Overweight and obesity are global multi-factorial health epidemics( 1 ) that are increasing in prevalence worldwide( 2 ). For example, in 2014–2015, 63 % of Australian adults were either overweight (BMI 25·0–29·9 kg/m2) or obese (BMI ≥30·0 kg/m2)( 3 , 4 ). Obesity is a known risk factor for many chronic diseases including CVD, type 2 diabetes, musculoskeletal disorders and cancer( 4 , Reference Pi-Sunyer 5 ).
The primary cause for overweight and obesity is a consistent positive imbalance between kJ consumed and energy expended( 4 ). A common dietary contributor is the replacement of nutrient-dense foods with energy-dense, nutrient-poor foods( 6 ), as seen in diets that are high in added sugar (AS) (>20 % of total energy intake)( Reference Cobiac, Record and Leppard 7 ). AS includes sucrose, fructose, dextrose, lactose and sugar syrups such as glucose syrup( 8 ), which are introduced either during manufacturing or by the consumer during food preparation( 8 ).
A meta-analysis of randomised controlled trials and cohort studies reported a parallel relationship between AS consumption and a corresponding change in body weight under ad libitum conditions (gain of 0·8 kg when increasing AS or reduction of 0·75 kg when reducing AS), over an intervention period of 2 weeks or more)( Reference Te Morenga, Mallard and Mann 9 ). One possible mechanism for weight gain is the metabolism of AS. It has been noted that fructose, a major constituent of AS( Reference Le, Lanaspa and Cicerchi 10 ), does not increase satiety when metabolised( Reference Lane and Cha 11 ), which may lead to overconsumption and thereby, in part, explain the association between AS and weight gain.
To date, many studies that investigate the impact of AS consumption focus on the general population. However, it is known that in the overweight or obese population, a modest weight loss (≥5 % initial body weight) reduces cardiovascular health risks associated with overweight and obesity( 12 ). This highlights a need to investigate dietetic strategies that may aid weight loss among overweight or obese individuals.
There is currently no known systematic literature review exploring the impact of AS consumption on appetite in overweight and obese individuals. The aim of this systematic review was to investigate whether increased AS consumption affects appetite in overweight or obese adults when compared with lower AS intakes. It is hypothesised that increased intakes of AS will affect appetite by reducing the feeling of satiety, resulting in an increased food intake.
Methods
This systematic literature review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement( Reference Moher, Liberati and Tetzlaff 13 ). The review was registered with PROSPERO, the international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO; registration number: CRD42017057777).
Searches
A systematic search was conducted across four databases (all years to 7 April 2017): Medline, Cochrane CENTRAL, Web of Science and CINAHL. Search terms and truncations included (‘overweight’ OR ‘obese*’ OR ‘obesity’) AND (‘added sugar*’ OR ‘sugar*’ OR ‘free sugar*’ OR ‘sucrose’ OR ‘refined sugar*’ OR ‘fructose’ OR ‘dextrose’) AND (‘appetite’ OR ‘hunger’ OR ‘food intake’ OR ‘satiety’ OR ‘satiat*’ OR ‘leptin’ OR ‘Ob protein’ OR ‘Ob gene’ OR ‘ghrelin’ OR ‘GHRL’ OR ‘Ppghrelin’).
To be included in this review, studies were limited to randomised controlled trials and cohort studies, published in English. Studies were required to meet the following inclusion criteria: (1) conducted in overweight or obese human adults (BMI ≥25 kg/m2); (2) assess associations between oral AS intake and appetite, with reference to food intake (including food intake measured at a subsequent meal or total intake including the AS treatment), self-reported satiety through a visual analogue scale (VAS), or appetite hormones (leptin or ghrelin); (3) report AS intake in comparison with a comparator group of lower AS content. In addition, the following exclusion criteria were applied: (1) samples of pregnant or breast-feeding women, (2) published as conference abstracts only and (3) studies conducted in animals or children.
Article screening
One review author (K. T.) conducted the literature search and assessed potential studies for inclusion. Inclusion of articles not clearly meeting the inclusion or exclusion criteria was discussed with two additional review authors (E. N. and K. C.), until consensus was reached.
Articles were initially screened based on title and abstract. Full-text articles were retrieved if an abstract was unavailable or provided insufficient information to determine inclusion in this review. These were then assessed for eligibility using the inclusion criteria. Where multiple articles reported results from the same study, results were merged in the summary table, to avoid duplication of the study population.
Data extraction
The following data were extracted from each study: citation, details of the study population (sample size, age, sex, BMI), intervention duration, intervention details, including comparison group and measured outcomes of interest (Table 1).
Table 1 Summary table of studies included in the systematic literature review on added sugar and appetite

NHMRC, National Health and Medical Research Council; AND, Academy of Nutrition and Dietetics; M, male; F, female; NR, not reported; I, intervention; C, control; CHO, carbohydrate; EER, estimated energy requirement; EEI, estimated energy intake.
* Exact volume of test beverage or food was not reported.
† Study included another intervention group which was not relevant to this review, only the relevant group was included in the summary table and review.
‡ Reports on hunger, satiety and desire to eat were pooled with lean individuals due to lack of difference in responses.
§ Number of males and females was not specified in the research paper.
|| Sugar content estimated from percentage of energy reported in test product.
¶ One subject excluded due to not completing the second respiratory chamber stay.
** Articles where authors did not specify whether allocation was randomised, studies were allocated to III-1.
Quality assessment
Study quality was assessed using the Academy of Nutrition and Dietetics Quality Criteria Checklist( 14 ) (online Supplementary material I). This checklist is a component of the Evidence Analysis Manual developed by the Academy of Nutrition and Dietetics to support systematic literature reviews in nutrition and dietetics. The checklist considers a number of aspects of study design that may impact on quality including participant selection, blinding, appropriateness of statistical analyses and risk of bias from funding sources. Studies were also classified according to the Australian National Health and Medical Research Council (NHMRC) level of evidence ranking( 15 ).
Results
A total of 2557 articles were identified using the search parameters. After removal of duplicates, articles were assessed for eligibility (n 1724). Following application of the inclusion and exclusion criteria, twenty-one articles describing nineteen studies were included in this review (Fig. 1). A total of two articles( Reference Raben, Møller and Flint 16 , Reference Sørensen, Vasilaras and Astrup 17 ) reported on a subgroup from a study already included in the review( Reference Raben, Vasilaras and Møller 18 ) and were therefore combined in the summary table.
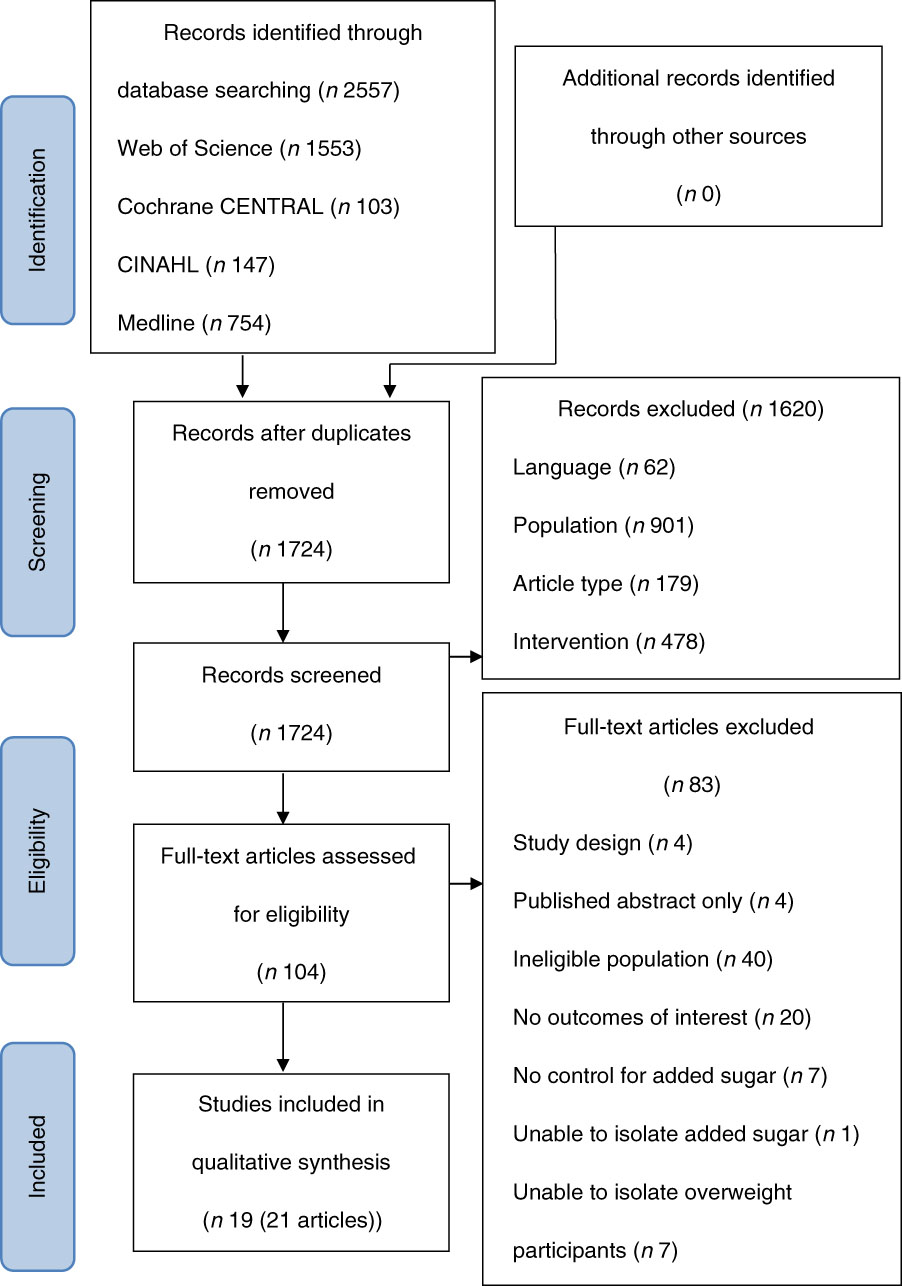
Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram( Reference Moher, Liberati and Tetzlaff 13 ) of the number of studies extracted for review.
A review of each article according to the quality criteria checklist( Reference Moher, Liberati and Tetzlaff 13 ) rated the quality of nineteen of the twenty-one studies as positive. A total of two studies were rated neutral as participant selection was not described( Reference Mazlan, Horgan and Whybrow 19 , Reference Overduin, Collet and Medic 20 ) (online Supplementary material I). Based on the NHMRC level of evidence( 15 ), all except three studies( Reference Rezvani, Cianflone and McGahan 21 – Reference Reid, Hammersley and Duffy 23 ) were randomised controlled trials (level II). A total of two studies( Reference Rezvani, Cianflone and McGahan 21 , Reference Reid, Hammersley and Duffy 22 ) did not state whether group allocation was randomised and, therefore, were considered to be pseudo-randomised controlled trials (level III-1). While prospective cohort study designs were also considered for this review, no cohort studies met the overall inclusion criteria.
Included studies evaluated the effects of AS consumption through food (n 5), beverages (n 12) or a combination of foods and beverages (n 2) over a period of time ranging from 1 d( Reference Overduin, Collet and Medic 20 , Reference Kasim-Karakas, Cunningham and Tsodikov 24 – Reference Wiessing, Xin and Budgett 32 ) to 6 months( Reference Saris, Astrup and Prentice 33 ). Characteristics of included studies are displayed in Table 1. Participants’ mean BMI ranged from 26·1( Reference Furchner-Evanson, Petrisko and Howarth 30 ) to 41·1 kg/m2 ( Reference Drewnowski, Massien and Louis-Sylvestre 31 ) and the mean age ranged from 22( Reference Hollis, Houchins and Blumberg 34 ) to 57 years( Reference Bowen, Noakes and Clifton 27 ). A total of three studies analysed only males( Reference Mazlan, Horgan and Whybrow 19 , Reference Bowen, Noakes and Clifton 27 , Reference Bowen, Noakes and Trenerry 28 ), eight only females( Reference Reid, Hammersley and Duffy 22 – Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Furchner-Evanson, Petrisko and Howarth 30 – Reference Wiessing, Xin and Budgett 32 , Reference Kasim-Karakas, Almario and Cunningham 35 , Reference Surwit, Feinglos and McCaskill 36 ) and the remainder involved both sexes( Reference Raben, Møller and Flint 16 – Reference Raben, Vasilaras and Møller 18 , Reference Overduin, Collet and Medic 20 , Reference Rezvani, Cianflone and McGahan 21 , Reference Dove, Hodgson and Puddey 25 , Reference Vozzo, Baker and Wittert 26 , Reference Maersk, Belza and Holst 29 , Reference Saris, Astrup and Prentice 33 , Reference Hollis, Houchins and Blumberg 34 ).
Studies evaluated energy consumption either through the amount of energy consumed post-treatment at a subsequent meal( Reference Overduin, Collet and Medic 20 , Reference Dove, Hodgson and Puddey 25 – Reference Wiessing, Xin and Budgett 32 ) or as total daily energy intake, including test products( Reference Raben, Møller and Flint 16 , Reference Sørensen, Vasilaras and Astrup 17 , Reference Mazlan, Horgan and Whybrow 19 , Reference Reid, Hammersley and Duffy 22 , Reference Reid, Hammersley and Duffy 23 , Reference Saris, Astrup and Prentice 33 – Reference Kasim-Karakas, Almario and Cunningham 35 ). Consumption of AS was reported either as prescribed doses, ranging from approximately 10( Reference Wiessing, Xin and Budgett 32 ) to 125 g( Reference Mazlan, Horgan and Whybrow 19 ), or as a set portion of dietary energy, with individual intakes varying( Reference Raben, Møller and Flint 16 – Reference Raben, Vasilaras and Møller 18 , Reference Rezvani, Cianflone and McGahan 21 , Reference Saris, Astrup and Prentice 33 ). A total of four studies( Reference Rezvani, Cianflone and McGahan 21 , Reference Vozzo, Baker and Wittert 26 – Reference Bowen, Noakes and Trenerry 28 ) reported more than one type of AS source, therefore all subsequent results are presented separately by comparisons between AS type. For example, one study explored the effect of both glucose and lactose on appetite( Reference Bowen, Noakes and Trenerry 28 ), which are examined separately in this review. A range of AS sources were reported, including sucrose( Reference Raben, Møller and Flint 16 – Reference Raben, Vasilaras and Møller 18 , Reference Overduin, Collet and Medic 20 , Reference Reid, Hammersley and Duffy 22 , Reference Reid, Hammersley and Duffy 23 , Reference Drewnowski, Massien and Louis-Sylvestre 31 , Reference Wiessing, Xin and Budgett 32 , Reference Surwit, Feinglos and McCaskill 36 ) (n 7 comparisons), glucose( Reference Rezvani, Cianflone and McGahan 21 , Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Vozzo, Baker and Wittert 26 – Reference Bowen, Noakes and Trenerry 28 , Reference Kasim-Karakas, Almario and Cunningham 35 ) (n 6), fructose( Reference Rezvani, Cianflone and McGahan 21 , Reference Vozzo, Baker and Wittert 26 , Reference Bowen, Noakes and Clifton 27 ) (n 3), lactose( Reference Bowen, Noakes and Trenerry 28 ) (n 1), fruit drink( Reference Dove, Hodgson and Puddey 25 , Reference Hollis, Houchins and Blumberg 34 ) (n 2), cola( Reference Maersk, Belza and Holst 29 ) (n 1), parfait( Reference Mazlan, Horgan and Whybrow 19 ) (n 1), dried plum( Reference Furchner-Evanson, Petrisko and Howarth 30 ) (n 1) and cookies( Reference Furchner-Evanson, Petrisko and Howarth 30 ) (n 1). In all, one study examined a whole-of-diet approach, reporting participants’ total intake of AS from a range of sources( Reference Saris, Astrup and Prentice 33 ). The effect of AS on appetite was explored through three study outcomes: energy consumption( Reference Raben, Møller and Flint 16 – Reference Overduin, Collet and Medic 20 , Reference Reid, Hammersley and Duffy 22 , Reference Reid, Hammersley and Duffy 23 , Reference Dove, Hodgson and Puddey 25 – Reference Kasim-Karakas, Almario and Cunningham 35 ) (n 16), satiety( Reference Sørensen, Vasilaras and Astrup 17 – Reference Overduin, Collet and Medic 20 , Reference Reid, Hammersley and Duffy 23 , Reference Dove, Hodgson and Puddey 25 – Reference Wiessing, Xin and Budgett 32 , Reference Hollis, Houchins and Blumberg 34 , Reference Surwit, Feinglos and McCaskill 36 ) (n 14) and appetite hormones (leptin( Reference Raben, Møller and Flint 16 , Reference Rezvani, Cianflone and McGahan 21 , Reference Kasim-Karakas, Almario and Cunningham 35 ) and ghrelin( Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Bowen, Noakes and Clifton 27 – Reference Furchner-Evanson, Petrisko and Howarth 30 )) (n 8).
Following AS consumption, significant reductions in subsequent energy intake, compared with controls, were reported in two comparisons( Reference Vozzo, Baker and Wittert 26 ), nine found no difference in energy intake( Reference Overduin, Collet and Medic 20 , Reference Bowen, Noakes and Clifton 27 – Reference Wiessing, Xin and Budgett 32 ) and two reported a significant increase in energy intake( Reference Dove, Hodgson and Puddey 25 , Reference Bowen, Noakes and Trenerry 28 ). When total daily energy intake was examined, including the AS source, four comparisons found no difference in energy intake( Reference Reid, Hammersley and Duffy 22 , Reference Reid, Hammersley and Duffy 23 , Reference Hollis, Houchins and Blumberg 34 , Reference Kasim-Karakas, Almario and Cunningham 35 ) and three reported a significant increase in energy intake( Reference Raben, Møller and Flint 16 , Reference Sørensen, Vasilaras and Astrup 17 , Reference Mazlan, Horgan and Whybrow 19 , Reference Saris, Astrup and Prentice 33 ). Consumption of AS significantly increased satiety in two comparisons( Reference Vozzo, Baker and Wittert 26 ), whilst twelve found no differences in reported satiety( Reference Mazlan, Horgan and Whybrow 19 , Reference Overduin, Collet and Medic 20 , Reference Reid, Hammersley and Duffy 23 , Reference Bowen, Noakes and Clifton 27 – Reference Wiessing, Xin and Budgett 32 , Reference Hollis, Houchins and Blumberg 34 , Reference Surwit, Feinglos and McCaskill 36 ) and four reported significantly reduced satiety( Reference Sørensen, Vasilaras and Astrup 17 , Reference Dove, Hodgson and Puddey 25 , Reference Bowen, Noakes and Clifton 27 , Reference Bowen, Noakes and Trenerry 28 ). In response to AS consumption, one comparison reported no change in leptin levels( Reference Kasim-Karakas, Almario and Cunningham 35 ), whereas three reported significantly increased leptin levels( Reference Raben, Møller and Flint 16 , Reference Rezvani, Cianflone and McGahan 21 ). Studies exploring the impact of AS on ghrelin reported an immediate drop in ghrelin levels (measured at 60 min post AS consumption), followed by a later rise (120–180 min)( Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Bowen, Noakes and Clifton 27 – Reference Furchner-Evanson, Petrisko and Howarth 30 ). Findings related to this rise in ghrelin levels varied, with four comparisons finding no difference between AS and control at 120( Reference Bowen, Noakes and Trenerry 28 , Reference Furchner-Evanson, Petrisko and Howarth 30 )–180 min( Reference Bowen, Noakes and Clifton 27 , Reference Maersk, Belza and Holst 29 ), whilst three reported a significantly higher ghrelin measure after AS consumption, compared with the control group at 120( Reference Bowen, Noakes and Trenerry 28 )–180 min( Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Bowen, Noakes and Clifton 27 ).
Discussion
This systematic review found inconsistent associations between AS intake and appetite in overweight and obese adults. Measures of appetite were examined through ad libitum energy intake, satiety (VAS) and appetite hormones (leptin and ghrelin). These measures have previously shown good intra-individual reproducibility and validity as measures of appetite( Reference Stubbs, Hughes and Johnstone 37 – Reference de Graaf, Blom and Smeets 40 ).
Changes in ad libitum energy consumption in response to AS intake were examined in twenty comparisons. A total of thirteen comparisons( Reference Overduin, Collet and Medic 20 , Reference Dove, Hodgson and Puddey 25 – Reference Wiessing, Xin and Budgett 32 ) examined single-day influences of AS at a following meal. Over half (n 9) of these comparisons found that AS consumption had no influence on subsequent energy intake( Reference Overduin, Collet and Medic 20 , Reference Bowen, Noakes and Clifton 27 – Reference Wiessing, Xin and Budgett 32 ). The lack of change in energy intake after AS consumption, despite differences in energy content of the pre-load( Reference Overduin, Collet and Medic 20 , Reference Bowen, Noakes and Clifton 27 – Reference Maersk, Belza and Holst 29 , Reference Drewnowski, Massien and Louis-Sylvestre 31 , Reference Wiessing, Xin and Budgett 32 ), aligns with previous findings of an incomplete compensation of energy intake following AS consumption in studies of both short and longer durations( Reference Mattes 41 , Reference DiMeglio and Mattes 42 ). A total of three long-duration studies (7 d to 6 months) reported an increased total daily energy intake when AS was compared with a control of lower energy value( Reference Raben, Møller and Flint 16 , Reference Sørensen, Vasilaras and Astrup 17 , Reference Mazlan, Horgan and Whybrow 19 , Reference Saris, Astrup and Prentice 33 ). A 2-month study( Reference Kasim-Karakas, Almario and Cunningham 35 ) reported no differences in total daily energy intake when AS was compared with an isoenergetic control. In all, two single-day comparisons reported a reduced energy intake following AS ingestion( Reference Vozzo, Baker and Wittert 26 ). However, when accounting for differences in energy values between the AS and control products, these analyses found that the significant reduction in energy intake at the following meal only equated to 40 % of the energy provided by the AS beverage, consequently resulting in a higher total energy intake across the day( Reference Vozzo, Baker and Wittert 26 ). Despite differing findings, most comparisons( Reference Raben, Møller and Flint 16 , Reference Sørensen, Vasilaras and Astrup 17 , Reference Mazlan, Horgan and Whybrow 19 , Reference Overduin, Collet and Medic 20 , Reference Vozzo, Baker and Wittert 26 – Reference Maersk, Belza and Holst 29 , Reference Drewnowski, Massien and Louis-Sylvestre 31 – Reference Saris, Astrup and Prentice 33 , Reference Kasim-Karakas, Almario and Cunningham 35 ) suggested that if energy intake over the day did not compensate for that provided by the AS, total daily energy intake would increase( Reference Wolff and Dansinger 43 ). This could explain the relationship between AS and body weight reported in ad libitum diets( Reference Te Morenga, Mallard and Mann 9 ).
Energy intake can be influenced by a diverse array of environmental, cultural, behavioural and economic factors( 4 , Reference Kopelman 44 ). Therefore, when analysing appetite, energy intake is most reliable when combined with another appetite measure such as a VAS( Reference Parker, Sturm and MacIntosh 45 ). In this review, changes in VAS responses were similar to changes in reported energy consumption (n 14)( Reference Sørensen, Vasilaras and Astrup 17 , Reference Overduin, Collet and Medic 20 , Reference Reid, Hammersley and Duffy 23 , Reference Dove, Hodgson and Puddey 25 – Reference Wiessing, Xin and Budgett 32 , Reference Hollis, Houchins and Blumberg 34 ). Only three comparisons( Reference Raben, Vasilaras and Møller 18 , Reference Mazlan, Horgan and Whybrow 19 , Reference Bowen, Noakes and Clifton 27 ) reported inconsistent findings between energy intake and the VAS scores.
A single-day study( Reference Bowen, Noakes and Clifton 27 ) reported that fructose consumption resulted in lower feelings of fullness (satiety) than was observed following consumption of a whey-control beverage of similar energy content. This finding may be explained by an incomplete fructose digestion at high fructose doses( Reference Ravich, Bayless and Thomas 46 ). However, these findings need to be further examined. Two longer duration comparisons (≥7 d)( Reference Raben, Møller and Flint 16 , Reference Raben, Vasilaras and Møller 18 , Reference Mazlan, Horgan and Whybrow 19 ) reported that AS consumption increased energy intake with no associated change in satiety. This inconsistency could be explained by an identified sensitivity issue of the VAS in longer-term studies( Reference Stubbs, Hughes and Johnstone 37 ). Despite the majority of comparisons between energy intake and VAS being consistent, the three identified discrepancies highlight the need to incorporate objective appetite measures, such as appetite hormones, when examining the relationship between AS and appetite( Reference Stubbs, Hughes and Johnstone 37 ).
Leptin is a hormonal response to food intake, thereby providing a physiological objective measure of appetite. Leptin, produced by adipose cells, inhibits hunger signals in the central nervous system, ensuring long-term regulation of energy balance( Reference de Graaf, Blom and Smeets 40 ). A total of three comparisons found a significant increase in leptin levels after 10 weeks of AS consumption( Reference Raben, Møller and Flint 16 , Reference Rezvani, Cianflone and McGahan 21 ), whereas one shorter duration comparison (2 months) found no difference in leptin levels in response to AS intake( Reference Kasim-Karakas, Almario and Cunningham 35 ). The differing leptin findings could be explained by a positive relationship between leptin concentrations and body fat stores( Reference Considine, Sinha and Heiman 47 ). Studies in this review identified that after adjusting for changes in body weight, the relationship between leptin levels and AS consumption was no longer significant( Reference Raben, Møller and Flint 16 , Reference Rezvani, Cianflone and McGahan 21 ). This explains the inconsistency in responses to AS intake in findings of self-reported satiety, energy intake and leptin levels when body weight was not accounted for.
Unlike leptin, ghrelin is associated with hunger ratings in individuals of all weight categories( Reference de Graaf, Blom and Smeets 40 ). Research suggests that ghrelin acts as a physiological meal initiator through a preprandial rise and postprandial fall of plasma ghrelin levels( Reference Cummings, Purnell and Frayo 48 ). This ghrelin response aligns with all seven ghrelin comparisons of AS in this review( Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Bowen, Noakes and Clifton 27 – Reference Furchner-Evanson, Petrisko and Howarth 30 ), with AS consumption resulting in an immediate drop in ghrelin levels (60 min), followed by a later rise (120–180 min)( Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Bowen, Noakes and Clifton 27 – Reference Furchner-Evanson, Petrisko and Howarth 30 ). Findings relating to the rise differed between studies. Only three comparisons( Reference Kasim-Karakas, Cunningham and Tsodikov 24 , Reference Bowen, Noakes and Clifton 27 , Reference Bowen, Noakes and Trenerry 28 ) reported a significantly higher final ghrelin measure after AS consumption, compared with the control. Each used glucose as the AS source, whereas the four comparisons that had no significant ghrelin response used lactose, fructose, dried plum, cookies or cola as the source of AS. These results may indicate that different sources of AS are digested differently, contradicting current research that compared AS sources and their influence on ghrelin( Reference Yau, McLaughlin and Gilmore 49 , Reference Teff, Elliott and Tschop 50 ). It should be noted that intakes of AS tended to be higher in the comparisons that reached significance (56( Reference Bowen, Noakes and Trenerry 28 )–75 g( Reference Kasim-Karakas, Cunningham and Tsodikov 24 )) compared with those that did not (33( Reference Furchner-Evanson, Petrisko and Howarth 30 )–65 g( Reference Bowen, Noakes and Clifton 27 )). These observations could suggest that ghrelin may respond to carbohydrate intake in a dose-dependent manner, as previously reported( Reference Teff, Elliott and Tschop 50 , Reference Blom, Stafleu and de Graaf 51 ). Inconsistency in ghrelin findings indicates that this area requires further research.
Although the present review followed a systematic process to provide an insight into the effect of AS intake on appetite in overweight or obese adults, when compared with lower intakes, there are some limitations. The results are limited by the substantial variation between studies, including the large range in doses, duration, control comparator and type of AS, possibly explaining the inconsistent results and impeding the ability to establish a dose effect. As a result of the variability between studies, it was not considered appropriate to pool the results in a meta-analysis. Many studies included in this review did not compare AS with an isoenergetic control( Reference Mazlan, Horgan and Whybrow 19 , Reference Overduin, Collet and Medic 20 , Reference Reid, Hammersley and Duffy 22 , Reference Reid, Hammersley and Duffy 23 , Reference Vozzo, Baker and Wittert 26 – Reference Maersk, Belza and Holst 29 , Reference Drewnowski, Massien and Louis-Sylvestre 31 – Reference Hollis, Houchins and Blumberg 34 , Reference Surwit, Feinglos and McCaskill 36 ), which may have confounded results, as suggested previously( Reference Te Morenga, Mallard and Mann 9 , Reference Wolff and Dansinger 43 ). The variety of mediums included could have influenced appetite, with liquid meals previously reported to be less satiating than solid meals, independent of energy density( Reference Mattes and Rothacker 52 , Reference Hulshof, De Graaf and Weststrate 53 ). Similarly, the variation in study duration, which included both acute and longer duration studies, may have resulted in some of the inconsistencies observed.
Conclusion
This review did not find a consistent relationship between AS intake and appetite measures, which may be partially explained by variation in study methodologies. The inconsistent results highlight a need for further randomised controlled trials that explore the impact of differing types of AS sources (including those replicating real-life consumption of AS) and different doses of AS on appetite in overweight and obese adults, to assist with targeting dietary messages for weight management.
Acknowledgements
The authors acknowledge that this project received no specific funding from any funding agency or from commercial or not-for-profit sectors.
The author attributes are as follows: K. T. and E. N. designed the study. K. T. collected and analysed the data. K. T., E. N. and K. C. interpreted the data. K. T. prepared the manuscript. E. N., K. C. and Y. P. were responsible for critically reviewing the study design and manuscript. All authors approved the final version of the paper submitted for publication.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003239





