The prevalence of human obesity, which is currently about 30 % in the USA, has increased dramatically over the last 30 years, consequently increasing medical costs for the treatment of related disease conditions( Reference Finkelstein, Trogdon and Cohen 1 – Reference Ogden, Yanovski and Carroll 3 ). Both visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) are affected in human obesity, but VAT shows the strongest correlation with inflammatory markers and risk factors for the metabolic syndrome( Reference Pou, Massaro and Hoffmann 4 ).
Numerous studies in rodents have already provided insights into the complex onset of obesity-related disorders; however, despite this abundant information, several topics, including the mechanism by which visceral obesity contributes more to the pro-inflammatory profile, are still subject to further investigation. As morbid obesity can be rapidly induced in rats, they may not be the most accurate model for human obesity research, which represents a more chronic obesity( Reference Bergman, Kim and Catalano 5 , Reference Symonds, Sebert and Budge 6 ). Dogs and cats are suggested to be more reliable models for human endocrine disorders than rodents, as the variation in the degree of obesity corresponds more to human obesity and genetic homology between humans and companion animals is greater than to rodents( Reference Bergman, Kim and Catalano 5 , Reference Hoenig 7 , Reference Kooistra, Galac and Buijtels 8 ).
As in humans, feline overweight and obesity have become a rising problem with a prevalence ranging between 19 (Western Australia) and 52 % (UK)( Reference Robertson 9 , Reference Russell, Sabin and Holt 10 , Reference Scarlett, Donoghue and Saidla 11 ). Obese cats tend to have a sedentary inactive lifestyle with offered food on an ad libitum basis, and obesity is mostly associated with owners who ‘humanise’ their pet( Reference Kienzle and Bergler 12 ). Additional similarities between cats and humans are postulated, as diabetes mellitus in cats strongly resembles human type 2 diabetes and dyslipidaemia also occurs with feline obesity( Reference Kooistra, Galac and Buijtels 8 , Reference Hoenig, Thomaseth and Waldron 13 ). Whether obesity causes an inflammatory state in cats that might predispose to diabetes mellitus, dyslipidaemia and lower urinary tract disorders has not been investigated so far( Reference German 14 ). In one study, a higher mRNA expression of TNF-α was demonstrated in the SAT of obese cats compared with lean cats( Reference Hoenig, McGoldrick and deBeer 15 ). Another study, however, concluded that obesity or subsequent weight loss did not alter plasma acute-phase proteins in cats( Reference Tvarijonaviciute, Ceron and Holden 16 ) and that systemic immune competence of obese cats showed no impairment compared with lean cats( Reference Jaso-Friedmann, Leary and Praveen 17 ). So far, no study has evaluated the impact of adipose tissue location on the inflammatory response to obesity in the domestic cat, which might elucidate a possible link between location-dependent inflammation and obesity-related disorders.
The present study evaluated adipokine mRNA expression and morphological features of SAT (abdominal and inguinal) and VAT (omental, bladder and renal) in chronically obese compared with lean control cats. The present hypothesis stated that the adipocyte cell size of chronically obese cats is larger compared with lean cats, and that adipose tissue in feline obesity attracts macrophages and T- and B-lymphocytes, consequently inducing an inflammatory response. Furthermore, location-dependent alterations of adipose tissue due to feline obesity are expected. As mentioned above, the results of the present feline study will help in determining the future suitability of cats as a model for human obesity.
Experimental methods
Animals and diet
A total of ten lean control (four males: one intact and three neutered; six females: one intact and five neutered) and ten chronically obese cats (five males and five females, all neutered) were included in the study. Obese cats were chronically obese (>2 years, except for the youngest cat that was only obese for 6 months). Obesity was a naturally occurring phenomenon and was not experimentally induced by the diet. All cats were group housed with an equal distribution of age in each group (lean cats: 5·8 (se 3·5) years; obese cats: 6·6 (se 3·0) years). All the cats underwent a general clinical examination and blood analysis (complete blood count and serum biochemistry) to ensure optimal health. At 4 weeks before the sampling procedure, an adaptation period was included, in which all cats received a commercial dry cat food (Friskies for neutered cat: rabbit and vegetables, Nestlé Belgilux NV; Nestlé Purina PetCare) that contained no fish oil (Table 1). Food was offered in groups of approximately eight cats at maintenance energy requirement (calculated for each cat individually and the total amount of food was calculated per group: lean cats 418 kJ/kg body weight (BW)0·67 and obese cats 544 kJ/kg BW0·4) according to the National Research Council guidelines( 18 ). Cats were weighed twice weekly to ensure a constant BW. Drinking water was available ad libitum.
Table 1 Analysed nutrient and labelled ingredient composition of the experimental diet*

NFE, N-free extract; TDF, total dietary fibre; ME, metabolisable energy.
* Ingredient list: grain, vegetable protein extracts, meat and animal by-products, vegetable by-products, oils and fats, yeast, minerals and vegetables.
† Derived by subtracting crude protein, diethyl ether extract, crude ash and crude fibre content from 100.
‡ Estimated using a four-step calculation( 18 ).
Study design
After the 4-week adaptation period, BW and body condition score were determined using a nine-point body condition scale, in which 1/9 reflects cachexia, 5/9 optimal body condition and 9/9 obesity( Reference Laflamme 19 ). Body composition was measured using the D2O dilution method( Reference Ferrier, Robert and Dumon 20 ). Under general anaesthesia, a fasting blood sample was taken from the jugular vein for the determination of serum leptin and the mRNA expression of adipokines. Samples of SAT (abdominal and inguinal) and VAT (omental, bladder and renal) were collected surgically for the determination of the mRNA expression of adipokines, and for histological and immunohistochemical (IHC) evaluation. The study design was approved by the ethical committee of the Faculty of Veterinary Medicine, Ghent University, Belgium and was in accordance with institutional and national guidelines for the care and use of animals (EC2011/131).
Anaesthetic protocol
After intramuscular pre-medication with 20 μg/kg of dexmedetomidine (Dexdomitor®; Janssen Animal Health), anaesthesia was induced with 1–2 mg/kg of propofol intravenously given to effect (Propovet®; Abbott Laboratories). Additional analgesia was provided by 0·1 mg/kg of methadone intravenously (Mephenon®; Sterop). After endotracheal intubation, anaesthesia was maintained with isoflurane (Isoflo®; Abbott Laboratories) in O2 given to effect using a paediatric circular system. During the procedure, all cats received a lactated Ringer's solution (Ringer lactate; B. Braun Medical NV/SA) at 10 ml/kg intravenously, and they were continuously monitored through pulse oximetry (N-20PA Portable Pulse Oximeter, Nellcor Puritan Bennett Inc.) and capnography (Capnomac Uttima; Datex Engstorn). After surgery, 0·05 ml atipamezole (Antisedan®; Pfizer Animal Health) was administered intramuscularly and post-operative analgesia was provided by an additional dose of methadone in combination with 0·3 mg/kg of meloxicam (Metacam®; Boehringer Ingelheim Vetmedica GmbH) subcutaneously.
Blood and adipose tissue sampling
A fasting blood sample was taken from the jugular vein and transferred to either a serum blood tube (Vacuette Z Serum Sep Clot Activator; Greiner bio-one) to measure leptin concentration or a PAXgene Blood RNA Tube (PreAnalytiX GmbH) and stored at − 20°C until analysis. Furthermore, samples of SAT (abdominal and inguinal) and VAT (omental, bladder and renal) were collected surgically through a ventral median celiotomy, utilising new biopsy material per location to prevent RNA contamination from previous samples, for the determination of the mRNA expression of several adipokines and for the microscopic evaluation of IHC and haematoxylin–eosin-stained sections. Samples of SAT and VAT were approximately 1 cm in diameter and were subdivided into pieces with a maximum diameter of 0·5 cm. Adipose tissue samples for mRNA expression were immediately stored in RNAlater tissue storage solution (Sigma-Aldrich). After an overnight incubation at 4°C, samples were stored at − 20°C until analyses were performed. Adipose tissue samples for IHC and haematoxylin–eosin staining were placed in neutral-buffered formalin for 48 h before embedding in paraffin and sectioning at 5 μm.
Analytical methods
Diet
The diet was analysed for DM, ash, crude protein, diethyl ether extract, crude fibre and total dietary fibre. DM and ash content were determined by drying to a constant weight at 103°C and combustion at 550°C, respectively. Crude protein (6·25 × N) was determined using the Kjeldahl method (ISO 5983-1, 2005) and diethyl ether extract was analysed with the Soxhlet method (ISO 1443, 1973). Crude fibre and total dietary fibre were determined using the Association of Official Analytical Chemists methods( Reference Cunnif 21 , Reference Cunnif 22 ).
Determination of body composition
Determination of body fat mass and lean mass was achieved by the D2O dilution technique and Fourier transform IR spectroscopy as described previously in dogs and cats( Reference Ferrier, Robert and Dumon 20 , Reference Backus, Havel and Gingerich 23 ). At 2 h before the start of the procedure, cats were deprived of food and water and a first blood sample (EDTA plasma tube, Vacuette K3E K3 EDTA; Greiner bio-one) was taken from the jugular vein. Then, 500 mg/kg BW of isotopic 99·9 % D2O (Euriso-Top SA) were injected subcutaneously and a second blood sample (EDTA plasma tube) was taken 4 h later. Plasma was obtained by centrifugation (15 min, 1620 g ) and immediately stored at − 20°C until analyses. Samples were analysed with Fourier transform IR spectroscopy as described previously( Reference Ferrier, Robert and Dumon 20 ).
Determination of serum leptin concentration
Serum was obtained by centrifugation (15 min, 1620 g ) and immediately stored at − 20°C until analyses were performed. Serum leptin was analysed using a commercially available multi-species RIA test kit (catalogue no. XL-85K; Linco Research, Inc.). This kit was developed to quantify leptin in the serum of several species, and has been validated for use in cats( Reference Backus, Havel and Gingerich 23 ).
Immunohistochemical staining of 5 μ_it;m paraffin-embedded sections
IHC staining on 5 μm paraffin-embedded sections of SAT and VAT was performed as described previously by Van der Heyden et al. ( Reference Van der Heyden, Vercauteren and Daminet 24 ), with some modifications. Primary monoclonal antibodies CD3 (A045201; Dako), CD20 (RB9013-P; Klinipath) and MAC387 (ab22506; Abcam) were applied at a dilution of 1:100, for the identification of T-lymphocytes, B-lymphocytes and macrophages, respectively. A positive control (inflamed intestinal tissue) was included in each test run to ensure specificity. Sections for CD3 and CD20 staining were treated in a pressure cooker with 0·01 m-citric acid (pH 6·0) (CBB 999; Klinipath) in distilled water in a microwave oven (15 min at 850 W followed by 15 min at 300 W). Staining was performed in an automated immunostainer (Dako). An EnVision™ + system-HRP mouse and rabbit (DAB) kit (K4007; Dako) was used to visualise MAC387 (anti-mouse) and CD3 and CD20 lymphocytes (anti-rabbit). This kit includes endogenous peroxidase block and antibody diluents (S302283; Dako).
Quantitative interpretation of immunohistochemical and haematoxylin–eosin staining
Stained sections were evaluated with a light microscope (Olympus BH2; Olympus Corporation). Adipose tissue was evaluated at the highest magnification of 400 × (high-power field; hpf), which corresponds to 0·2 mm2. The number of CD3+ T-lymphocytes, CD20+ B-lymphocytes and macrophages were evaluated in the IHC-stained sections and were counted in ten hpf. Adipocytes were evaluated in the haematoxylin–eosin-stained sections and were counted in five hpf. The mean number of both adipocytes and immune cells was expressed per mm2. Adipocyte size and the diameter of adipocytes (μm) were calculated by the following equations:
RNA isolation from blood and adipose tissue samples
Total RNA was isolated from the PAXgene tube using the PAXgene blood kit (PreAnalytix, GmbH) and from 100 mg fat ( ± 10 mg) using the Qiagen RNeasy Lipid Kit (Qiagen N.V.), according to the manufacturer's instructions including the on-column DNase digestion step. Fat samples were homogenised in a TissueLyser (Qiagen), using 1 ml of lysis buffer from the extraction kit and a 5 mm steel ball bearing in a 2 ml Safe-Lock tube (Eppendorf), with shaking at 20 Hz for 2 min. RNA was eluted in 2 × 50 μl of nuclease-free water with further DNase digestion carried out using RQ1 RNase-Free DNase (Promega Corporation) according to the manufacturer's instructions with the sample incubated for 15 min at room temperature. In order to remove DNase and reaction buffer from the purified RNA (both blood and fat), it was passed through the Macherey-Nagel NucleoSpin RNA 8 Isolation Kit (ABgene Limited) using the RNA clean-up protocol and was eluted in 2 × 40 μl of elution buffer (10 mm-Tris–HCl, pH 8·4).
The quantity and quality of the resulting RNA was assessed by automated gel electrophoresis (Experion; Bio-Rad Laboratories). RNA was stored at − 70 °C before use.
Primer and hydrolysis probe design
Primers and probes were designed using Primer 3( Reference Rozen and Skaletsky 25 ) (www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi) and M-Fold using the feline-specific GenBank sequences for potential housekeeping genes: β-actin (ACTB), β-2-microglobulin (B2M), hydroxymethyl-bilane synthase (HMBS), hypoxanthine phosphoribosyl-transferase 1 (HPRT1), ribosomal protein L32 (RPL32), ribosomal protein S7 (RPS7) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWAZ), as well as adiponectin (ADIP), leptin (LEP), IL-8, monocyte chemoattractant protein-1 (MCP-1), chemoligand-5 (CCL-5) as described previously (Table 2) ( Reference Peters, Helps and Hall 26 ). IL-6-, IL-10-, interferon-γ- and TNF-α-specific assays were the same as those published previously (Table 2)( Reference Nguyen, Taglinger and Helps 27 , Reference Vandesompele, De and Pattyn 28 ).
Table 2 Primer and probe sequences for cytokine quantification by RT-PCR
B2M, β-2-microglobulin; HPRT1, hypoxanthine phosphoribosyl-transferase 1; RPS7, ribosomal protein S7; YWAZ, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide; LEP, leptin; ADIP, adiponectin; CCL-5, chemoligand-5; MCP-1, monocyte chemoattractant protein-1; IFN-γ, interferon-γ.
Real-time PCR
Synthesis of complementary DNA (cDNA) was carried out with 500 ng of random hexamers using the ImProm-II Reverse Transcription System (Promega Corporation) with 300–500 ng (fat) and 600–1000 ng (blood) of total RNA (as measured by Experion; Bio-Rad Laboratories) in a final volume of 20 μl. All reactions were prepared in duplicate according to the manufacturer's instructions, giving a final magnesium chloride concentration of 3 mm with sample incubations carried out in a PTC-200 DNA engine (Bio-Rad Laboratories). All cDNA were diluted to a final volume of 120 μl (1:6 dilution) using EB buffer (10 mm Tris–HCl, pH 8·4; Qiagen Limited) and then stored at − 20 °C for future use. No template controls were performed by the addition of nuclease-free water in place of RNA.
Quantitative PCR was performed using GoTaq Colourless Master Mix (Promega Corporation) and 0·2 μm of each primer, 0·1 μm of probe Rox™ (1:5000; Invitrogen), 4·5 mm-MgCl2 and 5 μl of diluted cDNA in a final volume of 25 μl. Sample incubations were performed in an MxPro 3005P (Agilent) at 95 °C for 2 min, followed by forty-five cycles at 95 °C for 10 s and 60 °C for 30 s during which the fluorescence data were collected. Threshold values (C t) for the samples were calculated using MxPro qPCR software (version 4.1) with the multiple experiment analyser with run-to-run variations in C t normalised using a positive control of known copy number and ROX as a passive reference dye. The absence of genomic contamination of the RNA samples was confirmed before the RT reactions, and none of the samples showed evidence of amplifiable genomic DNA with the glyceraldehyde-3-phosphate dehydrogenase quantitative PCR assay. One quantitative PCR was run for each RT repeat, resulting in two C t values for each RNA sample.
In order to determine the most appropriate housekeeper genes for the study, all eight potential genes were quantified in twenty cDNA samples from the blood and twenty-five cDNA samples from fat. A mean C
t value was calculated for each sample using the two measured C
t values for each cat for each of the potential housekeeper genes. The mean C
t value was converted to a relative copy number value using the
![]() $$E ^{\Delta C _{t}} $$
method (E being the reaction efficiency as determined from a standard curve) with ΔC
t values calculated relative to the sample with the largest C
t (fewest gene copies). The geNorm VBA (visual basics for applications) applet for Microsoft Excel was used to determine the most stable genes from the set of the tested genes(
Reference Vandesompele, De and Pattyn
28
). The three most stable housekeeper genes for the blood samples were B2M, HPRT1 and YWAZ and for the fat samples were RPS7, RPL32 and YWAZ. As RPS7 and RPL32 are both ribosomal proteins, the next most stable housekeeper (HPRT1) was used in place of RPL32 to ensure that stable housekeeper genes with a variety of functions were used for normalisation. The three selected housekeeper genes were then used to normalise the results for the other genes quantified in all the samples tested.
$$E ^{\Delta C _{t}} $$
method (E being the reaction efficiency as determined from a standard curve) with ΔC
t values calculated relative to the sample with the largest C
t (fewest gene copies). The geNorm VBA (visual basics for applications) applet for Microsoft Excel was used to determine the most stable genes from the set of the tested genes(
Reference Vandesompele, De and Pattyn
28
). The three most stable housekeeper genes for the blood samples were B2M, HPRT1 and YWAZ and for the fat samples were RPS7, RPL32 and YWAZ. As RPS7 and RPL32 are both ribosomal proteins, the next most stable housekeeper (HPRT1) was used in place of RPL32 to ensure that stable housekeeper genes with a variety of functions were used for normalisation. The three selected housekeeper genes were then used to normalise the results for the other genes quantified in all the samples tested.
Relative expression data for each of the mRNA targets were calculated using the qBase applet for Microsoft Excel (http://medgen.ugent.be/qbase/). This applet calculates a relative copy number for each sample using individual assay efficiencies, normalised against the measured housekeeper mRNA, using the methods described by Vandesompele et al. ( Reference Vandesompele, De and Pattyn 28 ). The sample with the fewest gene copies (latest C t value) is given a relative copy number of 1 and all other samples are given values relative to this sample.
Statistical analysis
BW, body condition score, body composition, serum leptin concentration and mRNA expression in the blood were analysed using a one-way ANOVA, comparing the results of both groups. Histological, IHC and adipose tissue mRNA expression results were analysed using a univariate crossed factor ANOVA, with group and location as fixed factors and cat nested in group as the random factor. P values for group, location, cat nested in group and interaction effects are given in Table 3. A Bonferroni post hoc test was performed to distinguish differences annotated to biopsy location. Significance is set at P< 0·05 and values are given as means with their standard errors.
Table 3 P values* (univariate ANOVA with group† and location‡ as fixed factors and cat§ as the random factor)
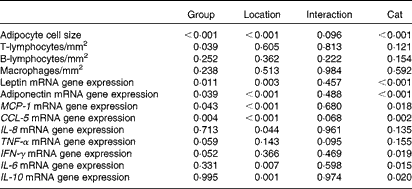
MCP-1, monocyte chemoattractant protein-1; CCL-5, chemoligand-5; IFN-γ, interferon-γ.
* P values are analysed with a univariate ANOVA, significance is set at P< 0·05.
† Group (ten obese v. ten lean cats) is a fixed factor.
‡ Location (abdominal and inguinal subcutaneous adipose tissue, and omental, bladder and renal visceral adipose tissue) is a fixed factor.
§ Cat nested in group is a random factor.
Results
As expected, BW (P< 0·001), body condition score (P< 0·001), serum leptin concentration (P= 0·019) and fat mass (P= 0·001 (kg) and P= 0·003 (%)) (Table 4) of obese cats were higher compared with lean cats.
Table 4 Body weight, body condition score (BCS), fat mass and serum leptin concentration in ten obese and ten lean cats (Mean values with their standard errors)
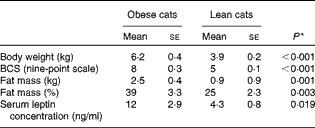
* P values were analysed with a one-way ANOVA, significance is set at P< 0·05.
Obese cats had larger adipocyte size compared with lean cats (P< 0·001) and adipocytes were the smallest in bladder VAT (P< 0·001) in both obese and lean cats (Fig. 1(A)). More T-lymphocytes were present in the adipose tissue of obese compared with lean cats (P= 0·039; Fig. 1(B)), whereas the number of B-lymphocytes (Fig. 1(C)) and macrophages (Fig. 1(D)) did not differ between the lean and obese cats. Adipose tissue location did not influence T-lymphocyte, B-lymphocyte and macrophage counts (Fig. 1(B)–(D)).
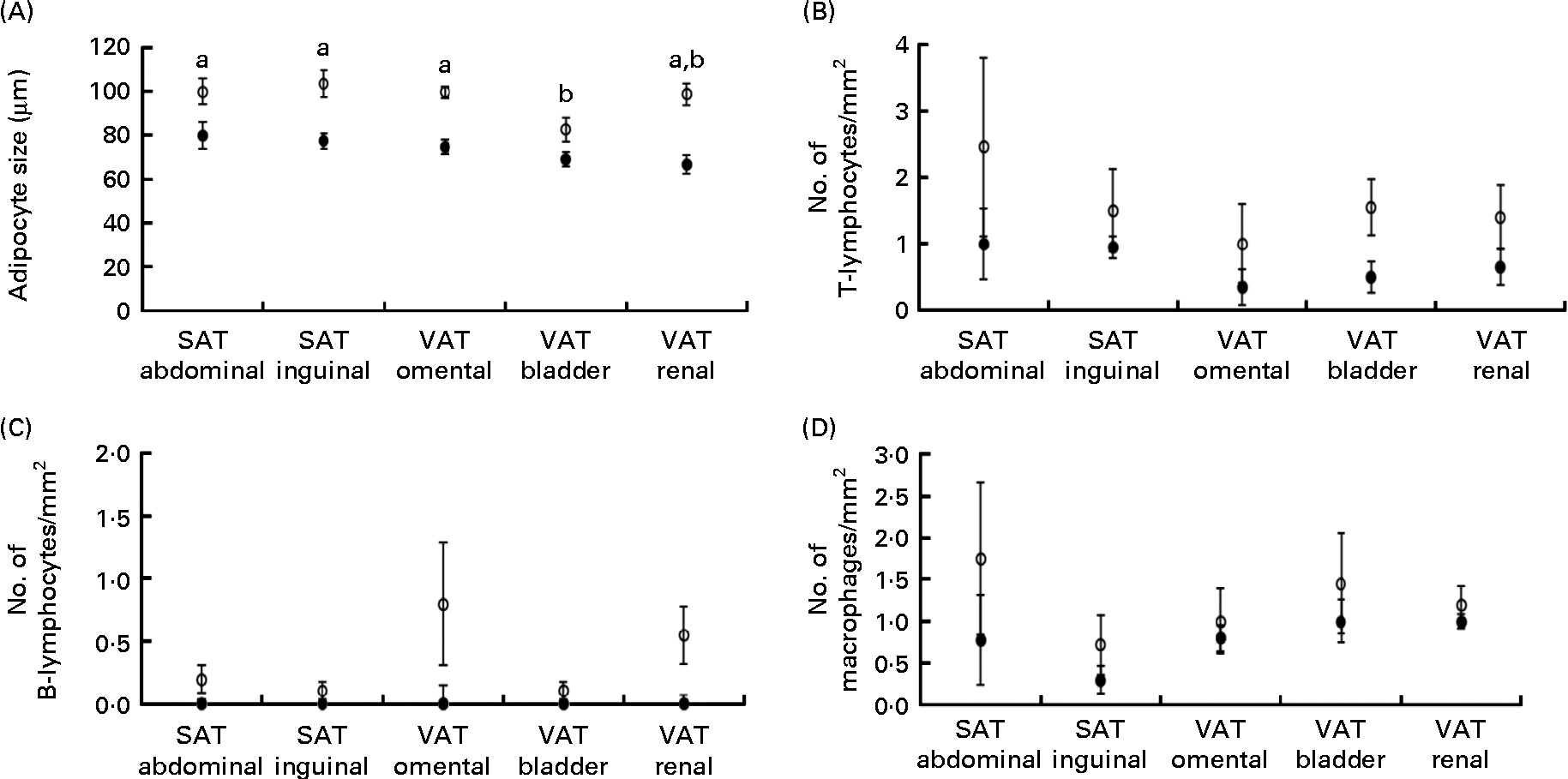
Fig. 1 (A) Adipocyte size, (B) number of T-lymphocytes/mm2, (C) number of B-lymphocytes/mm2 and (D) number of macrophages/mm2 in subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) in ten obese (○) and ten lean (●) cats. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different between the adipose tissue locations (P< 0·05).
Leptin mRNA expression was higher in the adipose tissue (P =0·011) and blood (P= 0·040) of obese cats compared with lean cats (Fig. 2(A) and (G), respectively). Compared with omental and bladder VAT, abdominal SAT showed a higher expression of leptin for both groups (P= 0·003). In contrast, the expression of total adiponectin mRNA was lower in the adipose tissue of obese cats compared with lean cats (P= 0·039). In both groups, the highest adiponectin expression was demonstrated for renal VAT (P< 0·001; Fig. 2(B)). Yet, body condition did not influence adiponectin expression in the blood (Fig. 2(G)).
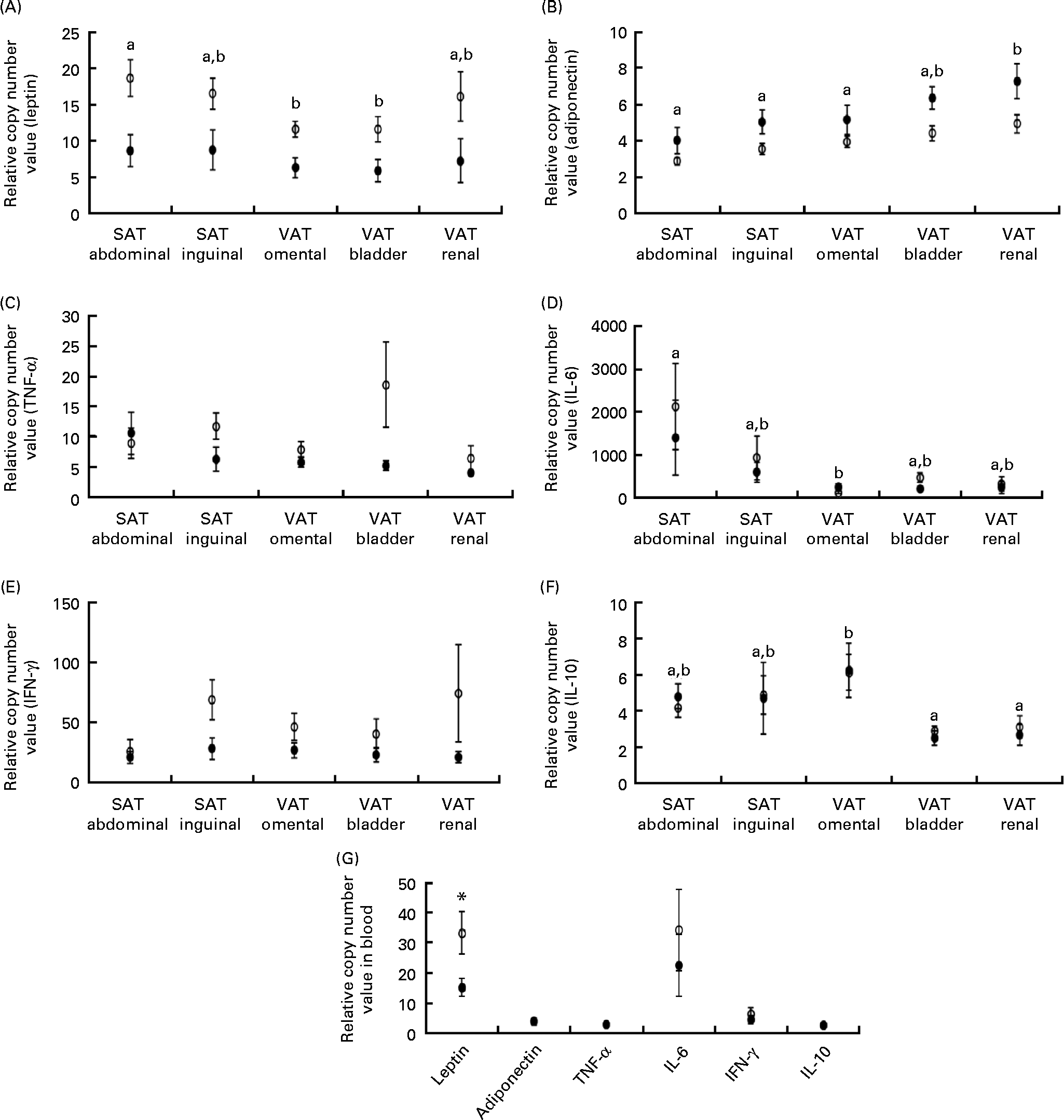
Fig. 2 Relative copy number value of (A) leptin, (B) adiponectin, (C) TNF-α, (D) IL-6, (E) interferon-γ (IFN-γ) and (F) IL-10 in subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) and (G) relative copy number value of the above-mentioned adipokines in the blood in ten obese (○) and ten lean (●) cats. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different between the adipose tissue locations (P< 0·05). * Mean values were significantly different between the two groups (P< 0·05).
TNF-α and interferon-γ tended to have a higher mRNA expression in the adipose tissue of obese cats compared with lean cats (P= 0·059 and 0·052, respectively), whereas the expression of IL-6 and IL-10 mRNA did not differ between the adipose tissue of both groups (Fig. 2(C)–(F)). Abdominal SAT of both groups had a higher expression of IL-6 mRNA compared with omental VAT (P= 0·007; Fig. 2(D)), and the expression of IL-10 mRNA of both obese and lean cats was higher in omental VAT compared with bladder and renal VAT (P= 0·001; Fig. 2(F)). In the blood, no differences between the lean and obese cats were detected for the expression of TNF-α, interferon-γ, IL-6 and IL-10 mRNA (Fig. 2(G)).
The expression of mRNA encoding the chemokines CCL-5 and MCP-1 did not differ between the lean and obese cats in the blood (Fig. 3(D)), but was elevated in the adipose tissue of obese cats compared with lean cats (P= 0·004 and P =0·043, respectively), and in both groups, the expression of CCL-5 and MCP-1 mRNA differed among the adipose tissue locations (P< 0·001) (Fig. 3(A) and (B), respectively). Abdominal SAT showed a greater expression of CCL-5 mRNA compared with bladder and renal VAT, and inguinal SAT had a higher expression compared with omental, bladder and renal VAT. The expression of MCP-1 mRNA was larger in inguinal SAT compared with omental, bladder and renal VAT. IL-8 mRNA revealed no difference in mRNA expression in the blood and adipose tissue between the groups (Fig. 3(C)).
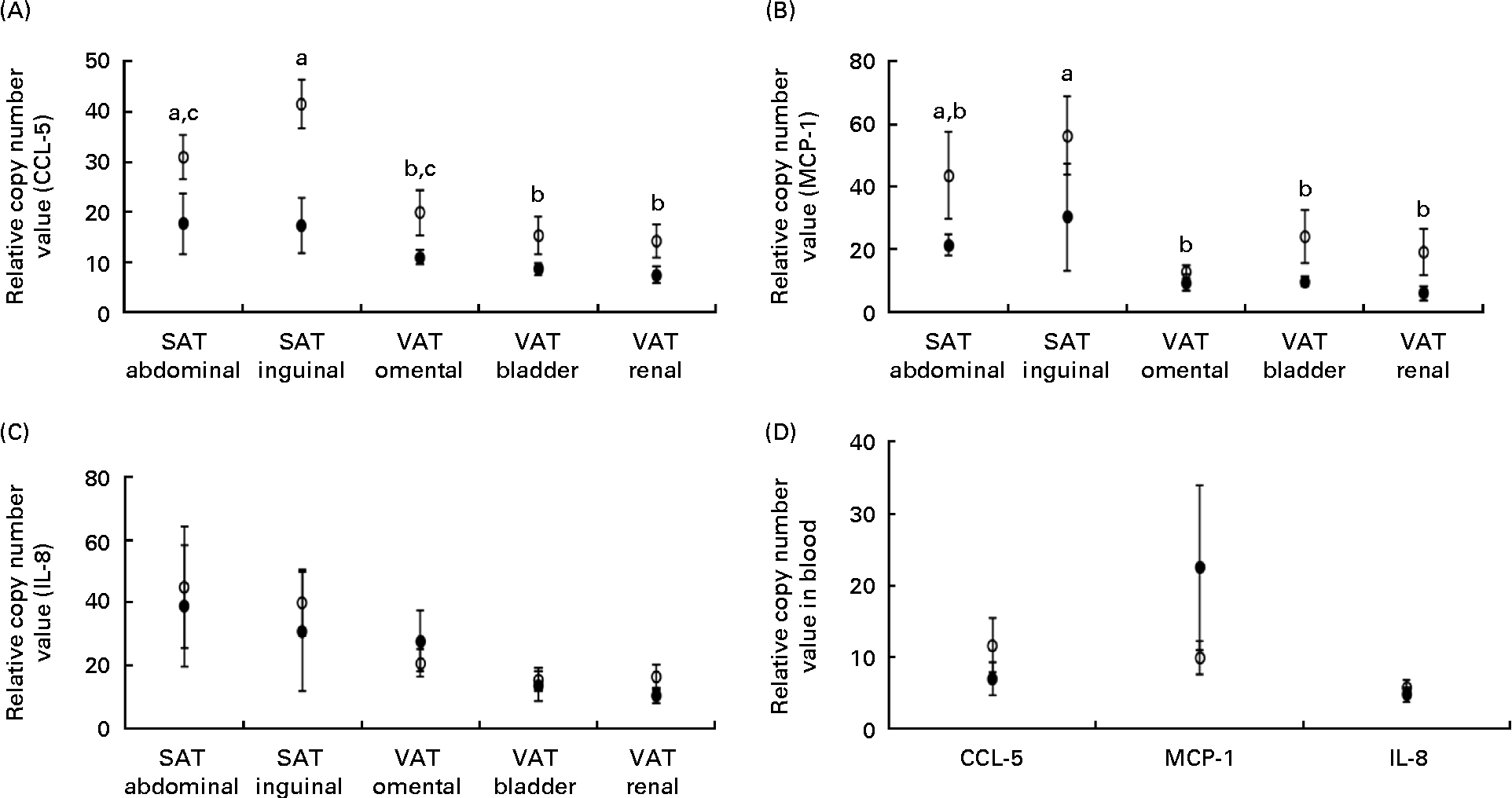
Fig. 3 Relative copy number value of (A) chemoligand-5 (CCL-5), (B) monocyte chemoattractant protein-1 (MCP-1) and (C) IL-8 in subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) and (D) relative copy number value of the above-mentioned chemokines in the blood in ten obese (○) and ten lean (●) cats. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different between the adipose tissue location (P< 0·05).
Discussion
The present study was designed to evaluate the inflammatory response of adipose tissue in obese cats compared with lean cats. It was hypothesised that chronically obese cats possess larger adipocytes and local inflammation in adipose tissue. As in humans, location-dependent alteration of gene expression and inflammatory profile was expected in obese cats, elucidating new insights for both human and feline research.
Feline obesity is characterised by increased adipocyte cell size
Technically, obesity is defined as a pathological condition in which an excessive amount of adipose tissue is deposited( Reference Klaus 29 ), which can be either due to cellular hypertrophy (enlargement of cell size) or hyperplasia (many small cells)( Reference Hoffstedt, Arner and Wahrenberg 30 ). In humans, hypertrophy of adipocytes causes alteration in the production of adipokines, leading to chronic low-grade inflammation( Reference Skurk, Alberti-Huber and Herder 31 ). In contrast, hyperplasia has been shown to exert a protective effect against obesity-related disorders, in particular insulin resistance( Reference Hoffstedt, Arner and Wahrenberg 30 ).
To our knowledge, no information exists on adipocyte cell size in cats. In the present study, adipocytes of obese and lean cats reached approximately 100 and 75 μm, respectively. These values are similar to a recent canine study of diet-induced obesity( Reference Kabir, Stefanovski and Hsu 32 ), in which adipocytes of VAT, larger than 75 μm, were highly associated with a prediction of insulin resistance. In addition, adipocytes of obese humans achieve a size of approximately 100 μm( Reference O'Connell, Lynch and Cawood 33 ). A limitation of the present study is the method of measuring adipose cell size. Adipose tissue was stained with haematoxylin–eosin and assessed with a morphological approach by counting the number of cells per hpf, which is probably a less sensitive technique than the isolation and individual measurement of adipocytes. Despite this limitation, a fairly good similarity in adipocyte size with human and canine studies was achieved. However, insulin sensitivity was not assessed in the present study.
Gene expression of adipokines is altered in feline obesity and induces inflammation and attraction of T-lymphocytes into adipose tissue
To our knowledge, mRNA expression of leptin and adiponectin has not previously been measured in the adipose tissue of cats. Gene expressions of these adipokines were increased and decreased, respectively, in obese cats compared with lean cats. The pattern of expression of these genes was as expected, since feline obesity is characterised by systemic hyperleptinaemia and hypo-adiponectinaemia( Reference Backus, Havel and Gingerich 23 , Reference Appleton, Rand and Sunvold 34 , Reference Ishioka, Omachi and Sasaki 35 , Reference Van de Velde, Janssens and Cox 36 ). However, adiponectin is manifested in several forms with different molecular weights, of which high-molecular-weight adiponectin has been suggested to be the most active form in humans( Reference Tilg and Moschen 37 ). Although low-molecular-weight and high-molecular-weight adiponectin have been measured in dogs and cats( Reference Tan, Rand and Morton 38 , Reference Verkest, Rand and Fleeman 39 ), their function has yet to be clarified in these species( Reference Verkest and Bjornvad 40 ). Validated species-specific techniques are mostly based upon the measurement of total adiponectin in dogs and cats( Reference Tvarijonaviciute, Ceron and Holden 16 , Reference Tvarijonaviciute, Martinez-Subiela and Ceron 41 ).
Gene expression of chemokines reflects the ‘homing’ capacity (chemoattraction of immune cells by chemokines) of inflamed adipose tissue. Indeed, gene expressions of MCP-1, a chemokine attracting monocytes/macrophages, and CCL-5, a chemokine attracting T-lymphocytes, were significantly increased in obese cats compared with lean cats. The higher CCL-5 expression was associated with an increased number of CD3+ T-lymphocytes in the adipose tissue of obese cats compared with lean cats. Despite the high expression of MCP-1 during feline obesity, an increased number of macrophages in the adipose tissue of obese cats compared with lean cats was not demonstrated. Although the presence of macrophages was expected, recent knowledge in rodents illustrates sequential influx of immune cells into the adipose tissue of obese subjects. Cells of adaptive immunity, such as T-lymphocytes, are proposed as initiators of an inflammatory cascade, followed by the production of MCP-1 and the subsequent attraction of macrophages( Reference Sell and Eckel 42 ). As the number of macrophages was not increased in obese cats, one might suggest that the degree of obesity in the present study was not severe enough to recruit macrophages into adipose tissue. However, the initial inflammatory response set up by T-lymphocytes and the increased expression of chemokines illustrate the ongoing homing of immune cells in these cats. As limited data are available concerning the order of immune cell attraction in obese adipose tissue from human subjects, the outcomes of the present study highlight the importance of this model to illustrate the mechanisms of ongoing obesity development.
Since only a few feline validated reagents are available, serum concentrations of cytokines were not measured and, consequently, the gene expression of cytokines in adipose tissue cannot be compared with the level of proteins. However, the gene expression of adipokines did not differ between the two groups in the blood, except for leptin, indicating the importance of determining mRNA expression of these molecules in adipose tissue. A greater mRNA expression of the pro-inflammatory cytokines TNF-α and interferon-γ was noted in the adipose tissue of obese cats compared with lean cats, illustrating a pro-inflammatory profile during feline obesity, similar to human and rodent studies. The expression of IL-6 and IL-10 mRNA was not different between the adipose tissue of obese and lean cats, but a location-dependent expression was shown for these cytokines. Despite the anti-inflammatory profile of IL-10, this cytokine can be co-elevated in human obesity, as a response to the increase of pro-inflammatory cytokines. The absence of increased numbers of macrophages in the adipose tissue of obese cats might explain the unaltered expression of IL-10, since activated cells are co-producers of this cytokine( Reference Spits and de Waal 43 ).
Altered gene expression of adipokines and increased cell size highlight the importance of subcutaneous adipose tissue in cats
Central obesity in humans is known as a state of obesity associated with a highly increased risk of metabolic and inflammatory diseases( Reference Pou, Massaro and Hoffmann 4 ). Until now, it was not known whether obese cats possess a similar profile to humans. By collecting adipose tissue at five different locations (abdominal and inguinal SAT, and omental, bladder and renal VAT), the present study provides valuable information regarding the impact of adipose tissue location on the inflammatory responses seen with feline obesity. This information is valuable since co-morbidities such as diabetes and dyslipidaemia are similar in both humans and cats, and a recent study suggested the cat as a model for human obesity( Reference Kooistra, Galac and Buijtels 8 ). The present study illustrated that adipocyte size in cats is smallest in bladder VAT, irrespective of their state of obesity. In contrast, one study in rats reported larger adipocytes in retroperitoneal adipose tissue compared with mesenterial adipose tissue and SAT( Reference Palou, Priego and Sanchez 44 ). Additionally, both in obese and non-obese human subjects, SAT was characterised by larger cells compared with omental VAT( Reference Fried, Russell and Grauso 45 , Reference Tchernof, Belanger and Morisset 46 ).
In the present study in cats, the expression of leptin, MCP-1 and IL-6 mRNA was higher in SAT compared with VAT in both groups, but differences depended on the location within SAT or VAT. CCL-5, the chemokine attracting T-lymphocytes, tended to illustrate an effect of obesity on adipose tissue location, meaning that this chemokine was increased more prominently in SAT by feline obesity. This is opposite to human obesity since in humans, VAT predominantly contributes to an inflammatory profile and consequently to obesity-related disorders. This dissimilar location-dependent response between humans and cats might be an interesting topic for further research and might have implications for the treatment of obesity-related disorders such as insulin resistance and diabetes mellitus in cats.
Conclusion
The present study is the first to illustrate that feline obesity is characterised by increased adipocyte cell size and altered adipokine gene expression. Moreover, the presence of T-lymphocytes in feline obese adipose tissue was demonstrated, which is in parallel with human obesity. However, SAT seems to be more involved in the development of an inflammatory response compared with VAT, which is in contrast with human obesity. The present study extends the knowledge on feline obesity and on the development of its related disorders, and highlights both similarities and differences compared with human obesity.
Acknowledgements
The present study is within the scope of the postgraduate study of the first author and is funded by the Institute for Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen), Belgium, grant no. 083253. The authors would like to thank Nestlé Belgilux NV, Nestlé Purina PetCare (Brussels, Belgium) for providing the cat food. Laura Statius and Saartje Van Beirs are kindly acknowledged for animal care taking and technical assistance, Herman De Rycke, Inge Vaesen and Daniel Vermeulen for performing the food and serum analyses. H. V. d. V. was responsible for the study design and accomplishment, sample analyses and data processing, and manuscript drafting. M. H. and G. P. J. J. were co-responsible for the study design, data processing and manuscript drafting. H. d. R., I. Po. and R. D. were co-responsible for the study accomplishment and manuscript drafting. I. Pe., P. N. and J. B. were co-responsible for the sample analyses and manuscript drafting. K. R., J. X. and A. V. cooperated in the study accomplishment and manuscript drafting. The authors declare no conflict of interest.









