The health benefits of nuts, mainly in relation to CVD as well as to other chronic conditions, have been widely demonstrated in both epidemiological(Reference Sabaté and Ang1) and clinical(Reference Estruch, Ros and Salas-Salvadó2, Reference Jenkins, Kendall and Banach3) trials. For this reason, the American Heart Association(Reference Lloyd-Jones, Hong and Labarthe4, Reference Stone, Robinson and Lichtenstein5), the Canadian Cardiovascular Society(Reference Anderson, Grégoire and Hegele6) and the US Food and Drug Administration(7) recommend the regular consumption of nuts to the general population, in the context of a healthy diet, to prevent the risk of CVD. Recently, nut consumption has also been inversely associated with total mortality(Reference Bao, Han and Hu8, Reference Guasch-Ferré, Bulló and Martínez-González9). Nuts are the rich sources of unsaturated fatty acids, fibre and protein, along with many vitamins (vitamins E and B6, niacin or folic acid), minerals (Mg, K and Cu) and other phytochemical constituents (stigmasterol, campesterol, resveratrol and catechins)(10). Compared with other nuts, pistachios have a lower fat (mostly from PUFA and MUFA) and energy content, and higher levels of fibre (both soluble and insoluble), K, phytosterols, γ-tocopherol, vitamin K, and xanthophyll carotenoids(10) (Table 1). Pistachios are among the top fifty foods with a high antioxidant potential(Reference Halvorsen, Carlsen and Phillips11). In addition, pistachios are the only nut that contains significant amounts of lutein and zeaxanthin(10). Polyphenols, xanthophylls and tocopherols from pistachios have been demonstrated to be rapidly accessible in the stomach, thus maximising the possibility of absorption in the upper small intestine, thereby contributing to the beneficial relationship between pistachio consumption and health-related outcomes(Reference Mandalari, Bisignano and Filocamo12).
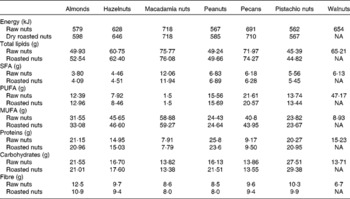
Table 1 Macronutrient contents of the selected nuts per 100 g (raw and dry roasted)*
NA, not available.
* US Department of Agriculture, Nutrient Database for Standard Reference, Release 26, 2013(10).
The present review examines the potential health effects of compounds in pistachios as well as epidemiological and clinical evidence supporting the health benefits of pistachio consumption.
Bioactive components of pistachios
Nuts and diet quality
Recent epidemiological studies conducted in children and adults have demonstrated a significant association between nut consumption and a higher diet quality score or improved nutrient intakes(Reference O'Neil, Keast and Fulgoni13, Reference O'Neil, Keast and Nicklas14). O'Neil et al. (Reference O'Neil, Keast and Fulgoni13), in a study of 13 292 adults participating in the 1999–2004 National Health and Nutrition Examination Survey, observed that tree nut consumers, defined as those consuming more than 7·09 g/d of nuts or tree nut butters, had a significantly higher intake of several nutrients as fibre, vitamins and minerals, and also a higher Total Healthy Eating Index-2005 score. Similarly, in an analysis including concatenated data from adults aged 2+ years participating in the National Health and Nutrition Examination Survey 1999–2000, 2001–02 and 2003–04, consumption of more than 7·08 g/d was associated with a healthier nutrient profile and higher Total Healthy Eating Index-2005 score in consumers of all age groups. Moreover, adult consumers showed a better metabolic risk profile(Reference O'Neil, Keast and Nicklas14). Furthermore, the results of a clinical trial conducted on 124 obese subjects demonstrated that nutritional dietary quality among nut consumers (those eating 42 g hazelnuts/d for 12 weeks) was appreciably improved compared with other groups consuming chocolate, potato crisps or no additional foods(Reference Tey, Brown and Gray15). Finally, the inclusion of nuts in energy-restricted diets reduced attrition and increased weight loss, supporting that nuts enhance palatability and compliance with diets without compromising beneficial health effects(Reference McManus, Antinoro and Sacks16).
Fat content
Pistachios, compared with other nuts, are relatively low in fat, containing 45·4 g total fat per 100 g pistachio kernel and consisting of 5·6 g SFA, 13·7 g PUFA and 23·8 g MUFA (Table 1)(10). Within fatty acids, oleic and linoleic fatty acids, both recognised for their cardiovascular-preventive properties(Reference Gillingham, Harris-Janz and Jones17), represent more than 60 % of the total fat content in pistachios.
The USA (California, Arizona and New Mexico), Iran and Turkey are the largest producers of pistachios, growing varieties that differ slightly in nutritional composition. Whereas US pistachios have less energy and contain higher amounts of lutein and zeaxanthin, Iranian pistachios are richer in linoleic acid(18) and Turkish pistachios in Ca(19) (Table 2). Fatty acid composition and nutritional profile characteristics also depend on the climate in which the pistachios are grown. For example, cultivars of pistachio nuts grown in hot temperatures (over 25°C) tend to produce a lower amount of a saturated fat such as palmitic acid(Reference Satil, Azcan and Baser20).
Protein
Pistachios are a good source of vegetable protein, which comprises about 20 % of total weight, with approximately 2 % l-arginine(Reference Ros21). This amino acid, also present in other nuts, is a precursor to the endogenous vasodilator NO, an important molecule involved in the cardiovascular system as a key regulator of vascular tone and in numerous pathological conditions such as hypertension, CVD and neurodegenerative disorders due to its pro-oxidant capacity(Reference Loscalzo and Welch22, Reference Jaffrey and Snyder23). NO synthase inhibitors based on arginine have been of special interest for experimental as well as clinical applications(Reference Víteček, Lojek and Valacchi24). Therefore, pistachios could play an important protective role in NO synthase-related diseases. On a per serving basis (28·35 g), pistachios provide 10·6 % US RDA of adult men and 12·9 % of adult women(25). Compared with the FAO- and WHO-recommended essential amino acid pattern for an adult, pistachios contain adequate amounts of all of the essential amino acids(Reference Sathe, Monaghan, Kshiesagar, Alsalvar and Shahidi26). Pistachios have an essential amino acid ratio (essential amino acid:total amino acid) of 39·1, higher than most of all the commonly consumed nuts (almonds, walnuts, pecans and hazelnuts). Pistachios also provide a high percentage of branched-chain amino acids (1·599 g leucine, 0·932 g isoleucine and 1·262 g valine per 100 g), higher than other tree nuts.
Carbohydrates and fibre
The amount of carbohydrate in pistachios, as in other nuts, is low to moderate (about 27·5 % by weight), but pistachios are rich in fibre, containing 10 % by weight of insoluble forms and 0·3 % of soluble forms. Pistachios provide 3 g or 12 % of RDA per serving basis (Table 1)(10). According to the US Department of Agriculture food composition tables, of all nuts, only almonds have similar amounts of fibre, with 13 % of weight. Fibre content is important because epidemiological and clinical studies have consistently demonstrated that fibre intake is inversely associated with weight gain(Reference Ye, Chacko and Chou27), diabetes(Reference Kaczmarczyk, Miller and Freund28), CVD(Reference Anderson, Hanna and Peng29) and some types of cancer(Reference Kaczmarczyk, Miller and Freund28). Moreover, pistachios have a low glycaemic index, which contributes to maintaining satiety longer and lowering postprandial blood glucose concentrations(Reference Kendall, West and Augustin30, Reference Gulati, Misra and Pandey31).
Vitamins and minerals
Pistachios are rich in Cu, Mg, Mn, vitamin A, vitamin C and B vitamins, with the exception of vitamin B12 (cyanocobalamin)(Reference Rothwell, Urpi-Sarda and Boto-Ordoñez32), compared with other nuts (Table 3). In particular, pistachios contain relatively high amounts of thiamin (vitamin B1), which is involved in intermediary carbohydrate metabolism, with 0·87 mg/100 g of pistachios (providing up to 50 % of the RDA). The amount of pyridoxine (vitamin B6) that is involved in the metabolism of amino acids and in the production of niacin is about 1·7 mg/100 g of pistachios, exceeding the RDA. Finally, the amount of folic acid in pistachios provides approximately 25 % of the RDA. Folic acid is necessary for the formation of structural proteins and Hb, and deficiency leads to an increase in the risk of CVD(Reference Carlsson33). Among nuts, pistachios also stand out for high vitamin K content, with approximately 13·2 μg/100 g (16 % of the RDA; Table 3). Beyond its role in bone metabolism(Reference Bulló, Estruch and Salas-Salvadó34–Reference Juanola-Falgarona, Salas-Salvadó and Estruch36), a higher dietary intake of vitamin K has been associated with a lower risk of several chronic diseases such as type 2 diabetes (T2DM)(Reference Bulló, Estruch and Salas-Salvadó34), cancer(Reference Nimptsch, Rohrmann and Kaaks37, Reference Juanola-Falgarona, Salas-Salvadó and Martínez-González38) and CVD(Reference Juanola-Falgarona, Salas-Salvadó and Martínez-González38), thus expanding the potential health benefits of pistachio consumption. The beneficial role of pistachios in inflammatory-related diseases may also be explained by the relatively high amount of γ-tocopherol they contain(Reference Dietrich, Traber and Jacques39).
Table 3 Micronutrient contents of the selected nuts per 100 g (raw and dry roasted)*

NA, not available.
* US Department of Agriculture, Nutrient Database for Standard Reference, Release 26, 2013(10). Polyphenol data were obtained from the Phenol-Explorer database (http://www.phenol-explorer.eu)(Reference Rothwell, Urpi-Sarda and Boto-Ordoñez32).
Pistachios are rich in several minerals such as K, Mg, Ca, Cu and Mn. Because of their mineral profile, pistachios could play a beneficial role in blood pressure (BP) regulation or in bone-related diseases. Pistachios also contain significant amounts of Zn and Se, both minerals with recognised antioxidant effects that are involved in the prevention of CVD and some types of cancer(Reference Huang, Caballero and Chang40, Reference Hercberg, Kesse-Guyot and Druesne-Pecollo41).
Phenol content
Pistachios, pecans and walnuts are rich sources of phenolic compounds, including anthocyanins, flavonoids, proanthocyanidins, flavonols, isoflavones, flavanones, stilbenes, phenolic acids and hydrolysable tannins, which are important as antioxidants and also for their chemopreventive, cardioprotective and vasoprotective properties(Reference Bulló, Lamuela-Raventós and Salas-Salvadó42, Reference Bolling, Chen and McKay43). Phenolic compounds may have protective effects against diseases related to free radical overproduction, such as CVD and cancer. A randomised, double-blinded, cross-over study with placebo v. a supplement of 640 mg anthocyanins daily during 4 weeks in pre-hypertensive men showed a significant increase in HDL-cholesterol (HDL-C) levels and also blood glucose levels after anthocyanin v. placebo treatment(Reference Hassellund, Flaa and Kjeldsen44). Furthermore, the hydrophilic extract from pistachios, which has high antioxidant activity, increases the resistance of human LDL-cholesterol (LDL-C) from healthy subjects to Cu-induced oxidation after 2 h of incubation(Reference Gentile, Tesoriere and Butera45).
According to Tomaino et al. (Reference Tomaino, Martorana and Arcoraci46), all phenolic groups found in pistachios, and in other nuts, are present in higher amounts in the skins than in the seeds. Pistacia vera L. (variety Bronte) skins contain cyanidin-3-O-galactoside (5865 mg/g), gallic acid (1453 mg/g), catechin (377 mg/g) and eriodictyol-7-O-rutinoside (366 mg/g). Pistachio kernels contain quercetin-3-O-rutinoside (98·1 mg/g), genistein (69·1 mg/g), genistein-7-O-glucoside (47·0 mg/g) and daidzein (42·4 mg/g). Therefore, the final content of total flavonoids in the skins is 70·27 (sd 5·42) mg of catechin equivalents/g of fresh weight, whereas in the seeds, it is only 0·46 (sd 0·03) mg of catechin equivalents/g of fresh weight(Reference Tomaino, Martorana and Arcoraci46). Pistachios are the only nut containing anthocyanins, phenolic compounds, in the skin. These phenolic compounds are known to bind metals through binding with o-diphenol groups, which is important in the inhibition of metal-induced lipid oxidation(Reference Dixon, Xie and Sharma47). Nonetheless, in a simulated human digestion model, more than 90 % of the pistachio polyphenols were released to the gastric compartment without differences between raw or roasted pistachios(Reference Mandalari, Bisignano and Filocamo12, Reference Nikzadeh and Sedaghat48).
Carotenoids
Lutein and zeaxanthin are two xanthophyll carotenoids responsible for giving colour to pistachio nuts. Raw pistachios contain 1405 μg lutein+zeaxanthin/100 g, about thirteen times more than the next highest nut type, hazelnuts, which contain only 92 μg (Table 3). The bioavailability of carotenoids depends on the source and interaction with other dietary components. Van Het Hof et al. (Reference Van het Hof, West and Weststrate49) demonstrated that the interaction of β-carotene and lycopene with the lipid matrix increases the bioavailability of carotenoids. Notably, almost 100 % of the bioaccessibility of lutein was found after in vitro duodenal digestion(Reference Mandalari, Bisignano and Filocamo12). Carotenoids have antioxidant properties and have been associated with a reduced risk of CVD and some types of cancer(Reference Van het Hof, West and Weststrate49). Moreover, lutein and zeaxanthin are concentrated in the retina where they thought to function as antioxidants and/or as a blue light filter, to protect the underlying tissues from phototoxic damage(Reference Carpentier, Knaus and Suh50). This has been proposed as an important factor in the pathophysiology of age-related macular degeneration(Reference SanGiovanni and Neuringer51).
Total phytosterols
Among nuts, pistachios have the highest phytosterol content, with 214 mg/100 g, including stigmasterol, campesterol and β-sitosterol. Phytosterols, structurally similar to cholesterol, have the same basic cyclopentanoperhydrophenanthrene ring structure but differ in the side chain at C24 and/or the position and configuration of unsaturated double bonds and the optical rotation at chiral carbons. Several studies have demonstrated a dose–response reduction of cholesterol mediated by phytosterols, even at lower levels similar to those found in plant-based diets with pistachios(Reference Ostlund, Racette and Stenson52). Although 500 mg of phytosterols per serving are needed to support the Food and Drug Administration (FDA) health claim, the levels of phytosterols in pistachio nuts may be sufficient to play a synergistic role with unsaturated fatty acids and the low SFA levels in helping to maintain normal cholesterol levels.
Effects of processing and storage on the final level of bioactive compounds
Roasting and steam roasting
Roasting and steam roasting are a common method of processing pistachios to increase the overall safety and palatability and enhance the flavour, colour, texture and appearance of the nuts(Reference Nikzadeh and Sedaghat48). However, this process may alter the bioactive compounds in pistachios(Reference Tomaino, Martorana and Arcoraci46). In this sense, it was demonstrated that the antioxidant capacity and total phenol content were reduced by 60 % in the same lot of Bronte's pistachio nuts, before and after exposure at 160°C for 40 min. Proanthocyanidin content was reduced by about 90 % and loss of vitamin C was observed, whereas isoflavones were not modified(Reference Gentile, Tesoriere and Butera45). Other antioxidants could be modified during the roasting processes as has been demonstrated in vegetables, in which, during a thermal process, the trans double bonds predominately present in carotenoids become susceptible to isomerisation, creating a cis configuration(Reference Lessin and Schwartz53) and lowering the total antioxidant content(Reference Updike and Schwartz54). Lutein, however, seems to be more stable with respect to degradation compared with other types of carotenoids.
Pistachios, as well as other types of nuts, contain several protein allergens that may trigger type I hypersensitivity reactions(Reference Fernández, Fiandor and Martinez-Garate55). Noorbakhsh et al. (Reference Noorbakhsh, Mortazavi and Sankian56) showed that the IgE-binding activity of pistachio nuts could be reduced by a steam-roasting process without any significant changes in the sensory quality of pistachios, due to the heat-induced denaturation of some proteins and/or reaction of these proteins to the food matrix.
Storage
Oxidation is one of the most serious problems in the storage of nuts. Oxidation causes the formation of hydroperoxides, which are colourless, tasteless and odourless. In addition, hydroperoxides increase water and soluble antioxidants by a degradation reaction of polymerised polyphenols to monomers. Fatty acid oxidation can be controlled by the application of antioxidants, using processing techniques that minimise the losses of tocopherols and other natural antioxidants; inactivate pro-oxidant metals and enzymes; reduce the exposure of nuts to oxygen, heat and light; promote hydrogenation of PUFA; and use an inert gas or vacuum packaging to expel atmospheric oxygen before long-term storage(Reference Haila, Lievonen and Heinonen57, Reference Subagio and Morita58).
Storage of nuts requires particular temperature, humidity/moisture and ventilation conditions. Bellomo et al. (Reference Bellomo, Fallico and Muratore59) tested the stability of lutein and oil in pistachio (P. vera L., variety Bronte) kernels stored up to 14 months at three temperatures: 10, 25 and 37°C. The samples were hermetically packaged using two films (nylon and ethylene vinyl alcohol) with and without oxygen scavengers. For each temperature, reference samples were packaged in open bags. After 14 months, the oil showed only a slight increase in acidity and peroxide value irrespective of storage temperature. As for lutein stability, the lowest concentrations were observed at 37°C with a degradation of about 57·5 %. At 10 and 25°C, the samples showed slight differences in lutein concentrations with a 37 % of degradation. Therefore, controlled storage is important for preserving pistachio quality. Oil stability is influenced only by the length of storage; lutein stability is also influenced by storage temperature and kinetic degradation. During storage, lutein showed good stability both at 10 and 25°C. In particular, a low storage temperature, such as 10°C, was the most important parameter because it guarantees good pistachio quality both for pigment and oil (acidity) stability and the absence of mould and bugs.
In vitro and animal studies
Recent in vitro studies and studies conducted on animals have suggested that the healthy properties of pistachios can be attributed partially to the content of the nut's dietary antioxidants. Gentile et al. (Reference Gentile, Allegra and Angileri60) evaluated the effects of a hydrophilic extract of P. vera L. on the production of reactive oxygen species in RAW 264.7 macrophage cells. A dose-dependent decrease in the production of Lipopolysaccharide (LPS)-induced reactive oxygen species was observed when the cells were incubated with different concentrations of hydrophilic extract, indicating proanthocyanidins as the bioactive components responsible for this effect. Similarly, the incubation of RAW 264.7 murine macrophages with a pistachio oil extract for 24 h decreased some LPS-induced inflammatory markers such as Ifit-2, TNF-α and IL-6(Reference Zhang, Kris-Etherton and Thompson61). This pistachio oil extract also reduced the expression of Ifirt-2, TNF-α, IL-6 and IL-1β by 78, 55, 58 and 35 %, respectively, in response to LPS stimulation of the same cells. In two studies on rats, increased antioxidant enzymatic activity was found in animals fed pistachios for 8 weeks(Reference Aksoy, Aksoy and Bagci62, Reference Alturfan, Emekli-Alturfan and Uslu63). In the first study, rats were divided into three groups of twelve animals and assigned to a control group fed a standard diet and two pistachio groups fed with a standard diet containing 20 or 40 % of the energy in the form of pistachios. A significant increase in the activities of Paraoxonase 1 (PON1) and arylesterase, both markers of antioxidant capacity, was shown in both groups supplemented with pistachios compared with the control group after 10 weeks of intervention(Reference Aksoy, Aksoy and Bagci62). In the second study, rats were assigned to a control diet (standard commercial chow); a control diet supplemented with 1·26 % of the total energy intake in the form of pistachios; a control diet with 1·63 % of cholesterol, 0·41 % of cholic acid and 16·3 % of sunflower oil (hyperlipidaemic diet); or a hyperlipidaemic diet supplemented with 1·26 % of the total energy intake in the form of pistachios. After 8 weeks, rats fed with the hyperlipidaemic diet supplemented with pistachios had higher total antioxidant activity, determined by thiobarbituric acid-reactive substances, than rats fed with the hyperlipidaemic diet alone(Reference Alturfan, Emekli-Alturfan and Uslu63). In another study, feeding 19-month-old rats with a 6 or 9 % walnut diet, which was approximately equivalent to a human eating 28 or 42 g, significantly inhibited the activation or phosphorylation of P38-Mitogen-activated protein kinase (MAPK) and the transcription factor NF-κB in brain tissues. Because both molecules are involved in the inflammatory response, these results suggest the potential attenuation of several inflammatory genes mediated by walnuts(Reference Poulose, Bielinski and Shukitt-Hale64).
Clinical trials in human subjects
Satiety and body-weight control
Despite the fact that nuts, including pistachios, contain a significant amount of fat and are energy-dense foods, several epidemiological studies have provided strong evidence that nut consumption is associated with neither weight gain nor an increased risk of obesity(Reference Bes-Rastrollo, Sabaté and Gómez-Gracia65–Reference Casas-Agustench, Bulló and Ros67). In addition, different clinical trials evaluating the effect of nuts on body weight have been conducted, but only a few have been designed to evaluate body weight as the main outcome. One of them, a 6-month cross-over study, assessed the impact of supplementing the habitual diet with 28–56 g of walnuts per d. In this study, walnut supplementation resulted in a much lower than expected weight gain(Reference Sabaté, Cordero-MacIntyre and Siapco68). Similar results were shown in a parallel, randomised, controlled trial conducted on 123 overweight and obese subjects assigned to an almond-enriched/low-energy diet (containing 56 g almonds to consume daily) or a free-nut/low-energy diet. After 6 months of follow-up, subjects in the almond-enriched diet lost slightly but significantly less weight than those in the free-nut diet, but no significant differences in body composition were observed after 18 months of follow-up(Reference Foster, Shantz and Vander Veur69). Most of the clinical trials that have assessed the influence of nuts on classical or emergent cardiovascular risk factors have also gathered and evaluated body-weight changes(Reference Tapsell, Gillen and Patch70, Reference Casas-Agustench, López-Uriarte and Bulló71). However, review of the available data suggests that adding nuts to habitual diets of free-living individuals does not lead to any appreciable weight gain(Reference Rajaram and Sabaté72–Reference Flores-mateo, Rojas-rueda and Basora78).
In three randomised, controlled clinical trials, the effect of pistachio consumption on body weight was evaluated(Reference Gulati, Misra and Pandey31, Reference Li, Song and Nguyen73, Reference Wang, Li and Liu76). In a 12-week weight-loss programme with hypoenergetic diets providing 2092 kJ less than energy recommendations, seventy overweight or obese individuals were randomly allocated to a pistachio-diet group (eating 53 g/d of pistachios) or to a pretzel-enriched diet group (eating 56 g/d of salted pretzels). The pistachios or pretzels were consumed as an afternoon snack. During the intervention, a significant reduction in BMI in the pistachio-supplemented group was observed ( − 4·3 % of the BMI). This reduction was higher than that observed in the pretzel-supplemented group ( − 2 % of the BMI)(Reference Li, Song and Nguyen73). Similarly, Wang et al. (Reference Wang, Li and Liu76) evaluated the impact of a 12-week normoenergetic diet intervention supplemented or not with two different doses of pistachio nuts (70 or 42 g/d) on total body-weight maintenance in ninety subjects with the metabolic syndrome. The results indicated that the consumption of any dosage of pistachios resulted in no changes in BMI or waist:hip ratio compared with the group of individuals following the American Heart Association Step I recommendations. More recently, a 24-week, randomised controlled trial, including sixty metabolic syndrome subjects randomised to either the pistachio (20 % of total energy in the form of pistachio nuts daily) or control group for 6 months, failed to find significant differences in body weight. However, Gulati et al. (Reference Gulati, Misra and Pandey31) observed a significant decrease in waist circumference and a trend towards a reduction in subcutaneous adipose tissue in the pistachio group compared with the control group.
Furthermore, five randomised feeding trials evaluated the effect of pistachio consumption on body weight and/or BMI as a secondary outcome. In all five studies, participants consumed at least 15 % of the total energy intake in the form of pistachio nuts. No significant effect on body weight and/or BMI was observed compared with participants assigned to the control diet group(Reference Edwards, Kwaw and Matud79–Reference Sari, Baltaci and Bagci83) (Table 4).
Table 4 Summary of cross-over, parallel and sequential intervention studies and their characteristics

M, male; F, female; RD, regular diet; PD, pistachio diet; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; VLDL-C, VLDL-cholesterol; CD, control diet; SBP, systolic blood pressure; AOP, antioxidant potential; MDA, malondialdehyde; IIEF, International Index of Erectile Function.
Several biological mechanisms may explain the unexpected null effect of nut consumption on adiposity. Nuts are rich in unsaturated fatty acids, and evidence suggests that MUFA and PUFA are more readily oxidised(Reference Reaven and Witztum84) and have a greater thermogenic effect(Reference Alper and Mattes85) than SFA, which can lead to less fat accumulation. Several lines of evidence also demonstrate that nuts have high satiety properties. Nuts are energy dense and a good source of fibre, protein and unsaturated fats, dietary factors that increase satiety ratings. Nuts exert a strong suppression of hunger and therefore subsequent food intake is curtailed(Reference Burton-Freeman86–Reference Hu88). In fact, two recent published studies have evaluated the satiating properties of pistachio nuts. The impact of consuming in-shell pistachios or pistachio kernels on fullness and energy intake was evaluated in a randomised, cross-over, controlled feeding trial including 140 university students aged 18–24 years. Consumption of in-shell pistachios resulted in a lower energy intake than consumption of kernels(Reference Honselman, Painter and Kennedy-Hagan89). The same authors, in a second cross-over feeding trial with 118 healthy individuals (mean age 47 (sd 10) years), demonstrated that the visual cue of the empty pistachio shells may have helped the participants to consume fewer pistachios and about 18 % less energy(Reference Kennedy-Hagan, Painter and Honselman90) (Table 5).
Table 5 Summary of acute intervention studies and their characteristics

WB, white bread; SM, specific meal; PD, pistachio diet; CHO, carbohydrate; GLP-1, glucagon-like peptide 1.
The physical structure of nuts may also contribute to their satiety effect; they are crunchy and must be mechanically reduced to particles small enough for swallowing. Mastication activates mechanical, nutrient and sensory signalling systems that may modify appetitive sensations(Reference Cassady, Hollis and Fulford91).
Furthermore, a small degree of fat malabsorption has been reported after nut intake, which is attributed to the fat being contained within walled cellular structures that are incompletely digested in the gut, an effect that can be compounded by incomplete mastication(Reference Ellis, Kendall and Ren92). In fact, a cross-over trial conducted on sixteen healthy volunteers consuming pistachios (42 and 84 g/d) or a free-nut diet for 3 weeks, as part of a controlled diet, demonstrated that the metabolisable energy of pistachios, calculated from differences in faecal energy excretion during the different dietary treatments, is 5 % less than the energy calculated by the Atwater general factors, suggesting that the energy from pistachios is not totally utilisable(Reference Baer, Gebauer and Novotny93).
Classical markers of CVD
In a pooled analysis of twenty-five intervention trials, participants who consumed an average of 67 g/d of nuts saw a 5 % decrease in total cholesterol, a nearly 7·5 % decrease in LDL-C levels and an 8 % decrease in the LDL-C:HDL-C ratio. The effects of nut consumption were dose-related, and different types of nuts had similar effects on blood lipid levels(Reference Sabaté, Oda and Ros94). The effect of pistachio consumption on cardiovascular risk markers has been evaluated in five randomised clinical trials as a primary outcome(Reference Edwards, Kwaw and Matud79–Reference Sari, Baltaci and Bagci83) and in other studies as a secondary outcome(Reference Li, Song and Nguyen73, Reference Wang, Li and Liu76, 95), giving from 10 to 20 % of energy or from 42 to 100 g/d as pistachios v. diets avoiding the consumption of nuts (Table 4). From them, in a total of five studies, the authors found significant reductions in plasma total cholesterol concentrations in the pistachio-supplemented group(Reference Edwards, Kwaw and Matud79, Reference Kocyigit, Koylu and Keles80, Reference Gebauer, West and Kay82, Reference Sari, Baltaci and Bagci83, 95), and in six of them, they found a significant reduction in the total cholesterol:HDL-C ratio and LDL-C:HDL-C ratio(Reference Edwards, Kwaw and Matud79–Reference Sari, Baltaci and Bagci83, 95). Moreover, LDL-C concentrations were decreased in the pistachio-supplemented group in three studies(Reference Gebauer, West and Kay82, Reference Sari, Baltaci and Bagci83, 95), whereas two studies reported no significant reductions in this recognised major cardiovascular risk factor(Reference Edwards, Kwaw and Matud79, Reference Kocyigit, Koylu and Keles80), although the levels decreased but not significantly in Kocyigit et al. (Reference Kocyigit, Koylu and Keles80). Only Wang et al. (Reference Wang, Li and Liu76), in a study of Chinese subjects with the metabolic syndrome, found an increase in plasma LDL-C levels after a 12-week period of dietary intervention with a normoenergetic diet including different amounts of pistachios compared with the normoenergetic diet alone. According to Wang et al. (Reference Wang, Li and Liu76), the nutrient content of the diet was underpowered to show changes in the secondary analyses of risk factors such as blood lipids. Notably, dietary intake was not controlled or reported, so it is difficult to ascertain the reason why LDL-C levels increased in the high-pistachio group. With respect to plasma HDL-C, only Sheridan et al. (Reference Sheridan, Cooper and Erario81) found a significant increase in this parameter in those subjects supplemented with pistachios.
A beneficial effect of pistachios on BP has also been demonstrated recently in a randomised, cross-over, clinical trial conducted on twenty-eight dyslipidaemic individuals. Participants were randomised to three 4-week interventions: a low-fat control diet; a diet containing 10 % of the total energy content in the form of pistachios; a diet containing 20 % of the total energy as pistachios. A dose-dependent reduction in systolic BP was observed in those subjects supplemented with pistachios, and a decrease in peripheral vascular dilation was observed in those supplemented with higher doses of pistachios(Reference West, Gebauer and Kay96). The BP-lowering effects of pistachios have also been evaluated in three additional controlled feeding trials as a secondary outcome showing non-significant differences in the changes in systolic or diastolic BP between those subjects supplemented with pistachios and those who did not receive supplementation(Reference Wang, Li and Liu76, Reference Sheridan, Cooper and Erario81, Reference Sari, Baltaci and Bagci83).
In conclusion, some evidence suggests that pistachios may improve the blood lipid profile and reduce BP, which could contribute to decreased cardiovascular risk.
Emerging risk factors of CVD
Pistachios are a rich matrix of fat-soluble antioxidants that could have important effects on the control of oxidative stress and a reduced risk of chronic diseases. In a study conducted on forty-four healthy men and women, half of the subjects were randomised to a regular diet group and the other half to a pistachio group (accounting for 20 % of their daily energy intake in the form of pistachios) for 3 weeks. The study showed an increased blood antioxidant potential determined by the production of thiobarbituric acid-reactive substances and decreased malondialdehyde levels, which is an important indicator of lipid peroxidation, in those volunteers consuming pistachios compared with those following a free-nut diet(Reference Kocyigit, Koylu and Keles80). A cross-over, randomised, controlled feeding trial conducted by Kay et al. (Reference Kay, Gebauer and West97) on twenty-eight hypercholesterolaemic adults showed that the consumption of diets containing 10 and 20 % of energy from pistachios (32–63 and 63–126 g/d, respectively) increased antioxidant concentrations in serum, such as γ-tocopherol, lutein and β-carotene, whereas it decreased oxidised LDL concentrations relative to the consumption of a control diet without pistachios. Finally, in a prospective study, Sari et al. (Reference Sari, Baltaci and Bagci83) assessed the effect of a traditional Mediterranean diet supplemented with pistachios by replacing the monounsaturated fat content constituting approximately 20 % of daily energy intake on thirty-two healthy young men for 4 weeks. They found a significant improvement in endothelium-dependent vasodilation, whereas endothelium-independent vasodilation remained unchanged compared with the Mediterranean diet. An increase in total antioxidant status and superoxide dismutase and a decrease in inflammation and other oxidative markers were also observed. Taken together, these results provide evidence of the beneficial effects of pistachios on the risk of CVD beyond the lipid-lowering effect.
Insulin resistance and type 2 diabetes
Diabetes mellitus is one of the most common diseases worldwide, largely the result of an increase in the prevalence of obesity and physical inactivity. Moreover, T2DM is a recognised risk factor for CVD and other chronic conditions and diseases, and is thus becoming a serious public health burden(Reference Sarwar, Gao and Seshasai98, Reference Shaw, Sicree and Zimmet99). Data from epidemiological and interventional studies suggest that the frequency of nut consumption is inversely related to an increased risk of T2DM, mainly attributed to the fibre, healthy fats, antioxidants and anti-inflammatory compounds(Reference Rajaram and Sabaté72, Reference Jenkins, Hu and Tapsell100–Reference Pan, Sun and Manson104) in nuts. In addition, among all nuts, pistachios have a low glycaemic index, suggesting a possible effect on reducing postprandial glycaemia and insulinaemia, thereby potentially decreasing the risk of diabetes. The effect of pistachios, consumed alone or combined with meals, on postprandial glycaemia has been evaluated(Reference Kendall, West and Augustin30, Reference Kendall, Josse and Esfahani105) (Table 5). Thus, whereas pistachios consumed alone had a minimal effect on postprandial glycaemia, the addition of pistachios (56 g) to foods with a high glycaemic index (pasta, parboiled rice and instant mashed potatoes) reduced, in a dose-dependent manner, the total postprandial glycaemic response by 20–30 %(Reference Kendall, Josse and Esfahani105). In a recent randomised, cross-over study conducted on twenty subjects with the metabolic syndrome, 85·04 g of pistachios consumed with bread reduced postprandial glycaemia levels and increased glucagon-like peptide levels compared with bread alone(Reference Kendall, West and Augustin30).
In three clinical studies, the effect of pistachio supplementation on glucose concentrations as a secondary outcome was evaluated, with contradictory results. In a controlled, cross-over, clinical trial, participants were randomised to a Mediterranean diet or a Mediterranean diet supplemented with 20 % of energy intake as pistachios for 4 weeks in each arm. Subjects in the intervention period showed a significant decrease in fasting plasma glucose concentrations in comparison to the control period(Reference Sari, Baltaci and Bagci83). The second study evaluated the effect of the American Heart Association Step I diet supplemented with 42 or 70 g/d of pistachios compared with the effect of a control diet (American Heart Association Step I), in Chinese subjects with the metabolic syndrome using a randomised, parallel-group, controlled study design. After 12 weeks of intervention, no differences in fasting plasma glucose or insulin levels were observed between the groups, although compared with baseline values, blood glucose levels increased significantly in the control group at week 12 but not in the two pistachio groups(Reference Wang, Li and Liu76). Finally, in a third parallel study conducted on sixty subjects with the metabolic syndrome randomised to either an unsalted pistachios diet (20 % energy) or a control diet for 24 weeks, a significant decrease in glucose levels but not in blood insulin levels was observed(Reference Gulati, Misra and Pandey31).
In addition to the fibre, healthy fats and low available carbohydrate content, the effect of pistachios on glucose metabolism may be a result of the rich content of carotenoids. A 9-year longitudinal study conducted on 1389 healthy elderly volunteers demonstrated a 58 % lower risk for the development of impaired fasting glucose levels or T2DM mellitus in subjects in the highest quartile of total plasma carotenoids than in those in the lowest quartile, even after adjusting for possible confounding variables(Reference Akbaraly, Fontbonne and Favier106). In a randomised controlled study, the intake of 75 g/d of mixed nuts (including pistachios) in 117 T2DM subjects during 3 months as a replacement for carbohydrate-containing foods in comparison to the intake of healthy whole-wheat muffins, or half portions of both, demonstrated for the first time a significant decrease in HbA1c levels, even though the subjects were on oral antidiabetic medication. Additionally, and despite the subjects consuming statins, an improvement in total cholesterol was observed(Reference Jenkins, Kendall and Banach3).
Despite the positive results observed for glucose metabolism in fasting conditions or postprandial status, more studies are necessary to evaluate the long-term effects of pistachio consumption on insulin resistance, secretion or diabetes control.
Summary and conclusions
Pistachios are nutrient-dense nuts with a healthy nutritional profile including fibre, healthy fats, phytosterols and antioxidant compounds, contributing to a reduced risk of heart disease. Growing evidence suggests that consumption of nuts, including pistachios, improves diet quality and provides several bioactive compounds with recognised properties for weight management, glycaemic control and vascular health.
Acknowledgements
The authors thank Carles Munné (Rovira i Virgili University, Reus, Spain) for his help as editor assistance.
Institut d'Investigació Sanitària Pere Virgili (IISPV) received research funding from American Pistachios Growers (USA) and Paramount Farms International.
All the authors contributed equally to this work.
M. B., M. J.-F. and P. H.-A. have no conflict of interest. J. S.-S. is a non-paid member of the Scientific Advisory Council of the International Nut Council.








