The category of ‘schizotypal disorder’ was introduced in classifications in 1980 (American Psychiatric Association) and in 1992 (World Health Organization), although the concept of subclinical forms of schizophrenia coexisted with the concept of schizophrenia itself from its emergence (with various designations, e.g. schizoid personality, latent, simple, pseudo-neurotic, ambulatory and borderline schizophrenia). Contemporary literature on the subject is vast. One problem confronting the reader is that the reported samples are very diverse (e.g. comprising college students with high scores on psychometric scales targeting assumed schizotypal dimensions, persons identified in epidemiological–genetic studies of schizophrenia and patients diagnosed in clinical settings). The psychopathological profile of individuals with schizotypy in patient populations is almost never described in detail, despite the fact that this diagnosis (or some of its aspects) may represent a pre-schizophrenic condition (Meehl, Reference Meehl1962, Reference Meehl1989), thus possessing obvious relevance for early detection research and for clinical work with first-contact populations.
Schizotypy is located on Axis I in the ICD–10 as a ‘syndrome’ listed immediately after somatic disorders and schizophrenia. In the DSM–IV (American Psychiatric Association, 1994), it is a personality disorder, and as such, it may in principle be associated with any syndromatic diagnosis (with the exception of schizophrenia).
The purpose of this article is twofold: (a) to present a detailed psychopathological description of patients diagnosed as having the ICD–10 schizotypal disorder in a consecutive series of first hospital admissions; (b) to discuss the bearing of these findings on the epidemiology and the taxonomic status of schizotypy.
METHOD
The sample initially comprised 155 consecutively first-admitted patients aged under 40 years to the Department of Psychiatry at Hvidovre Hospital. The catchment population is 130 000 in the city of Copenhagen. The study took place between 1 September 1998 and 1 September 2000. The patients with clear-cut affective disorders (melancholic depression, bipolar disorder), somatic disorders, severe substance misuse as a primary diagnosis, or a clinically dominating comorbid condition were excluded from the study. Patients who were severely psychotic and aggressive or involuntarily admitted were not included due to ethical concerns or because they were considered to be unable to undergo the full examination. Four patients were excluded after the data collection was completed because they had a somatic disorder which was not detected at inclusion, thus leaving a total of 151 patients for investigation.
All the individuals were assessed with a semi-structured interview lasting 3–5 h, consisting of the Operational Criteria for Psychotic Illness (OPCRIT) checklist (Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991) expanded with several items used in the Copenhagen High Risk Study (Reference Parnas, Cannon and JacobsenParnas et al, 1993), the Danish version of the Bonn Scale for the Assessment of Basic Symptoms (BSABS; Reference Gross, Huber and KlosterkotterGross et al, 1987), the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OpierKay et al, 1987), the Premorbid Adustment Scale (PAS; Reference Cannon-Spoor, Potkin and WyattCannon-Spoor et al, 1982) and the DSM–IV Global Assessment of Functioning scale (GAF–F; Reference Endicott, Spitzer and FleissEndicott et al, 1976). All interviews were carried out by the first author (P.H.). Each diagnosis was allocated following agreement between P.H. and the second author (J.P.). Another clinician collected family history data with the Family History Method (Reference Andreasen, Endicott and SpitzerAndreasen et al, 1977). All patients were also assessed using the standardised Danish version of the National Adult Reading Test (NART; Reference Nelson and O'ConnellNelson & O'Connell, 1978), a measure of premorbid IQ.
In order to condense the extensive psychopathological data, a number of rational a priori scales were constructed. The content of these scales was selected to reflect essential aspects of schizophrenia-spectrum psychopathology, i.e. disorder of emotional contact and formal thought disorder. Moreover, five scales (predominantly derived from the BSABS) were specifically created to measure several domains of anomalous subjective experience believed to be pertinent to the schizophrenia-spectrum disorders (Reference Parnas and HandestParnas & Handest, 2003): perplexity (loss of meaning), cognitive disorders, subjective disorders (anomalies in subjective experience), perceptual disorders and cenesthesias (anomalous bodily experience). In addition, a scale targeting affective symptoms was created. Details of the scales as well as their α-coefficients (Reference CronbachCronbach, 1951) are shown in the Appendix. In addition, the PANSS positive and negative symptoms scales were included for comparative purposes. P values were calculated with non-parametric tests (Kruskall–Wallis–ANOVA, Mann–Whitney test, logistic regression and multivariate logistic regression) because the data were mainly ordinal in nature and usually not normally distributed. Two-tailed P values <0.05 were considered statistically significant.
RESULTS
Diagnostic distribution
The diagnostic characteristics of the sample were grouped into three main categories: (a) schizophrenia and other non-affective psychosis; (b) schizotypal disorders; and (c) other diagnoses (Table 1).
Table 1 Characteristics of the sample in the study
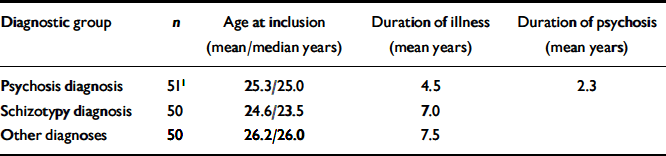
| Diagnostic group | n | Age at inclusion (mean/median years) | Duration of illness (mean years) | Duration of psychosis (mean years) |
|---|---|---|---|---|
| Psychosis diagnosis | 511 | 25.3/25.0 | 4.5 | 2.3 |
| Schizotypy diagnosis | 50 | 24.6/23.5 | 7.0 | |
| Other diagnoses | 50 | 26.2/26.0 | 7.5 |
The group with psychosis comprised 41 patients with schizophrenia; the remainder comprised individuals with various acute psychoses, one case of delusional disorder and one case of schizoaffective psychosis. The other diagnoses group had a range of diagnoses (affective illness, obsessive–compulsive disorder, anxiety, eating disorder and personality disorder). There were no statistically significant gender differences, but the categories of schizotypy and other diagnoses had more females than males (which is caused by a bias operating through exclusion criteria, especially substance misuse and aggression). There were no significant age differences. Patients with psychosis had the lowest global level of functioning (GAF–F=35.1, s.d.=11.5). This was statistically significant compared with the group with schizotypy (GAF–F=51.7, s.d.=9.07) and with the group with other diagnoses (GAF–F=59.8, s.d.=8.1). Patients with schizotypy had a significantly lower GAF–F than the group with other diagnoses (P <0.001 for all these comparisons). Patients with schizotypy had a longer duration of untreated illness (DUI) than those with psychosis but this difference disappeared if they were only compared with the patients with schizophrenia from the group with psychosis. The duration of social and work dysfunction tended to be longer among patients with psychosis. There were no significant correlations between several duration variables (e.g. DUI) and the concurrent GAF–F or severity measures of psychopathology. There were no significant IQ differences between the groups or differences in educational levels. Family history of schizophrenia was similar in groups with psychosis and schizotypy and more frequent than among other diagnoses (Table 2).
Table 2 Family history of schizophrenia in the study

| Diagnostic group | Patients with affected 1st-degree relative n (%) | Patients with affected 1st- and/or 2nd-degree relative n (%) |
|---|---|---|
| Psychosis diagnosis (n=51) | 5 (9.8) | 11 (21.6) |
| Schizotypy diagnosis (n=49) | 3 (6.1) | 9 (18.4) |
| Other diagnoses (n=48) | 1 (2.1) | 4 (4.8) |
| P=0.0441 | P=0.071 |
Of the patients with schizotypy, 92% had had at least one psychiatric treatment contact prior to admission (median number of treatment attempts was three) compared with 67% of patients with psychosis and 92% of patients from the group with other diagnoses. The variable ‘pre-admission management’ included several psychiatric therapies provided by the general practitioner, psychologist or psychiatrist, high-school or university psychological counselling facilities, etc. The vast majority of those treated pre-admission in all groups received antidepressant drugs, perhaps because of a diagnostic possibility of affective illness. We have no systematic data on the efficacy of these treatments but they did not prevent subsequent hospitalisation.
Schizotypal criteria
Among the 50 patients with schizotypy diagnosis (4 of a possible 9 criteria), there were 47 combinations of criteria. All schizotypal criteria also occurred among patients in the category of other diagnoses with a frequency ranging between 10% and 50%. Among the patients with schizotypy, the least frequent symptoms were eccentricity and suspiciousness/paranoid ideation (36–38%) and the most frequent symptoms were odd speech and perceptual disorder (76–78%). The number of schizotypal criteria across the combined patient groups with schizotypy and other diagnoses (n=100) was not normally distributed but displayed a surplus aggregation of patients in the range of 2–6 criteria (Kolmogorov–Smirnoff test). If the ICD–10 diagnostic threshold for schizotypy were lowered to 2 criteria or elevated to 6 criteria, these changes would have correspondingly resulted in 86 or 14 patients receiving a diagnosis of schizotypy. Factor analysis (with VARIMAX rotation) of the ICD–10 schizotypy criteria in the 100 patients with no psychosis resulted in four factors (criteria loading highly on the factors are given in parentheses) with eigenvalues >1: interpersonal/negative (isolation, constricted/inadequate affect), disorganised (eccentric, odd speech), perceptual/positive (‘micropsychosis’, perceptual disorders) and paranoid (suspiciousness, paranoid ideation).
Patterns of psychopathology
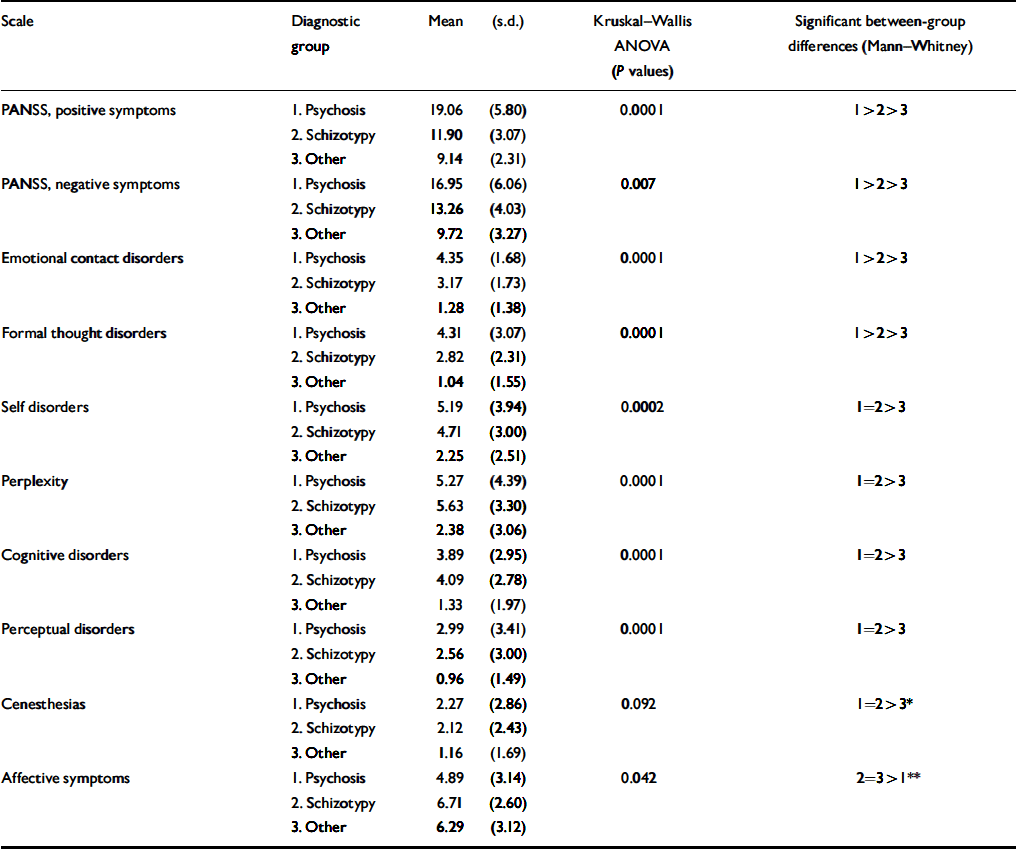
Symptom profiles are shown in Table 3 and provide the scores on all scales across the diagnostic groups. It should be noted that for the PANSS positive and negative symptom scales as well as for the a priori scales targeting contact disorder and formal thought disorder, the distribution of scores is linear: psychosis scores significantly more than schizotypy, which in turn scores higher than other diagnoses. On all scales targeting anomalies of subjective experience (e.g. perplexity, cognitive and perceptual disorders), patients with psychosis and schizotypal disorders had the same scores which were significantly higher than other diagnoses. Because the psychosis and schizotypy scales were positively intercorrelated, we compared all the scales using a multivariate logistic regression model with a binary outcome (schizotypy v. other diagnoses). The self-disorders and cognitive disorders remained significant at P <0.01 in separating the outcome. Affective symptoms were less pronounced in psychosis than in schizotypy and other diagnoses, whereas the last two did not differ from each other.
Table 3 Symptom score across diagnoses in the study
| Scale | Diagnostic group | Mean | (s.d.) | Kruskal—Wallis ANOVA (P values) | Significant between-group differences (Mann—Whitney) |
|---|---|---|---|---|---|
| PANSS, positive symptoms | 1. Psychosis | 19.06 | (5.80) | 0.0001 | 1>2>3 |
| 2. Schizotypy | 11.90 | (3.07) | |||
| 3. Other | 9.14 | (2.31) | |||
| PANSS, negative symptoms | 1. Psychosis | 16.95 | (6.06) | 0.007 | 1>2>3 |
| 2. Schizotypy | 13.26 | (4.03) | |||
| 3. Other | 9.72 | (3.27) | |||
| Emotional contact disorders | 1. Psychosis | 4.35 | (1.68) | 0.0001 | 1>2>3 |
| 2. Schizotypy | 3.17 | (1.73) | |||
| 3. Other | 1.28 | (1.38) | |||
| Formal thought disorders | 1. Psychosis | 4.31 | (3.07) | 0.0001 | 1>2>3 |
| 2. Schizotypy | 2.82 | (2.31) | |||
| 3. Other | 1.04 | (1.55) | |||
| Self disorders | 1. Psychosis | 5.19 | (3.94) | 0.0002 | 1=2>3 |
| 2. Schizotypy | 4.71 | (3.00) | |||
| 3. Other | 2.25 | (2.51) | |||
| Perplexity | 1. Psychosis | 5.27 | (4.39) | 0.0001 | 1=2>3 |
| 2. Schizotypy | 5.63 | (3.30) | |||
| 3. Other | 2.38 | (3.06) | |||
| Cognitive disorders | 1. Psychosis | 3.89 | (2.95) | 0.0001 | 1=2>3 |
| 2. Schizotypy | 4.09 | (2.78) | |||
| 3. Other | 1.33 | (1.97) | |||
| Perceptual disorders | 1. Psychosis | 2.99 | (3.41) | 0.0001 | 1=2>3 |
| 2. Schizotypy | 2.56 | (3.00) | |||
| 3. Other | 0.96 | (1.49) | |||
| Cenesthesias | 1. Psychosis | 2.27 | (2.86) | 0.092 | 1=2>3* |
| 2. Schizotypy | 2.12 | (2.43) | |||
| 3. Other | 1.16 | (1.69) | |||
| Affective symptoms | 1. Psychosis | 4.89 | (3.14) | 0.042 | 2=3>1** |
| 2. Schizotypy | 6.71 | (2.60) | |||
| 3. Other | 6.29 | (3.12) |
Prodromal symptoms in psychosis and schizotypy
We examined the diagnostic values on 16 prodromal symptoms reported retrospectively, which are frequently cited as being typical prodromal features of schizophrenia (Reference Hafner and NovotnyHäfner & Novotny, 1995). We compared psychosis and schizotypy with logistic regression and odds ratio (OR) statistics. There were five significant differences: patients with schizotypy scored higher on depression (OR=6.65, 95% CI 2.52–17.60) and sleep disturbances (OR=4.91, 95% CI 1.65–14.57) and lower on suspiciousness (OR=0.28, 95% CI 0.12–0.63), loss of role functioning (OR=0.17, 95% CI 0.06–0.44) and odd behaviour (OR=0.42, 95% 0.19–0.95). The remaining symptoms (anxiety, hypochondria, neurosis-like symptoms, irritability, isolation/withdrawal, lack of initiative, neglect, emotional indifference, perceptual disturbances, magical thinking, poverty of speech) were equally frequent in the history of patients with psychosis and schizotypy.
Polydiagnostic assessment: ICD–10 v.ICD–8/9
The entire sample of 151 patients has undergone a polydiagnostic assessment reported elsewhere (Reference Jansson, Handest and NielsenJansson et al, 2002). It is of interest to note in this context that a computer-based operationalised algorithm for the ICD–8/9 schizophrenia diagnosis resulted in ICD–8/9 schizophrenia among 37 out of 50 patients with schizotypy (74%). These 37 patients diagnosed with schizotypal disorder scored numerically higher on all scales listed in Table 3 than the 13 with schizotypy who were not diagnosed with ICD–8 schizophrenia (the difference was statistically significant for emotional contact and formal thought disorders and perplexity and the PANSS negative symptom scale). The corresponding rates for ICD–8/9 schizophrenia in the remaining sample were n=48 (94%) among patients with psychosis and n=4 (8%) among other diagnoses. In other words, the ICD–8/9 concept of schizophrenia corresponds quite well to the ICD–10 concept of schizophrenia spectrum (psychosis and schizotypy).
DISCUSSION
Is the sample representative?
We will first address the question of our sample's comparability with our department's usual diagnostic composition. In a separate, intradepartmental study we allocated operational diagnoses to 100 consecutive first admissions to our department aged under 40 years on the basis of their clinical records: 37% were diagnosed as non-affective psychotics, 25% had schizotypal disorder, 36% had disorders outside the schizophrenia spectrum, and 2% had somatic disorders. These frequencies did not differ statistically from those reported in Table 1. However, the clinical diagnostic practice of diagnosing the schizophrenia spectrum deviate dramatically from the rates identified when applying strict diagnostic operational criteria (e.g. as in the present study). Thus, the frequencies of schizophrenia and schizotypy as a principal diagnosis among patients discharged during 2001 and 2002 from seven, mutually independent, psychiatric departments (jointly serving Greater Copenhagen, i.e. Copenhagen City and County) ranged from a low of 17% (schizophrenia) and 0.4% (schizotypy) to a high of 36% (schizophrenia) and 10% (schizotypy; mean=2.7%) (statistical data from the Institute of Psychiatric Demography in Risskov). These differences cannot be explained by differential socio-economic factors across the departments’ catchment areas, nor is the low frequency of schizotypy at a given site reflective of a more frequent use of a diagnosis of schizophrenia (in the sense that schizotypy cases were simply absorbed by the schizophrenia diagnosis). On the contrary, there is a positive and statistically significant association between the tendencies (high or low) to use both diagnostic categories within each department (n=7, Spearman's rho=0.818, P=0.024). In other words, at a given site, the less frequent the diagnosis of schizophrenia, the less frequent is the diagnosis of schizotypy. It appears then that the daily clinical application of the ICD–10 categories of the schizophrenia spectrum is problematic in the following respects: (a) the diagnosis rates of schizophrenia-spectrum disorders vary across different clinical sites in the same region of a small, homogenous country; and (b) the clinical use of the schizotypy diagnosis is, on average, incommensurably lower than its ‘true’ operational frequency. This suggests that clinicians either do not know of or do not use this diagnosis, or both. Both points in conjunction question a widely held assumption that modern, criteria-based diagnostic systems have improved everyday clinical reliability.
The notion of a spectrum of disorders
The present study seems to support the spectrum concept of schizophrenic disorders as it is presented in the ICD–10. There is a gradation of schizophreniform symptomatology with its fading out in the category of other diagnoses. The schizotypal disorder–especially in the dimensions clearly reflective of the ICD–10 diagnostic criteria of schizophrenia (contact and formal thought disorder and PANSS positive and negative symptoms)–occupies a tautologically intermediate position between non-effective psychosis and other diagnoses. However, a strong similarity observed between patients with psychosis and those with schizotypal disorders (Table 3) on the scales measuring qualitative alterations of subjective experience (perplexity, cognitive, self-disorders and perceptual disorders) provides additional and independent validation of schizotypy as a part of the schizophrenic spectrum of disorders.
Anomalies of subjective experience have already been described in the classical literature as characteristic of schizophrenia and were considered of paramount diagnostic significance (Reference BerzeBerze, 1914; Reference MinkowskiMinkowski, 1927; Reference Berze and GruhleBerze & Gruhle, 1929; Reference ConradConrad, 1958; Reference HuberHuber, 1966). More recent empirical work has rediscovered these phenomena. Thus, it seems that certain anomalies of subjective experience (Reference BlankenburgBlankenburg, 1971; Reference Cutting and DunneCutting & Dunne, 1989; Parnas et al, Reference Parnas, Jansson and Sass1998, Reference Parnas, Handest and Saebye2003; Reference Möller and HusbyMöller & Husby, 2000; Reference MeehlMeehl, 2001), especially anomalies of self-awareness (Reference Parnas and HandestParnas & Handest, 2003; Reference Sass and ParnasSass & Parnas, 2003), represent a fundamental nucleus of schizophreniform symptomatology. These symptoms may be the most sensitive and specific clinical phenotypes currently available in the context of early detection (Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001). The frequency of typical ‘prodromal symptoms’ in the history of patients with schizophrenia and those with schizotypal disorder is mainly suggestive of psychopathological similarity.
The distribution of family history of schizophrenia across the diagnostic groups (Table 2) supports the categorical affinity of schizophrenia and schizotypy, as does the fact that 76% of patients with schizotypy were diagnosed with ICD–8/9 schizophrenia.
In summary, the data point to an overall psychopathological similarity of schizophrenia and schizotypy. Elevated levels of Bleulerian fundamental symptoms (Reference Bleuler and AchaffenburgBleuler, 1911) and anomalies of subjective experience characterise both groups. It is mainly the severity of psychosis (a diagnostic requirement for an ICD–10 diagnosis of schizophrenia) that marks the distinction of schizophrenia from schizotypy (in the latter group only ‘micro-psychotic’ experiences are allowed).
Affective symptoms and schizotypy
Population studies suggest that compensated patients with schizotypal disorder are rarely treated (Reference Parnas, Cannon and JacobsenParnas et al, 1993) and those who are display apparently affective symptoms, substance misuse and actingout behaviour (Reference Parnas and TeasdaleParnas & Teasdale, 1987). This may explain the findings of elevated levels of affective symptoms among the patients with schizotypy in this sample (Table 3), as well as the frequent reporting of depression as a pre-admission symptom in the history of illness. It points perhaps to a relative preservation of affectivity in patients with schizotypy as opposed to patients with schizophrenia, although the clinical overlap between schizotypal or schizophrenic anhedonia (and other so-called negative symptoms) and genuine depressive–affective complaints makes any such interpretation quite tentative (see Reference Parnas and HandestParnas & Handest, 2003, and Reference Sass and ParnasSass & Parnas, 2003, for a phenomenological analysis of initial complaints in schizophrenia). None the less, it is striking that most ‘pre-admission’ treatments involved antidepressant medication. It appears that clinicians become quickly impressed by the affective complaints of their patients.
APPENDIX

| Psychometric scales used in the study | |
|---|---|
| Formal thought disorders (α=0.652) | Contact disorders (α=0.605) |
| Speech difficult to understand | Autism |
| Incoherence | One-way emotional contact |
| Positive formal thought disorder (including semantic changes) | Withdrawn/shy |
| Negative formal thought disorder (including vagueness) | |
| Perplexity (α=0.682) | Cognitive disorders (α=0.672) |
| Derealisation | Thought interference |
| Disorder of impressive speech | Thought pressure |
| Diminished ability to discriminate between perception and imagination | Thought blockage |
| Disturbance of thought initiative or thought intentionality | |
| Diminished ability to discriminate between imagination and memory | |
| Disturbance of expressive language function | |
| Disturbance in grasping the significance of observed objects | |
| Heightened perception | |
| Abnormal attention to a detail | |
| Loss of automation of movement | |
| Hyper-reflexivity | |
| Perceptual disorders (α=0.593) | Self-disorders (α=0.654) |
| Blurred vision | Mirror-related phenomena (Spiegel-phänomen) |
| Partial vision | Physical depersonalisation |
| Momentary blindness | Psychic depersonalisation |
| Sensitivity to light or sound | Diminished sense of identity |
| Near- and tele-vision | Transitivism (permeable ego boundary) |
| Micro- and macropsia | Spatialisation of inner experience |
| Abnormally long-lasting retinal after-image | I-split |
| Changes in perception of intensity or quality of acoustic stimuli | Disturbance of awareness of continuity of own actions |
| Metamorphopsia | |
| Metachromopsia | |
| Movements of objects experienced as related to own movements | |
| Diplopia, oblique vision | |
| Disturbance in estimation of distances or size | |
| Disintegration in perception of linearity of contours | |
| Affective symptoms (α=0.698) | Cenesthesias (α=0.573) |
| Dysphoric mood | Migrating sensations |
| Morning depression/oppression | Electric sensations |
| Mood swings during the day | Thermal sensations (heat or cold) |
| Agitation | Sensations of movement, pulling or pressure inside the body or on its surface |
| Diminished activity | |
| Hypersomnia | Kinesthetic sensations |
| Anergia | Sensations of abnormal heaviness, lightness or emptiness, of falling or sinking, levitation or elevation |
| Diminished sense of pleasure | |
| Reduced libido | |
| Difficulty in falling asleep | Sensations of extension, diminution, shrinking, enlargement or constriction |
| Interrupted sleep | |
| Early wakening | Vestibular sensations |
| Reduced appetite | |
| Increased appetite | |
| Suicidal thoughts | |
Schizotypal criteria
The study shows the arbitrary nature of the four criteria needed for the ICD–10 schizotypy diagnosis. The distribution of criteria among 100 patients with no psychosis follows a steep symmetrical curve, where any number of criteria between 2 and 6 might be chosen as an appropriate cut-off level for schizotypy. Moreover, a dimensionality of schizotypy, as demonstrated through the factor analysis (and in agreement with the results from many other studies, e.g. Reference Vollema and van den BoschVollema & van den Bosch, 1995; Reference Venables and RectorVenables & Rector, 2000; Reference Fossati, Maffei and BattagliaFossati et al, 2001) suggests a methodological inaccuracy of a purely polythetic diagnostic approach with each criterion possessing equivalent diagnostic value. Such an approach becomes highly problematic when the criteria are not independent but are correlated in sets.
Relevance for early detection of schizophrenia
The ICD–10 schizotypy, as it appears in this study, can be considered as being a diluted schizophrenia, and as such not a ‘pre-onset condition’. Thus, the gradual transition of the ICD–10 schizophrenia-spectrum criteria complicates the issue of pre-onset diagnosis and early intervention in schizophrenia, because it challenges the concept of schizophrenia as a clearly demarcated condition. As also demonstrated by the polydiagnostic studies, schizophrenia has variable borders, changing with the diagnostic perspective (Reference Jansson, Handest and NielsenJansson et al, 2002). Thus, despite a widely held illusion of a tremendous recent progress in psychiatric classification (Reference Parnas, Zahavi, Maj, Gaebel, Lopez-Ibor and SartoriusParnas & Zahavi, 2002), there is still an acute need for serious work on the conceptual validity (also called ‘non-empirical’ validity, Reference KendlerKendler, 1990) of such categories as ‘schizophrenia’ or ‘psychosis’ (see also Parnas, this issue). From a more optimistic perspective, we may conclude that schizophrenia and schizotypy are associated with certain characteristic anomalies of subjective experience (the so-called basic symptoms in German terminology) which may be potentially useful for early clinical detection of individuals at risk for schizophrenia-spectrum disorders. We are now conducting a 4-year follow-up of this particular sample, expecting additional schizophrenia cases to emerge mainly, but not only, from the schizotypal group. These longitudinal data will shed more light on the diagnostic significance of anomalies of subjective experience.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ ICD–10 schizotypal disorders are nearly as frequent as schizophrenia among first-admitted patients.
-
▪ Schizophrenia and schizotypy are qualitatively similar on most psychopathological dimensions.
-
▪ The daily clinical use of schizotypy diagnosis is highly variable at different psychiatric sites.
LIMITATIONS
-
▪ There is no comparison group of population-sampled individuals with schizophrenia.
-
▪ There is a need for a diagnostic follow-up of the present sample to substantiate some of the claims.
-
▪ There is a need for a more comprehensive assessment scheme for self-disorders.
Acknowledgements
Supported by a grant from the University of Copenhagen (P.H.). We thank Professor Povl Munk-Jørgensen for providing statistical information on hospital discharge diagnoses for the region of Greater Copenhagen.






eLetters
No eLetters have been published for this article.