This difference might be attributable to the fact that the seroprevalence method could underestimate the prevalence of LSD [Reference Gari7]. However, the study conducted around Nekemt town reported a similar result and it was in the 95% confidence interval range of our finding [Reference Regassa21]. Apparent mortality in the present study was 2·12% which agreed with the previous reports by Davies and Babiuk et al. [Reference Davies4, Reference Babiuk9], whose results ranged between 1% and 3% and with the previous studies done in Ethiopia.
The potential risk of agro-climate variations in LSD occurrence showed that midland and lowland agro-climates were more likely to be at risk for LSD occurrence than the highland agro-climate. This association might be attributed to the availability and abundance of effective mechanical vector insects. The temporal association between LSD occurrence and the biting-fly population found in our study suggested that mechanical vector insects might play a major role in the epidemiology of LSD, and agro-climate variation could be the basis for the type and abundance of speculated mechanical vector insects. The warm and humid climate in midland and lowland agro-climates has been considered a more favourable environment for the occurrence of large populations of biting flies than the cool temperature in the highlands [Reference Zumpt33–Reference Kettle35]. The use of insecticides to control biting flies is a rare practice in Ethiopia except for few areas in tsetse-infested zones where pour-on drugs are used.
Regarding the extensive livestock production system, communal grazing and watering point resource utilization was dominant in all agro-climate zones. Herd contact and mixing is likely to occur in communal grazing and watering points. However, in districts like Fentale where irrigation-fed agriculture has emerged as means of subsistence in some PAs, cattle were kept on private grazing plots which relatively lowered the percentage of communal grazing and watering resource management in lowland agro-climate zones (80·6%). Herd mixing is assumed to be less likely to occur in private grazing plots.
For the midland agro-climate, introduction of new cattle was associated with an increase in the risk of disease introduction to the herd, as already reported for infectious diseases such as tuberculosis and paratuberculosis [Reference Tiwari36, Reference Tschopp37]. Communal grazing and watering point utilization were found to be significantly associated with LSD occurrence. Sharing watering points, grazing plots and post-harvest fields would allow contact and intermingling of different herds that would probably increase the risk of exposure and enhance the virus transmission through the speculated mechanical vectors such as Stomoxys spp. and mosquitoes (Aedes aegypti) [Reference Chihota10, Reference Kitching and Mellor38, Reference Waret-Szkuta and Newton39]. The host's reaction to the piercing pain from the fly's bite would interrupt the insects' feeding which would lead to the flies looking for other nearby hosts to complete their feeding, allowing the transmission of the infection from infected to susceptible animals [Reference Waret-Szkuta and Newton39, Reference Desquesnes and Dia40]. Contamination of the pasture and water could be considered as a potential risk in communal grazing and watering point utilization despite the fact that contagious transmission is considered to be inefficient route of transmission [Reference Davies4, Reference Babiuk8, Reference Lefévre, Gourreau, Lefévre, Blancou and Chermette30].
Cattle movement due to farming system was not significantly associated with LSD occurrence in contrast with the findings of Munyeme et al. and MacPherson [Reference Munyeme28, Reference MacPherson41] who reported the transhumant system as a risk factor for infectious disease transmission. This could imply that cattle movement due to farming system might not have a significant role for the spread of LSD due to the inefficient contagious transmission nature of the LSDV [Reference Kitching and Mellor38].
Our study shows that LSD has been extensively circulating across diverse agro-climatic zones with large variations between districts that could be attributed to their respective agro-ecological zones and farming practices. Moreover, factors such as virus isolates, the breed of cattle, the immunological status of the population and the vector insects involved in the transmission should also be considered for the variations observed in our findings [Reference Davies4, Reference Babiuk9, 12]. Further study is required to elucidate vector insects incriminated in the transmission of LSDV and their dynamics in different agro-ecologies. Hence it is likely that improved awareness by farmers and veterinary services on the potential disease transmission associated with shared use of grazing areas and watering points as well as promotion of biosecurity consciousness in the management of the introduction of new animals may assist in the control and prevention of infectious diseases in herds in Ethiopia. Knowledge-driven risk maps could also be built based on better knowledge of risk factors associated with LSD and could help target disease surveillance and control activities. These findings are also important to direct future studies in other countries where LSD is an important livestock disease problem.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
This work was financed by the General Directorate for Development and International Cooperation, French Ministry of Foreign Affairs, French Embassy in Ethiopia through PSF No. 2003-24 LABOVET project. The authors are grateful for the technical and practical support given by the Sebeta NAHDIC. We also thank Dr Amsalu Demissie from MoARD Veterinary Section for information provided and the district agricultural offices and veterinary section staff that facilitated the data collection in the field, and finally Veronica Marsch (RVC, London) who improved the manuscript's English.
DECLARATION OF INTEREST
None.







