Ethnic inequalities in type 2 diabetes in the UK
It is estimated that one in eleven adults worldwide is living with diabetes; this equates to 425 million people, a figure which is predicted to rise to 629 million by 2045(1). The alarming rate at which diabetes is increasing makes it a global public health priority. In the UK, 3·7 million people have diagnosed diabetes, although the actual prevalence is thought to be nearer to 5 million when undiagnosed cases are considered(2). Ethnic inequalities in diabetes have been reported consistently in the scientific literature. The present paper will focus specifically on type 2 diabetes (T2D) as it is the burden of this disease that is most evident among ethnic minority groups.
In 2011, approximately 14 % of the UK population identified as from a minority ethnic background; about half were of South Asian ancestry (originating mainly from India, Pakistan, Bangladesh and Sri Lanka), and approximately 25 % were of black, African, Caribbean or other black ancestry(3). Among ethnic minority groups, the prevalence of T2D is estimated to be about three to five times higher than in people of white European ethnicity(Reference Becker, Boreham and Chaudhury4). The London SABRE multi-ethnic cohort estimated that by age 80 years, 40–50 % of South Asian and black African-Caribbean men and women will have T2D, which is at least twice the proportion of their age-matched white European counterparts(Reference Tillin, Hughes and Godsland5). An earlier onset of T2D is also particularly noticeable. A recent analysis of UK primary care data showed the age of diagnosis to be 10–12 years younger, on average, in South Asians and black African-Caribbeans compared to white Europeans(Reference Paul, Owusu Adjah and Samanta6). Moreover, a significantly greater proportion of people from ethnic minority backgrounds develop T2D before age 40 years compared to white-Europeans: 30 % of South Asians and 23 % of black African-Caribbeans with T2D are under age 40 years compared to only 9 % of white Europeans (Fig. 1)(Reference Paul, Owusu Adjah and Samanta6).
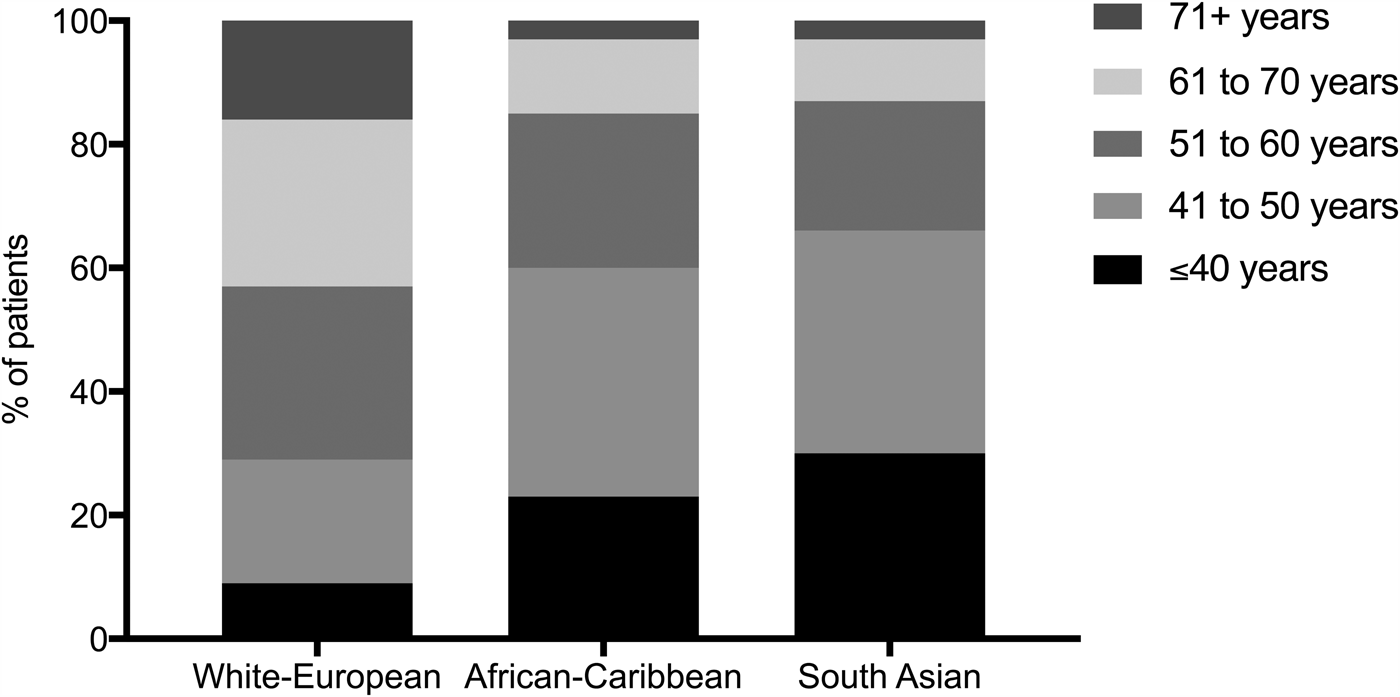
Fig. 1. Age distribution of people with type 2 diabetes in White-European, African-Caribbean and South Asian ethnic groups in the UK. Reproduced from Paul SK et al.(Reference Paul, Owusu Adjah and Samanta6).
Following the recognition that 90 % of adults with T2D are overweight or obese, and T2D is five times more prevalent amongst adults with an obese BMI compared to those with a healthy weight(Reference Abdullah, Peeters and de Courten7), obesity is now recognised to be one of the strongest contributors to the development of T2D. In the UK, about 60 % of adults are overweight or obese (BMI ≥25 kg/m2) and 25 % are obese (BMI ≥30 kg/m2)(8). In some ethnic groups, obesity rates are high, for example, black African-Caribbean and Pakistani women, but in others (e.g. black African-Caribbean, Indian, Pakistani and Bangladeshi men), the rates are no different from white Europeans or the general population(Reference Becker, Boreham and Chaudhury4). This pattern of obesity being more common among ethnic minority populations and particularly among ethnic minority females is replicated in America, in which African-American and Hispanic women suffer the highest rates of obesity(Reference Arroyo-Johnson and Mincey9–Reference Cossrow and Falkner11). The reasons for these ethnic and sex disparities are complex and not fully understood but are believed to involve both biological and environmental factors(Reference Zhang and Wang12). Recently several UK multi-ethnic cohort studies have identified a higher risk of T2D at lower levels of obesity among ethnic minority groups compared with white Europeans(Reference Paul, Owusu Adjah and Samanta6,Reference Ntuk, Gill and Mackay13,Reference Tillin, Sattar and Godsland14) . Modelling of UK Biobank data has demonstrated that the T2D risk associated with a BMI of 30 kg/m2 in white Europeans is equivalent to a BMI of 22 kg/m2 in South Asian groups and a BMI of 26 kg/m2 in black African-Caribbean groups(Reference Ntuk, Gill and Mackay13). Data from The Health Improvement Network, a UK longitudinal general practice dataset, demonstrate that 38 % of South Asians and 29 % of black African-Caribbeans with T2D have a BMI below 30 kg/m2 compared to only 26 % of white Europeans. Furthermore, both South Asians and black African-Caribbeans have a significantly higher probability of developing T2D in the normal and overweight BMI categories compared to white Europeans(Reference Paul, Owusu Adjah and Samanta6). This evidence suggests that the commonly used clinical definitions for obesity, that are derived from populations of white European descent (BMI ≥30 kg/m2; waist circumference ≥88 cm in women and ≥102 cm in men), may not be appropriate for screening diabetes risk in non-white groups. Accordingly, the WHO and International Diabetes Federation have proposed that overweight is defined as BMI >23 kg/m2, and obesity >27·5 kg/m2, in Asian adults, with waist circumference cut-offs of 80 cm for Asian women and 90 cm for Asian men(15,16) , although there is pressure for lower cut-offs to be introduced(Reference Misra, Chowbey and Makkar17). Currently there are no agreed specific cut-offs for men and women of black African-Caribbean ethnicity and European thresholds remain in use for these populations.
The development of diabetes often manifests itself, clinically, as the metabolic syndrome. First described in the 1980s, the metabolic syndrome is a clustering of metabolic abnormalities, namely abdominal obesity, hypertension, dyslipidaemia (low HDL-cholesterol and high TAG concentrations) and hyperglycaemia, which are associated with insulin resistance and commonly precede the onset of T2D(Reference Reaven18). In 2006, the International Diabetes Federation published a consensus worldwide definition to aid detection of metabolic syndrome(16). Using these criteria, it is estimated that about 22 % of adults have the metabolic syndrome and will go on to develop T2D(Reference O'Neill and O'Driscoll19). Studies that have investigated the prevalence of metabolic syndrome among high-risk ethnic groups have identified that the diagnostic criteria fail to detect T2D risk in populations of black African ancestry(Reference Sumner20–Reference Sumner, Finley and Genovese23). Looking in more detail, several interesting differences from other ethnic groups have been described, and a distinct metabolic phenotype is emerging. Consistently, black African-Caribbean populations have been reported to exhibit pronounced insulin resistance(Reference Haffner, D'Agostino and Saad24–Reference Osei and Cottrell27) and higher rates of hypertension compared to other ethnic groups(Reference Chaturvedi, Marmot and McKeigue28,Reference Chaturvedi, McKeigue and Marmot29) but in the absence of abdominal obesity(Reference Haffner, Howard and Mayer25,Reference Chaturvedi, McKeigue and Marmot30–Reference Park, Zhu and Palaniappan32) and the characteristic dyslipidaemia of the metabolic syndrome(Reference Zoratti, Godsland and Chaturvedi26,Reference Chaturvedi, McKeigue and Marmot30,Reference Goff, Griffin and Lovegrove33) . Raised fasting TAG and low HDL-cholesterol concentrations are the principal lipid abnormalities associated with insulin resistance and T2D and are included in the diagnostic criteria for the metabolic syndrome. Often the TAG:HDL ratio is used as a lipid metric to detect T2D risk but there is extensive evidence to show that it fails to detect risk in populations of black African-Caribbean ethnicity(Reference Sumner20–Reference Sumner, Finley and Genovese23). These findings from large epidemiological studies have provided evidence to suggest there are ethnic distinctions in the pathophysiology of T2D among black African-Caribbean ethnic groups, which have been investigated in detail in recent years.
Pathophysiology of type 2 diabetes
The main processes underlying the development of T2D are well described and emphasise the role of obesity, visceral and ectopic fat accumulation, insulin resistance and insulin secretory failure(Reference Defronzo34). Originally insulin resistance was proposed as the primary abnormality(Reference Reaven18) but earlier defects within adipose tissue are now believed to trigger a cascade of metabolic abnormalities, involving the liver, skeletal muscle and pancreas(Reference Scheen35). Three separate, but not mutually exclusive, theories explain how dysfunctional adipose tissue, and its inability to buffer excess fat, is proposed to be a primary defect underlying the development of T2D (Fig. 2). The spillover theory proposes that it is a limited capacity of subcutaneous adipocytes to store fatty acids which results in an overflow of fatty acids to the visceral compartment and expansion of this depot(Reference Lewis, Carpentier and Adeli36). The portal theory proposes that hypertrophic dysfunctional visceral adipocytes are highly lipolytic and have a greater flux of fatty acids than subcutaneous adipocytes, which are released into the portal circulation and become deposited in the liver, leading to ectopic fat accumulation in the liver and hepatic insulin resistance(Reference Bergman and Ader37). The twin-cycle hypothesis proposes that increased hepatic fat accumulation leads to increased export of VLDL-TAG from the liver, which then deposits in other organs and tissues, particularly the pancreatic β-cells, leading to the β-cell failure that underlies the development of frank T2D(Reference Taylor38). It is the accumulation of TAG within these ectopic depots that is believed to play an integral role in the development of T2D by causing metabolic disturbances within the organs/tissues in which it resides, termed lipotoxicity. Ectopic depots of importance include intrahepatic lipid (IHL), intramyocellular lipid and intrapancreatic lipid (IPL). In response to hepatic and peripheral insulin resistance, compensatory hypersecretion of insulin ensues to maintain normoglycaemia. Eventually ‘β-cell exhaustion’ or ‘burn-out’ occurs whereby the β-cells are unable to secrete sufficient insulin and a hyperglycaemic state develops(Reference Scheen35). Heterogeneity in the relationship between insulin sensitivity and insulin secretion has been recognised whereby glucose tolerance can be maintained, even in the presence of severe insulin resistance, if the insulin secretory capacity of the β-cells is able to balance the degree of insulin resistance. Conversely, an individual can become hyperglycaemic with a relatively low level of insulin resistance if they possess a relatively low β-cell secretory capacity(Reference Kahn39).
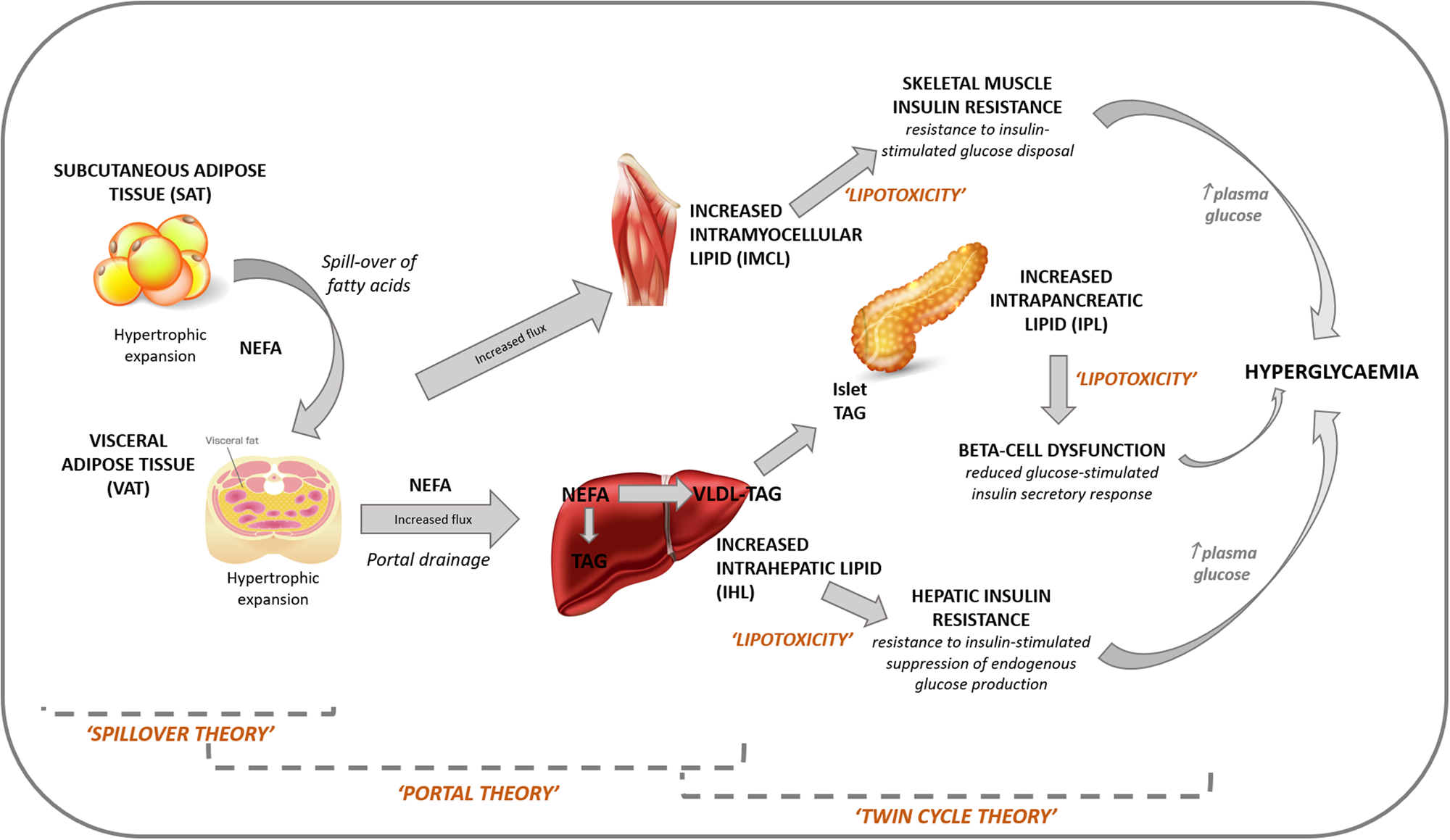
Fig. 2. Role of adipose tissue dysfunction and ectopic fat accumulation in the pathogenesis of type 2 diabetes. The spillover theory proposes that it is a limited capacity of subcutaneous adipocytes to store fatty acids which results in an overflow of fatty acids to the visceral compartment and expansion of this depot. The portal theory proposes that hypertrophic dysfunctional visceral adipocytes are highly lipolytic and have a greater flux of fatty acids, which are released into the portal circulation and become deposited in the liver, leading to ectopic fat accumulation in the liver and hepatic insulin resistance. The twin-cycle hypothesis proposes that increased hepatic fat accumulation leads to increased export of VLDL-TAG from the liver, which then deposits in other organs and tissues, particularly the pancreatic β-cells, leading to the β-cell failure that underlies the development of type 2 diabetes.
Ethnic distinctions in type 2 diabetes pathophysiology: a focus on black African-Caribbean populations
Insulin resistance
The role of insulin resistance in the development of T2D in black African-Caribbean populations has been investigated in many studies. Large cohort studies have consistently reported exaggerated insulin resistance among black African-Caribbean populations(Reference Zoratti, Godsland and Chaturvedi26,Reference Chaturvedi, McKeigue and Marmot30) . The first of these was the insulin resistance atherosclerosis study(Reference Haffner, D'Agostino and Saad24,Reference Haffner, Howard and Mayer25) , which was conducted in America in the 1990s. A limitation of these epidemiological studies is their use of indirect methods for assessing insulin sensitivity, such as the homeostatic model assessment(Reference Matthews, Hosker and Rudenski40) and the intravenous glucose tolerance test, which provide just an estimate of insulin sensitivity, and only measure at a whole-body level. The euglycaemic–hyperinsulinaemic clamp is considered the gold standard method for assessing insulin sensitivity(Reference DeFronzo, Tobin and Andres41), which, when used with the addition of stable isotopes, enables specific measurement of hepatic and skeletal muscle insulin sensitivity, thus providing greater insight as to where the defects in insulin action are occurring. There have been several studies investigating in vivo tissue-specific insulin resistance using the euglycaemic–hyperinsulinaemic clamp method with isotopes in black and white communities. These studies have primarily focused on women and high-risk adolescents, e.g. those with morbid obesity or prediabetes. In adolescents, peripheral insulin sensitivity has been shown to be lower(Reference Lee, Boesch and Kuk42–Reference Arslanian, Saad and Lewy44) or no different(Reference Hannon, Bacha and Lin45–Reference Lee and Arslanian47) in black compared to white populations with no clear reasoning as to why they are different. In terms of hepatic insulin sensitivity, black populations show no difference(Reference Lee, Boesch and Kuk42–Reference Hannon, Bacha and Lin45,Reference Burns, Kelsey and Arslanian48–Reference Schuster, Kien and Osei51) or greater sensitivity(Reference Bacha, Saad and Gungor46) compared to white adolescents. The greater hepatic insulin sensitivity in black adolescents is acknowledged as a surprise to the authors who believe it may be explained by lower visceral adipose tissue (VAT). Studies in women assessing peripheral insulin sensitivity show either no ethnic differences(Reference Goedecke, Keswell and Weinreich52) or reduced sensitivity(Reference DeLany, Dube and Standley53) in black compared to white populations. The differences here are likely due to the choice of female adiposity status (obese v. lean participants, respectively). Studies in women, which have assessed hepatic insulin sensitivity, show no difference(Reference DeLany, Dube and Standley53,Reference Chung, Courville and Onuzuruike54) , greater(Reference Goedecke, Keswell and Weinreich52) or lower(Reference Ellis, Alvarez and Granger55) sensitivity in black compared to white populations. The inconsistences here are likely due to methodological differences, where hepatic insulin resistance has been measured either at basal or during a hyperinsulinaemic state, or the obesity status of the participant populations. The very limited mixed sex studies have revealed no ethnic differences in peripheral(Reference Stefan, Stumvoll and Weyer56,Reference Pratley, Wilson and Bogardus57) or hepatic(Reference Stefan, Stumvoll and Weyer56) insulin sensitivity in black compared to white populations. Within black populations, women have been shown to display lower insulin sensitivity in comparison to men(Reference Goedecke, George and Veras58,Reference Falkner, Hulman and Kushner59) . They also present with a different body composition which suggests a sex-specific pathophysiology may be present. Overall these inconsistent findings could imply that the methods to assess insulin sensitivity are affected by ethnicity or that insulin resistance is not the sole driver for the increased T2D risk in black populations.
Insulin secretory function
Unlike the assessment of insulin sensitivity, there is no widely accepted gold standard methodology for the determination of insulin secretory function; therefore, the exploration of ethnic differences in this area has been complicated by the wide variety of techniques used. The most common techniques measure the insulin response to stimulation of the β-cell by glucose, either intravenously (as in the case of the hyperglycaemic clamp or intravenous tolerance test) or orally (as in the case of the oral glucose tolerance test or the mixed meal tolerance test). Surrogate indices are also used, which may be derived from fasting glucose and insulin, such as homeostasis model assessments of β-cell function(Reference Matthews, Hosker and Rudenski40), or from the oral glucose tolerance test or mixed meal tolerance test, such as the insulinogenic index. Each method has its strengths and limitations, for example, oral glucose and mixed meal tests are highly physiological while intravenous techniques allow specific assessment of the β-cell by excluding the modulating effect of the incretin hormones(Reference Ferrannini and Mari60,Reference Cobelli, Dalla Man and Toffolo61) . Most of the studies in the literature have employed the intravenous glucose tolerance test in their measurement of insulin secretion. A recent review of these studies concluded that black African-Caribbeans exhibit a higher insulin response to glucose compared to white Europeans(Reference Kodama, Tojjar and Yamada62). This has been demonstrated in studies in black populations both in African countries(Reference Osei, Schuster and Owusu63,Reference Goedecke, Dave and Faulenbach64) and across the globe(Reference Haffner, D'Agostino and Saad24,Reference Goff, Griffin and Lovegrove33,Reference Chiu, Chuang and Yoon65) . Furthermore, this higher insulinaemic response can be demonstrated from early childhood(Reference Arslanian, Suprasongsin and Janosky66–Reference Uwaifo, Nguyen and Keil68).
In a widely accepted paradigm of T2D, increased insulin secretion represents a compensation for insulin resistance in order to maintain normoglycaemia. Therefore, it is important to note that when studies make an adjustment for the prevailing insulin sensitivity of black populations (e.g. through the calculation of a disposition index(Reference Kahn, Prigeon and McCulloch69)), their insulin response to glucose remains greater than that of white Europeans, suggesting that the phenomenon is not simply a compensatory response to increased insulin resistance(Reference Arslanian, Saad and Lewy44,Reference Hannon, Bacha and Lin45,Reference Osei and Gaillard70,Reference Goree, Darnell and Oster71) . It has therefore been speculated that β-cell function may be upregulated in black populations, contributing to their increased risk of T2D by predisposing to early β-cell exhaustion(Reference Hannon, Bacha and Lin45).
Hepatic insulin clearance
Circulating concentrations of insulin are determined by a balance between the rate of insulin secretion from the pancreatic β-cells and insulin degradation, which occurs predominantly in the liver. Upon its secretion from pancreatic β-cells, insulin reaches the liver through the portal circulation. Approximately 80 % of endogenous insulin is cleared by the liver during the first portal passage prior to reaching the systemic circulation. While the liver is the primary site of insulin clearance, the kidneys and skeletal muscle are also involved in its degradation(Reference Duckworth, Bennett and Hamel72). To quantify and differentiate the processes of insulin secretory function and hepatic insulin clearance, it is necessary to measure both c-peptide and insulin. C-peptide is co-secreted with insulin in equimolar concentrations but undergoes negligible metabolism in the liver; therefore, the measurement of circulating c-peptide provides a true assessment of the rate of insulin secretion(Reference Polonsky, Jaspan and Pugh73). Looking at all the studies of β-cell function, it is important to note that only a small minority include the measurement of c-peptide and are therefore able to differentiate between insulin secretory function and hepatic insulin clearance. There is increasing evidence that the hyperinsulinaemic response to glucose in black subjects is due to ethnic differences in rates of insulin clearance as well as β-cell insulin secretion. These studies have consistently observed lower rates of insulin clearance in black subjects compared to white(Reference Arslanian, Saad and Lewy44,Reference Uwaifo, Nguyen and Keil68,Reference Weiss, Dziura and Burgert74) . Furthermore, recent advances in modelling techniques have demonstrated that this is due to differences in hepatic rather than extra-hepatic insulin clearance(Reference Piccinini, Polidori and Gower75) and that reduced hepatic insulin clearance in black subjects can also be demonstrated from early childhood(Reference Piccinini, Polidori and Gower76).
Visceral and ectopic fat deposition
The observation that abdominal/visceral fat is a more sensitive predictor of insulin sensitivity than BMI has led to considerable interest in visceral fat in black populations. These studies have recognised that, despite being at high risk of T2D, black populations exhibit significantly less visceral fat than other ethnic groups(Reference Lovejoy, de la Bretonne and Klemperer77), a phenomenon that has been coined the African paradox. In a large pooled analysis of black women, Sumner et al. demonstrated a lesser increase in visceral fat per unit increase in waist circumference in black compared to white women(Reference Sumner, Micklesfield and Ricks78), raising a question regarding the role and importance of visceral fat in the development of T2D in black populations(Reference Goedecke, Mtintsilana and Dlamini79).
Ectopic fat, defined as the deposition of TAG within cells of non-adipose tissue, is proposed to be central to the development of T2D by causing metabolic disturbances in the organs/tissues in which it resides(Reference Gastaldelli and Basta80). Ectopic fat accumulation typically occurs during increasing adiposity due to reduced expandability of adipocytes within subcutaneous adipose tissue (SAT), which leads to a spillover of NEFA into other organs(Reference Unger81). However, individuals who have lipodystrophies, where genetic factors result in an inability to adequately store fat in SAT depots, also have high ectopic fat accumulation and develop insulin resistance at severely low BMI levels(Reference Melvin, O'Rahilly and Savage82). Therefore, an increase in ectopic fat is also considered to be a marker of a metabolic state of overwhelmed dysfunctional SAT caused by its limited expandability. This state is characterised by several features of SAT including hypertrophic adipocyte expansion, increased infiltration of macrophages, reduced insulin sensitivity and increased release of inflammatory cytokines, which are an indicator of chronic low-grade inflammation of SAT(Reference Bays, Gonzalez-Campoy and Bray83). The degree of expandability of SAT, which determines the point at which it dysfunctions, is mostly influenced by intrinsic factors, such as sex, as women have a greater capacity to store SAT leading to lower ectopic fat storage and a lower risk of T2D(Reference Cuthbertson, Steele and Wilding84). Recent research in South Asians has indicated that ethnicity may also influence the genetic factors that determine SAT expandability and ectopic fat storage(Reference Trouwborst, Bowser and Goossens85).
Ectopic fat has been studied in ethnic minority groups to understand the impact of ethnicity on ectopic fat deposition and its role in the development of T2D. South Asian populations exhibit greater levels of VAT and IHL at similar levels of whole-body adiposity compared to white populations(Reference Wulan, Westerterp and Plasqui86,Reference Petersen, Dufour and Feng87) . This has commonly been used to explain the greater risk of T2D in South Asians since they have a reduced capacity to store excess energy in SAT leading to increased ectopic fat deposition(Reference Sniderman, Bhopal and Prabhakaran88). However, the same cannot be said for black populations who, despite being at greater risk of T2D, have lower levels of ectopic fat compared to their white counterparts(Reference Goedecke, Mtintsilana and Dlamini79,Reference Alderete, Toledo-Corral and Goran89) . This phenomenon has previously been coined the African ectopic fat paradox(Reference Goran90).
The advancement of imaging technologies, such as computerised tomography and MRI, has allowed the direct quantification of regional fat deposition. Thereafter, several studies have reported ethnic differences in regional fat deposition between black and white populations. From as early as the 1990s, several studies have reported lower levels of VAT in black populations compared to white populations which has become a well-accepted phenomenon(Reference Goedecke, Mtintsilana and Dlamini79,Reference Alderete, Toledo-Corral and Goran89) . Furthermore, with increasing whole-body adiposity, VAT has been shown to increase to a lesser extent in blacks compared to whites, indicating a greater capacity to store excess energy in the more favourable SAT depot in blacks(Reference Nazare, Smith and Borel91).
While the pathogenic potential of VAT is well accepted in T2D risk, several reports have shown that the accumulation of IHL is more detrimental and plays a greater role in the development of T2D(Reference Okamura, Hashimoto and Hamaguchi92–Reference Lee, Chung and Kang94). Similarly to VAT, IHL has also been reported to be consistently lower in black compared to white populations(Reference Guerrero, Vega and Grundy95,Reference Liska, Dufour and Zern96) . Furthermore, several large cohort studies have shown that the prevalence of non-alcoholic fatty liver disease is considerably lower in black populations and, in some studies, the prevalence is half that of white populations(Reference Rich, Oji and Mufti97,Reference Pan and Fallon98) . Lower levels of IHL, along with more favourable lipid profiles and reduced metabolic disturbances of the liver, have led researchers to question the understanding of the role of metabolic disturbances of the liver in the development of T2D in black populations(Reference Chung, Courville and Onuzuruike54,Reference D'Adamo, Northrup and Weiss99) .
Unlike VAT and IHL, there has been less investigation of IPL and intramyocellular lipid in black populations, which may be due to limitations in the techniques used to determine these depots, or the lack of clarity regarding their role in the development of T2D. Current investigations show that while intramyocellular lipid does not appear to differ between black and white populations, it is inversely associated with insulin sensitivity in white populations but not black populations, which may indicate a lesser role for intramyocellular lipid in insulin resistance in black individuals(Reference Ingram, Lara-Castro and Gower100,Reference Lawrence, Newcomer and Buchthal101) . Investigations of IPL in black populations provide inconclusive findings; while IPL appears lower in black populations compared to whites(Reference Szczepaniak, Victor and Mathur102,Reference Le, Ventura and Fisher103) , some reports find it to be inversely associated with β-cell insulin secretory function in blacks(Reference Szczepaniak, Victor and Mathur102), while others report no such association(Reference Le, Ventura and Fisher103). In a study investigating the role of IHL and IPL in blacks, Toledo-corral et al. showed IPL predicted prediabetes status in blacks while IHL did not, indicating IPL may be more detrimental than IHL in black populations(Reference Toledo-Corral, Alderete and Hu104).
The South London Diabetes and Ethnicity Phenotyping study
The South London Diabetes and Ethnicity Phenotyping (SouL-DeEP) study is being conducted to provide a detailed comparison of the principal pathophysiological processes involved in the development of T2D between men of black west African and white European ethnicity, to test the hypothesis that T2D is driven by relatively early failure of β-cell insulin secretory function in black west African men. Highly sophisticated techniques are being used: hyperglycaemic clamp and meal tolerance test methods enable the assessment of β-cell function, hepatic insulin clearance and incretin secretion; the euglycaemic–hyperinsulinaemic clamp with stable isotope infusions enables the assessment of insulin sensitivity at a whole-body level as well as distinguishing hepatic, skeletal muscle, and adipose tissue insulin sensitivity; and MRI and spectroscopy methods are being used to assess visceral fat and ectopic fat deposition in the skeletal muscle, liver and pancreas. Our study focuses on men as there is consistent evidence for sex differences in T2D pathophysiology in populations of black African ancestry(Reference Carnethon, Palaniappan and Burchfiel105,Reference Harris, Cowie and Gu106) . While the development of T2D in black women has been reasonably well studied(Reference Goedecke, Keswell and Weinreich52,Reference Goedecke, Dave and Faulenbach64,Reference Ellman, Keswell and Collins107) , there is a relative lack of studies conducted in men, despite high rates of T2D, which we aim to address through our work.
In our study of men with T2D, we have demonstrated greater insulin secretory deficits in black west African men compared with white Europeans(Reference Mohandas, Bonadonna and Shojee-Moradie108). Moreover, we have recognised that black west African men exhibit a comparable circulating insulin concentration to that of white Europeans through a greater reduction in hepatic insulin clearance, which acts to preserve circulating insulin concentrations(Reference Mohandas, Bonadonna and Shojee-Moradie108). Importantly, we have recognised that these ethnic differences in β-cell function and hepatic insulin clearance are not in response to differences in insulin sensitivity, as our clamp methods demonstrate comparable whole body, hepatic and peripheral insulin sensitivity(Reference Bello, Ladwa and Marathe109,Reference Bello, Mohandas and Shojee-Moradie110) .
Our imaging methods have enabled us to quantify visceral, liver and pancreatic fat and to investigate the associations between these measures and the metabolic processes. The black west African men have been found to have significantly lower visceral, hepatic and pancreatic fat compared to the white European men(Reference Hakim, Billoo and Sunderland111–Reference Hakim, Bonadonna and Mohandas114), yet they exhibit comparable insulin resistance(Reference Bello, Mohandas and Shojee-Moradie110) and greater β-cell failure(Reference Mohandas, Bonadonna and Shojee-Moradie108). We have investigated whether there are ethnic differences in the associations between the fat depots and the metabolic variables and have found that whilst visceral fat associates with insulin resistance in both ethnic groups(Reference Bello, Ladwa and Marathe109,Reference Bello, Mohandas and Shojee-Moradie110) , hepatic fat associates with hepatic insulin resistance in only the white European men(Reference Hakim, Bello and Bonadonna113) but associates with hepatic insulin clearance in only the black west African men(Reference Hakim, Bello and Bonadonna113). Pancreatic fat has been found to associate with β-cell function but only in the white European men(Reference Hakim, Bonadonna and Mohandas114). These findings, taken together, show clear evidence of ethnic distinctions in the pathophysiology of T2D.
Our findings challenge the relevance of existing theories that put ectopic fat at the centre of the pathophysiology of T2D (Fig. 2), to populations of black African-Caribbean ethnicity. We have shown lower visceral and hepatic fat in black west African men, yet comparable insulin sensitivity; furthermore, the association between hepatic fat and insulin resistance that was seen in white European men was not evident in black west African men. Pancreatic fat is believed to be a principal driver of β-cell failure, according to the twin cycle hypothesis(Reference Taylor38), yet β-cell function was associated with pancreatic fat only in our white European men. These findings lead us to hypothesise that insulin resistance and β-cell failure develop independently of ectopic fat accumulation in black west African populations, and that ectopic fat is not the primary defect underlying the development of T2D. We are currently extending our work to investigate these mechanisms in men who are normally glucose tolerant and in men with impaired glucose tolerance to provide a greater understanding of how they are implicated in the progression to T2D. Data from our normal glucose-tolerant participants show that black men exhibit a pronounced hyperinsulinaemia compared to white men, and that this is driven by significantly lower hepatic insulin clearance rates rather than upregulated insulin secretion(Reference Ladwa, Bello and Shojaee-Moradie115). This leads us to hypothesise that hyperinsulinaemia is a primary defect in black west African men that occurs as a result of lower rates of hepatic insulin clearance, rather than in response to relative reductions in insulin sensitivity. We await further data on ectopic fat deposition and tissue-specific insulin sensitivity in our cohort of healthy men and in the men with impaired glucose tolerance, which will enable us to shed further light on our hypothesis and a much greater understanding of ethnic distinctions in the pathophysiology of T2D.
Conclusions
Populations of black African-Caribbean ethnicity are disproportionately affected by T2D. While the mainstream understanding of T2D pathophysiology focuses on the role of visceral and ectopic fat accumulation leading to insulin resistance and insulin secretory failure, there is compelling evidence for distinct processes in black populations. In populations of black African-Caribbean ethnicity, there is less central obesity and absence of the dyslipidaemia associated with insulin resistance. Furthermore, the metabolic syndrome criteria have little clinical utility for recognising T2D risk in black populations. More detailed investigations, showing lower VAT and hepatic fat deposition in black populations, provide evidence to suggest that ectopic fat plays a less central role in the development of insulin resistance in black populations. Hyperinsulinaemia is present, even in a healthy, normal glucose-tolerant state, driven by lower rates of hepatic insulin clearance rather than upregulated insulin secretion, and may be a primary defect in black populations. A greater understanding of these processes and the mechanisms underlying the development of T2D in black populations may lead to much needed improvements in prevention and treatment strategies.
Acknowledgements
The staff of the NIHR-Wellcome Trust King's Clinical Research Facility supported the SouL-DeEP study. Andrew Pernet, Bula Wilson and Ines De Abreu (research nurses, Diabetes Research Group, King's College Hospital, UK), Anne-Catherine Perz (King's College London, UK), Daniel Curtis (University of Surrey, UK), Tracy Dew (ViaPath, UK), Elka Giemsa (CRF manager, King's College Hospital, UK), Maddalena Trombetta (University of Verona, Italy) assisted with data collection and data analysis.
Financial Support
The SouL-DeEP study was funded by Diabetes UK (grant numbers 12/0004473 and 14/0004967).
Conflict of Interest
None.
Authorship
L. M. G. was lead author and took overall responsibility for the preparation of the manuscript; M. L. contributed to the writing of the insulin secretion and clearance sections; O. H. contributed to the writing of the ectopic fat section; O. B. contributed to the writing of the insulin resistance section.




