Lycopene (LYC) is a carotenoid pigment that gives tomatoes and tomato products their red colour. Both its dietary intake and blood concentration have been negatively associated with prostate cancer( Reference Kristal and Cohen 1 – Reference Wang, Jacobs and Newton 6 ) and CVD( Reference Müller, Caris-Veyrat and Lowe 7 – Reference Mordente, Guantario and Meucci 9 ) risk.
LYC is very hydrophobic but paradoxically also poorly soluble in fat. This explains, at least in part, its low bioavailability, which has been estimated at about 23 % when pure LYC was mixed with olive oil( Reference Moran, Cichon and Riedl 10 ) and at about 5 % when it was ingested from tomato juice( Reference Cooperstone, Ralston and Riedl 11 ). LYC absorption efficiency is also highly variable because it is affected by numerous factors( Reference Borel 12 ). These factors can be either dietary, for example, the effect of the food matrix in which LYC is absorbed( Reference Reboul, Richelle and Perrot 13 , Reference Reboul, Borel and Mikail 14 ), or linked to the physiological/genetic characteristics of the individual, for example, genetic polymorphisms that are involved in inter-individual variability in LYC bioavailability( Reference Borel, Desmarchelier and Nowicki 15 ).
LYC absorption is assumed to be closely related to that of dietary fat. After its extraction from the food matrix, LYC gets transferred to dietary fat( Reference Tyssandier, Reboul and Dumas 16 ) and then to mixed micelles( Reference Tyssandier, Lyan and Borel 17 ). These particles carry LYC to the enterocyte where it is taken up via scavenger receptor class B type I (SR-BI)( Reference Moussa, Landrier and Reboul 18 ) or perhaps via other apical membrane proteins( Reference Moussa, Gouranton and Gleize 19 , Reference Reboul and Borel 20 ). The fraction of LYC incorporated into mixed micelles during digestion is very low – that is, between 0·1 and about 2·7 %( Reference Reboul, Richelle and Perrot 13 , Reference Tyssandier, Cardinault and Caris-Veyrat 21 ) – and is affected by numerous factors( Reference Borel 12 ) including the presence of other nutrients, for example, fat, fibres and minerals. As species of fatty acids present in mixed micelles can affect carotenoid micellarisation( Reference Gleize, Tourniaire and Depezay 22 ), also known as bioaccessibility, it has been hypothesised that Ca, which can lead to insoluble fatty acid–soap complex formation( Reference Chappell, Clandinin and Kearney-Volpe 23 ), could impair carotenoid micellarisation. This hypothesis is supported by a recent in vitro study that has shown that LYC micellarisation was diminished in the presence of Ca( Reference Corte-Real, Iddir and Soukoulis 24 ). As LYC micellarisation is assumed to be a key step for LYC bioavailability, the authors have suggested that if a similar interaction occurs in vivo – that is, in the human intestinal lumen – this can lead to diminished LYC absorption efficiency.
The objectives of this study were therefore to assess whether a nutritional dose of Ca can impair LYC bioavailability in healthy humans and, if so, to study the mechanism(s) involved.
Methods
Subject number and characteristics
In all, ten healthy, non-obese subjects (five males, five females) were recruited for the study. As this was the first study dedicated to assess the effect of Ca on LYC bioavailability in humans, it was not possible to perform a statistical power analysis to calculate the number of subjects required to observe a significant effect. Thus, the number of subjects was chosen on the basis of the team’s knowledge on the variability of the postprandial LYC plasma response( Reference Reboul, Borel and Mikail 14 , Reference Borel, Desmarchelier and Nowicki 15 , Reference Cardinault, Tyssandier and Grolier 25 , Reference Richelle, Lambelet and Rytz 26 ), and on the hypothesis that a very significant effect can be expected because in vitro studies have shown that Ca is able to strongly impair LYC bioaccessibility( Reference Corte-Real, Iddir and Soukoulis 24 ). Subjects had no history of chronic disease, hypocalcaemia, hyperlipidaemia or hyperglycaemia and were not taking any medication known to affect LYC or lipid metabolism (e.g. tetrahydrolipstatin, ezetimibe, phytosterols, cholestyramine, fibrates) the month before the study or during the study period. The present study was approved by the local Ethical Review Board (Comité de Protection des Personnes Sud-Est VI, approved study no. 2013-A00096-39). Procedures followed were in accordance with the Declaration of Helsinki of 1975 as revised in 1983. Objectives and requirements of the study were fully explained to all participants before starting the study, and written informed consent was obtained from each subject. Subject baseline characteristics are reported in Table 1.
Table 1 Characteristics of the subjectsFootnote * (Mean values with their standard errors)
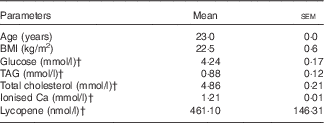
* n 10 (five males, five females), all Caucasians.
† Fasting plasma variables.
Postprandial experiments
In order to assess the effect of Ca on LYC bioavailability, we measured the postprandial plasma LYC response to two LYC-rich test meals, separated by at least 3 weeks, and which contained either no supplement or a supplement that provided 500 mg Ca as calcium carbonate (Cacit 500®; Warner Chilcott). This dose of Ca is about half the RDA for a healthy male adult( 27 ). Subjects were asked to refrain from the consumption of LYC supplements and LYC-rich foods 48 h before the postprandial experiments (an exclusion list was provided by a dietitian). In addition, subjects were asked to eat dinner between 19.00 and 20.00 hours the day before the postprandial experiments, and to then abstain from any food or beverage consumption with the exception of water. After the overnight fast, subjects arrived at the local Centre for Human Nutrition (Laboratory of Human Nutrition). A fasting blood sample was collected 20 min before administration of the meal. The subjects then consumed the test meal with or without the Ca supplement. The test meal provided 19 (sem 1·3) mg (all-E)-LYC in 40-g tomato paste. The tomato paste (triple concentrated, refractive index 30·8° Brix), which came from the same barrel, was prepared using a cold-break process by Conserve de France S.A. The meal also contained 90-g cooked pork meat, 80-g pasta (dry weight; Lustucru® torti), 10-g olive oil and 250-ml mineral water (Volvic, which provided about 2·5-mg Ca and in which the effervescent supplement of calcium carbonate was mixed). Subjects were asked to consume the meal at a steady pace, with one half of the meal consumed in about 7–8 min and the remainder of the meal consumed within 15 min (to diminish the variability due to different rates of intake, and thus gastric emptying). No other food or water was permitted over the following 7 h. Blood samples were collected at 20, 40, 60, 90, 120, 180, 240, 300, 360 and 420 min after meal consumption via evacuated purple-top glass tubes containing lithium heparin (BD Vacutainer®; Becton, Dickinson and Company). Tubes were immediately placed on ice and covered with aluminium foil to avoid light exposure. Plasma was isolated by centrifugation (10 min at 4°C and 2000 g ) <2 h after collection and stored at −80°C until analysis.
In vitro digestion experiments
Corte-Real et al. ( Reference Corte-Real, Iddir and Soukoulis 24 ) have recently reminded us that in the intestinal lumen Ca ions can be complexed by other molecules such as phosphate from animal products or phytic acid/oxalic acid from plant matrices. This complexation could attenuate the effect of Ca on several processes including its hypothesised impact on LYC micellarisation. We therefore used an in vitro digestion model that contained both an animal product and a plant matrix, meat and potato, respectively, in addition to a vegetable oil, olive oil( Reference Reboul, Richelle and Perrot 13 , Reference Gleize, Tourniaire and Depezay 22 ). We also used the same source of LYC as was used in the clinical study, that is, tomato paste. The amounts of added Ca ranged between 10 and 80 mg/meal, which were equivalent to concentrations between 175 and 1400 mg/l. Note that the ratio mg Ca:g tomato paste in the meal used in the clinical study was 12·5, which amounted to 50 mg added Ca in the in vitro digestion test meal, as it contained 4 g of tomato paste. The in vitro digestion experiments were adapted from Reboul et al. ( Reference Reboul, Richelle and Perrot 13 ) with minor modifications( Reference Gleize, Tourniaire and Depezay 22 ). After the digestion experiments, the aqueous fractions, which contained the mixed micelles, were separated from oil droplets and food particles by centrifugation (2200 g for 1 h at 10°C). They were then collected and passed through a 0·8- and a 0·22-µm filter (Millipore) in order to discard the remaining small food particles and to obtain clear solutions. Aliquots of these solutions, which contained LYC in mixed micelles, were stored at −80°C until LYC analysis.
Lycopene extraction and analysis
Tomato paste LYC extraction and analysis were carried out as described by Page et al. ( Reference Page, Van Stratum and Degrou 28 ). LYC in plasma and in samples from the in vitro experiments was extracted and analysed as described by Gleize et al. ( Reference Gleize, Steib and Andre 29 ). In brief, up to 2 ml of the samples was de-proteinated by adding 1 volume of ethanol, which also contained echinenone as an internal standard. After adding 2 volumes of hexane, the mixture was vortexed for 10 min and centrifuged at 1200 g for 10 min at 4°C. The upper phase (containing LYC and echinenone) was collected, and the sample was extracted a second time with hexane following the same procedure. The hexane phases were pooled and evaporated to dryness under N gas. All extractions were performed at room temperature under dimmed light to minimise light-induced damage. The dried residue was dissolved in 200 μl of methanol–dichloromethane (65:35, v/v). A volume of 150 μl was used for HPLC analysis.
The HPLC system comprised a Dionex separation module (P680 HPLC Pumps and ASI-100 Autosampler; Dionex) and a UVD340U Dionex photodiode array detector (detection at 472 nm, spectral analysis between 300 and 600 nm). LYC and echinenone were separated using a 10×4·0-mm, 5-μm C30 guard column (Chromoptic) followed by a 250×4·6-mm internal diameter, 5-μm particle size, YMC C30 column (Chromoptic) held at 35°C. The mobile phase was composed of methanol, methyl tert-butyl ether and water. A flow rate of 1 ml/min was used. All-E-LYC and cis-LYC isomers were integrated together to obtain the sum of all LYC isomers. Quantification was performed using Chromeleon software (version 6.80 DU 13a; Dionex) comparing peak area with standard (all-E-LYC and echinenone) reference curves. All solvents used were of HPLC grade (Carlo Erba).
Measurement of mixed micelles size and zeta potential
The intensity-weighted mean hydrodynamic radius and the zeta potential of the mixed micelles were determined by photon correlation spectroscopy at 25°C (Zetasizer Nano ZS; Malvern Instruments), directly following their isolation after the in vitro digestions.
Calculation and statistical analysis
The trapezoidal approximation method was used to calculate the AUC of the postprandial plasma LYC concentration over 7 h. Micellarisation was defined as the percentage of LYC recovered in the micellar phase after in vitro digestion, in relation to the amount of LYC recovered in the digestive medium just before addition of artificial saliva.
Data are expressed as mean values with their standard errors. Means were compared by non-parametric tests, either the Mann–Whitney U test or the Wilcoxon signed-rank test when two groups, respectively, unpaired and paired, were compared. When more than two groups were compared, the Kruskal–Wallis test followed by Dunn’s test as a post hoc test was used. Statistical dependence between two variables was assessed by Spearman’s rank-correlation coefficient. For all tests, the bilateral alpha risk was α=0·05. Statistical analyses were performed using Statview and SAS softwares (SAS Institute).
Results
Lycopene and calcium status of the subjects
Baseline characteristics of the subjects are shown in Table 1. The fasting plasma LYC concentration of the subjects was in the range of values previously reported for healthy, French subjects( Reference Borel, Desmarchelier and Nowicki 15 , Reference Borel, Moussa and Reboul 30 , Reference Borel, Moussa and Reboul 31 ).
Effect of dietary calcium on lycopene bioavailability
The mean postprandial plasma LYC concentrations measured after consumption of the control and Ca test meals are shown in Fig. 1(a). It is noteworthy that the plasma LYC concentration increased 2 h after intake of the control meal, whereas it did not significantly increase until 5 h after intake of the Ca meal. In addition, the postprandial plasma LYC response, as evaluated by the AUC of the 0–7-h plasma LYC concentration, was significantly lower following consumption of the test meal with added Ca than following consumption of the test meal without added Ca (−83 %, P=0·047) (Fig. 1(b)), and nine out of the ten subjects exhibited a decrease in their response (Fig. 1(c)).
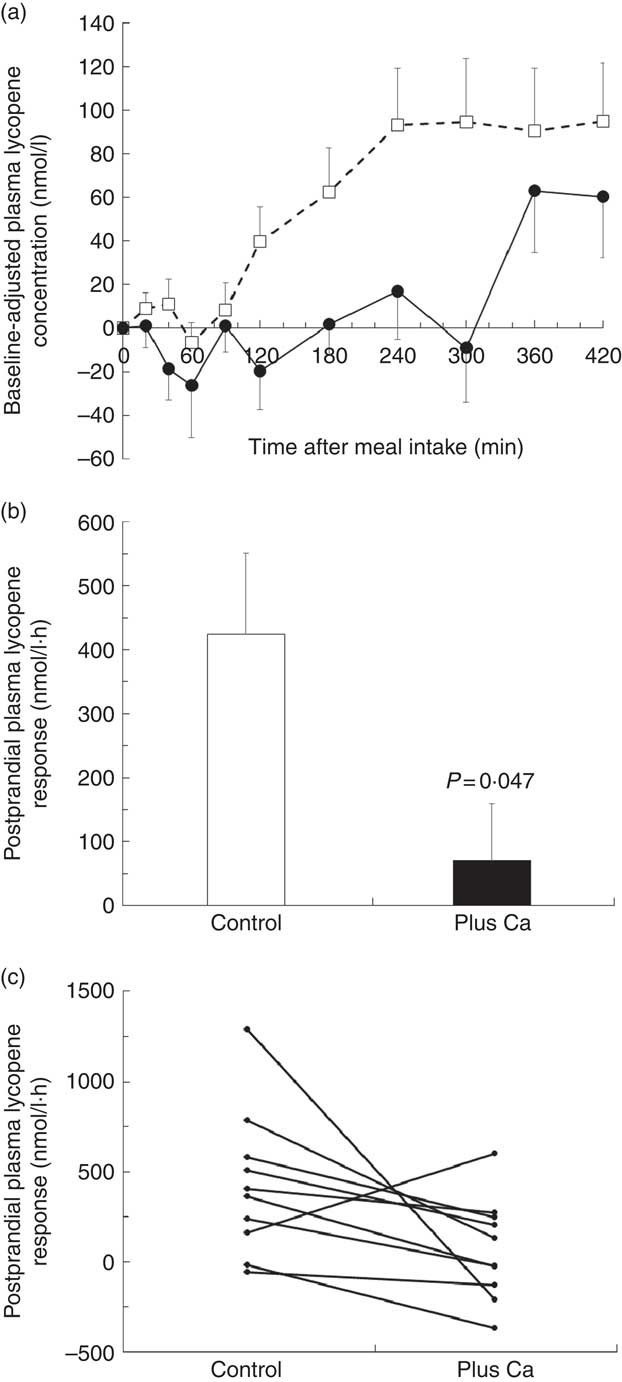
Fig. 1 Postprandial plasma lycopene (LYC) responses to a LYC-rich meal that contained either no added calcium or a calcium supplement. (a) Baseline-adjusted plasma LYC concentration over 7 h after consumption of the LYC-rich meals (control meal (□): meal without added calcium, meal plus calcium (●): meal with 500 mg calcium carbonate). For each subject, postprandial plasma LYC concentrations were baseline adjusted by using the fasting plasma LYC concentration. (b) Mean AUC of the postprandial plasma LYC responses obtained after intake of either the control or the calcium-supplemented meal. Means were compared by Wilcoxon’s signed-rank test. (c) Individual AUC of the postprandial plasma LYC responses (0–7 h AUC) after the control meal and after the meal that contained the calcium supplement.
Effect of calcium on tomato paste lycopene micellarisation during in vitro digestions
The micellarisation of LYC in the in vitro digestion experiment without added Ca was very low, about 0·6 % of the amount present in the tomato paste. Furthermore, it was not significantly affected by the addition of Ca, up to 1400 mg/l, in the in vitro test meal (P=0·262, Kruskal–Wallis test; data not shown).
Effect of calcium on the size and zeta potential of particles produced during in vitro digestions
It should first be noted that only particles whose size corresponded to that of mixed micelles, that is, mean hydrodynamic diameter <10 nm, were observed in the micellar phase. The effect of Ca concentration on the size and zeta potential of the mixed micelles produced during the in vitro digestions is shown in Fig. 2. The mean diameter of the mixed micelles was positively, although non-significantly, correlated to Ca concentration (ρ=0·90, P=0·072). Nevertheless, there was no significant effect of Ca concentration on micelle size (P=0·162, Kruskal–Wallis test) (Fig. 2(a)). The zeta potential of the mixed micelles recovered after digestion of the control meal was negative, about −12 mV, and its absolute value was significantly diminished (P=0·049, Kruskal–Wallis test), down to about −5 mV, when Ca was added in the digestive medium (Fig. 2(b)). Nevertheless, there was no dose effect of the concentration of Ca on the zeta potential of mixed micelles.
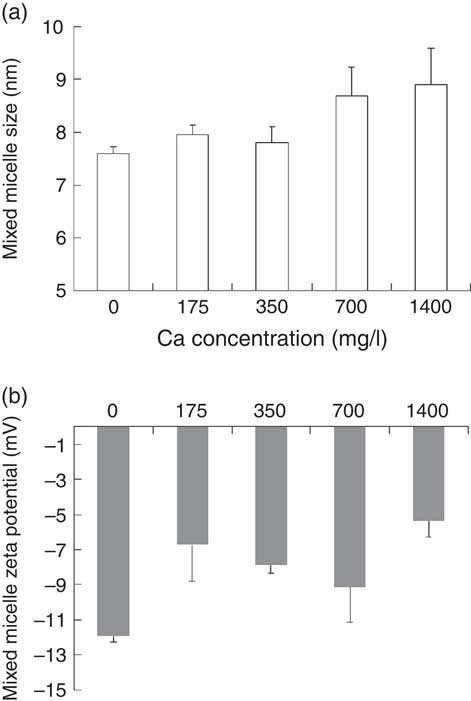
Fig. 2 Effect of calcium concentration on physico-chemical parameters of mixed micelles produced during in vitro digestions. (a) Mixed micelle size. (b) Mixed micelle zeta potential. These two parameters were measured by photon correlation spectroscopy at 25°C. Means were compared by the Kruskal–Wallis test followed by Dunn’s test as a post hoc test when the Kruskal–Wallis test was significant.
Discussion
This study was initiated following a recent study that showed that Ca diminishes LYC micellarisation in vitro ( Reference Corte-Real, Iddir and Soukoulis 24 ), suggesting that it could diminish LYC bioavailability. As both LYC status and LYC intake have been negatively associated with prostate cancer risk( Reference Wertz, Siler and Goralczyk 3 , Reference Jian, Du and Lee 4 , Reference Graff, Pettersson and Lis 32 ), this interaction could partly explain the positive association observed between Ca intake and prostate cancer risk( Reference Aune, Navarro Rosenblatt and Chan 33 , Reference Butler, Wong and Koh 34 ), although this association is still under debate( Reference Wilson, Shui and Mucci 35 ).
Our first objective was therefore to perform a clinical study dedicated to assess whether a dietary dose of Ca can significantly affect LYC bioavailability in healthy subjects. The results obtained showed that adding 500 mg Ca to a meal that contained a food source of LYC significantly impaired its bioavailability. This is the first time that such an effect is described, and this led us to perform in vitro studies to identify the mechanism(s) involved.
The first suspected mechanism was an inhibition of carotenoid micellarisation, as this was described by Bohn’s group( Reference Corte-Real, Iddir and Soukoulis 24 , Reference Biehler, Hoffmann and Krause 36 ). However, we did not observe this effect in our experiments. This apparent discrepancy could be due to the fact that we used a test meal where LYC, together with other nutrients, was brought by foods( Reference Reboul, Richelle and Perrot 13 ) that contain molecules that can bind Ca, for example, phosphates in meat and oxalic acid in tomato paste, whereas Bohn’s group used purified LYC incorporated in rapeseed oil without adding any food in their test meals. Yet, as suggested by Corte-Real et al. ( Reference Corte-Real, Iddir and Soukoulis 24 ), foods contain chemicals that can bind Ca, leading to partial or even total masking of its inhibitory effect on LYC micellarisation. Of note, we verified the adequacy of our methods to detect a change in LYC micellarisation by measuring LYC bioaccessibility when only purified LYC incorporated in colza oil (i.e. no other food item) was present in our in vitro digestion model. We indeed observed a significant 84·7 % decrease (P=0·006; data not shown) in LYC bioaccessibility when Ca (1000 mg/l) was added to the model. Thus, differences between the in vitro digestion protocols used (e.g. presence of an oral phase, no sonication before digestion, shorter gastric and duodenal phases with slightly different pH in our model) could also partly explain the differences between our results and the results obtained by Bohn’s group. Nevertheless, an inhibitory effect of Ca on LYC micellarisation could occur at higher Ca concentrations, depending on the Ca-binding ability of foods co-ingested with the food source of LYC.
We also studied the effect of Ca on the physico-chemical properties of mixed micelles, that is, their zeta potential and size, as changes thereof could affect the uptake of micellarised LYC by intestinal cells. We indeed observed that adding Ca to the in vitro digestions significantly diminished the absolute value of the zeta potential of the mixed micelles. We suggest that Ca cations bind to the negatively charged micelles and consequently diminish their electrical charge. This effect was maximal when 10-mg Ca was added, suggesting that only a limited number of the negative charges of the micelles was available for the Ca cations. The Ca-induced modification of micelle electrical charge could in turn affect LYC uptake by enterocytes as it has been shown that the apex region of the membrane protein involved in LYC uptake, that is SR-BI( Reference Moussa, Landrier and Reboul 18 ), exhibits a significant accumulation of cationic residues and that electrostatic interaction contributes to the binding of SR-BI to its ligands, impacting micelle interactions with the surface receptor loop( Reference Goncalves, Gontero and Nowicki 37 ). Obviously, other mechanisms can be involved in the observed effect of Ca on LYC bioavailability. For example, we can suggest an effect on SR-BI expression, as SR-BI expression has been shown to be significantly diminished in the presence of Ca in human keratinocytes( Reference Tsuruoka, Khovidhunkit and Brown 38 ). Nevertheless, other studies are required to verify this hypothesis.
In conclusion, this study shows for the first time that a dietary dose of Ca, that is, half the RDA for a male adult, ingested together with a food source of LYC, that is, tomato paste, can significantly impair LYC bioavailability. It also suggests that this is due, at least partly, to an effect of Ca ions on mixed micelle zeta potential. The pathophysiological consequences of this interaction are not known, but these results suggest that the long-term consumption of high doses of Ca, such as those found in dairy products or in some mineral waters, can diminish body LYC status. As LYC status has been inversely related to prostate cancer and CVD risk, we hypothesise that the positive association between Ca intake and incidence rate of these diseases is due, at least in part, to the negative effect of Ca on LYC bioavailability. Obviously, further experiments are required to verify the validity of this hypothesis. Finally, these results call for a thorough assessment of the effects of Ca, or other divalent minerals, on the bioavailability of other carotenoids and lipophilic micronutrients.
Acknowledgements
The authors are very grateful to the physician and nurses of the Human Nutrition Research Centre of Auvergne and to Patrice Reiling for their technical assistance.
The present study has received research funding from the European Community’s seventh Framework Programme (FP7 2007–2013). The funding was attributed to the Dream project (grant agreement no. FP7-222654-2), which was an integrated project within the framework of the ‘Food Quality and Safety’ programme. This publication reflects only the view of the authors. The Dream community is not liable for any use that may be made of the results.
P. B., J. L. S. and D. R. designed the study; C. Buisson and C. Buffière conducted the clinical study; D. P. analysed tomato paste lycopene; U. D. and C. D. performed the in vitro studies; C. H. analysed plasma lycopene; P. B. drafted the paper; P. B. and C. D. analysed the results and had primary responsibility for final content of the manuscript.
The authors declare that there are no conflicts of interest.






