Norovirus is the most common cause of infectious intestinal disease (IID) in the community in high-income countries [Reference Amar1]. Norovirus infection has also been identified in a substantial proportion of individuals with no IID symptoms in several community-based studies, with crude prevalences of up to 16% reported in high-income countries [Reference Amar1–Reference Karsten3]. Volunteer studies have demonstrated the occurrence of norovirus infection with no concurrent IID after experimental inoculation [Reference Atmar4]. While these volunteer individuals experienced no IID symptoms, some reported other non-specific symptoms such as headache, fever, muscle ache, abdominal pain and nausea. The objectives of this study was to describe the age and seasonal distribution of norovirus infection without IID (hereafter referred to as ‘asymptomatic norovirus infection’) in the community in England and to describe the characteristics of these infections.
We used data from participants in the Study of Infectious Intestinal Disease in England, conducted between 1993 and 1996 [Reference Sethi5]. These individuals were recruited as controls for a case-control study, either from a prospectively followed community cohort, or from the registration lists of general practitioners participating in the study [Reference Sethi5]. Informed consent was obtained at the time of recruitment [Reference Sethi5]. The inclusion criteria specified that participants should have no recent history of diarrhoea (any loose stools) or significant vomiting (⩾2 vomiting episodes per 24 h) prior to recruitment [Reference Sethi5].
At recruitment, participants submitted stool specimens for microbiological testing, in order to detect a range of 18 bacterial, viral and protozoal gastrointestinal pathogens. Norovirus was detected by electron microscopy in the original study [Reference Tompkins6]. Stool specimens were archived and subsequently retested, using real-time reverse transcription–polymerase chain reaction (RT–PCR) to detect norovirus [Reference Amar1, Reference Tompkins6]. In the current study, participants were classified as having norovirus infection if they tested positive either by electron microscopy or real-time RT–PCR, or both. The real-time RT–PCR assay has separate sets of primers and probes for genogroup I and genogroup II noroviruses, making it possible to distinguish the genogroup of norovirus present in the positive specimens. No further genotyping was performed.
Participants provided details of gastrointestinal and non-specific symptoms in the previous 3 weeks in an epidemiological questionnaire (although details of fever and nausea were not collected). Adults completed the questionnaire themselves; a parent or guardian completed the questionnaire on behalf of children aged <16 years [Reference Sethi5]. For this analysis, participants who had been free of diarrhoea and vomiting for at least 10 days prior to recruitment were considered asymptomatic with respect to IID, although they may have experienced other symptoms during that period.
Stool specimens were received from 2205 asymptomatic participants and 2065 returned the questionnaire providing information on recent symptoms. Of the 2205 asymptomatic participants, 361 had an asymptomatic norovirus infection and 1844 tested negative for norovirus; the age- and season-specific prevalence of asymptomatic norovirus infection was based on these 2205 participants. Of the 2065 asymptomatic participants who returned questionnaires, 344 had an asymptomatic norovirus infection and 1721 were norovirus negative; these 2065 participants were used for the analysis of recent symptoms.
The age-adjusted prevalence of asymptomatic norovirus infection in the community in England was calculated by standardizing against the age-stratified mid-1994 population estimate for England, obtained from the Office for National Statistics, UK. Symptoms that were in excess in asymptomatic norovirus infections compared to norovirus-negative participants are presented. The analysis of symptoms is intended to be exploratory, to generate hypotheses for future work; the original study was not designed or powered to examine differences in symptom profiles between asymptomatic norovirus infections and norovirus-negative participants. Accordingly, confidence intervals are provided for symptom prevalences, prevalence differences and prevalence ratios, but no hypothesis tests (or P values) are presented.
The age-adjusted, community prevalence of asymptomatic norovirus infection was 12% [95% confidence interval (CI) 11–14], with the highest prevalence in children aged <5 years, although more than 5% of individuals in older age groups were infected (Fig. 1). The prevalence of asymptomatic infection showed a wintertime peak of 20% during November, December and January (Fig. 2); the seasonal pattern was less distinct for children aged <5 years compared to older children and adults (data not shown).
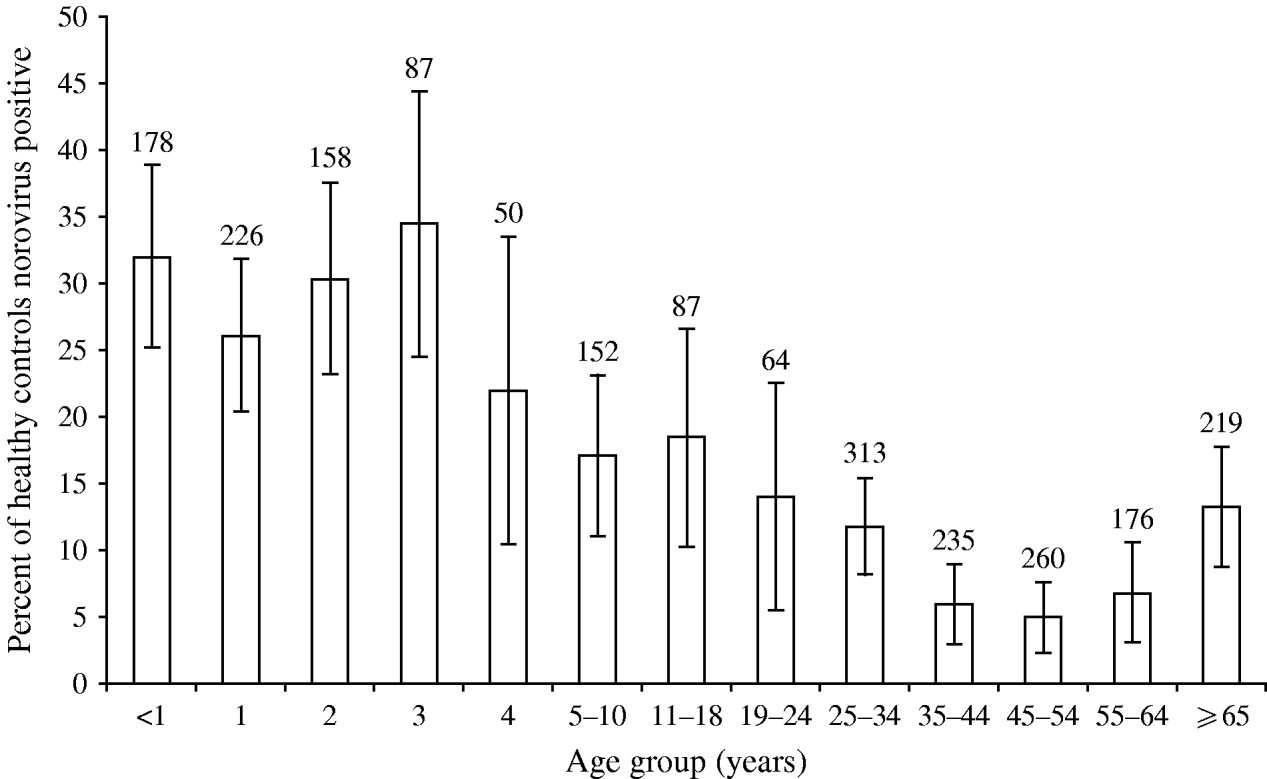
Fig. 1. Age-specific prevalence of asymptomatic norovirus infection in the Study of Infectious Intestinal Disease, England (1993–1996). Numbers above the histograms show the number of participants tested in each age group. Black bars (![]() ) show the 95% confidence intervals.
) show the 95% confidence intervals.
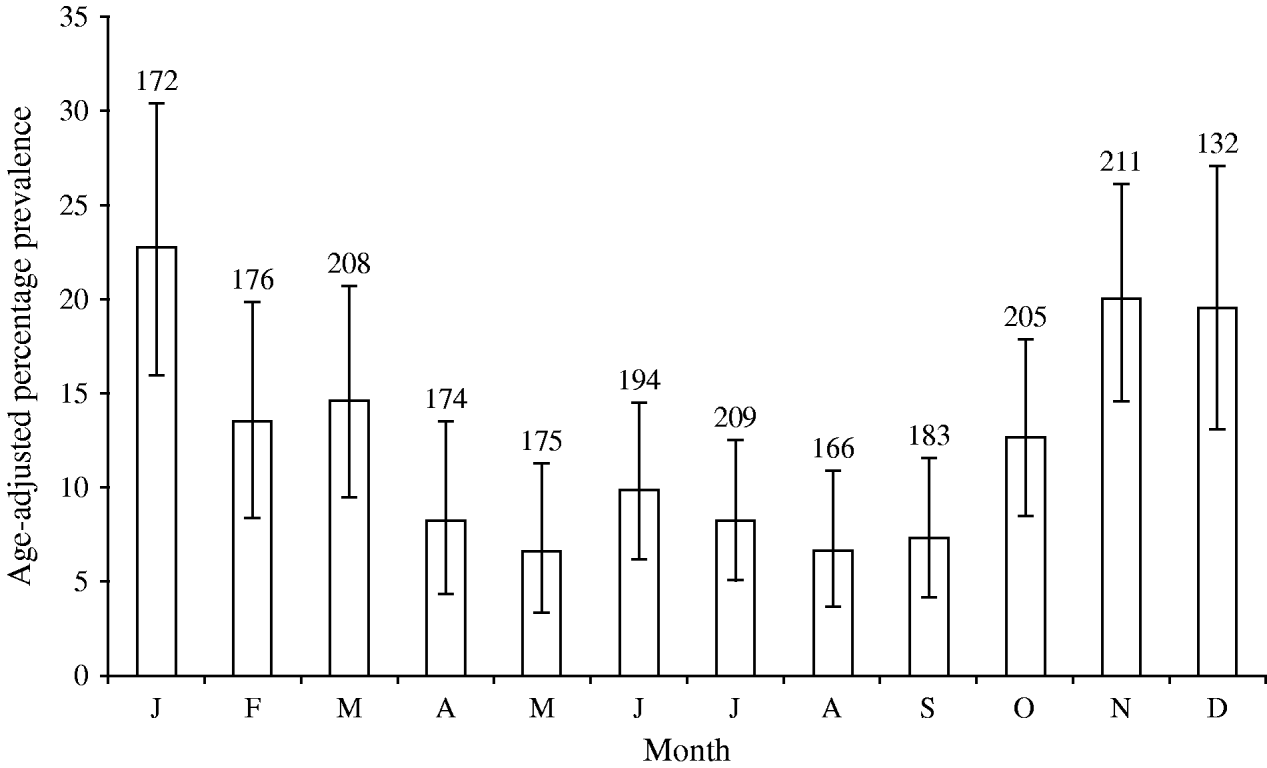
Fig. 2. Age-adjusted monthly prevalence of asymptomatic norovirus infection in the Study of Infectious Intestinal Disease, England (1993–1996). Numbers above the histograms show the number of participants tested in each month. Black bars (![]() ) show the 95% confidence intervals.
) show the 95% confidence intervals.
Genogroup II noroviruses were most common, representing 78% of the 361 asymptomatic norovirus infections, with 13% of specimens positive for genogroup I and 9% positive for both genogroups. The prevalence of genogroup II, compared to genogroup I and mixed genogroup infections, varied between 63% and 86% over the year, with the highest prevalence during October–December, and in April and May. However, the number of asymptomatic infections occurring per month was <40 throughout most of the year, so some of this variation could be due to sampling error.
During the 3 weeks preceding questionnaire completion, a cough, sore throat and other cold-like symptoms were reported by 61% of participants aged <5 years with asymptomatic norovirus infection (95% CI 54–68), compared to 52% (95% CI 47–56) of norovirus-negative participants in this age group [prevalence difference 9% (95% CI 0·7–17); prevalence ratio adjusted for month of the year 1·2 (95% CI 1·0–1·4)]. There was a smaller excess of cold-like symptoms in older children and adults with asymptomatic norovirus infection; the prevalence in individuals with asymptomatic norovirus infection was 12% (95% CI 7–17) and 9% in norovirus-negative participants (95% CI 7–10) [prevalence difference 3% (95% CI −2 to 8); prevalence ratio adjusted for month of the year 1·3 (95% CI 0·8–2·0)]. No other non-gastrointestinal symptoms were found to be in excess in participants with asymptomatic norovirus infection.
Nine percent of participants with asymptomatic norovirus infection experienced diarrhoea and/or vomiting prior to the 10-day exclusion period, but within 3 weeks of questionnaire completion (95% CI 6–12). The prevalence was higher in participants with asymptomatic norovirus infection compared to norovirus-negative participants, for both children aged <5 years [asymptomatic norovirus infection 10% (95% CI 6–15); norovirus negative 7% (95% CI 5–10); prevalence difference 3% (95% CI −2 to 8)], and older children and adults [asymptomatic norovirus infection 8% (95% CI 4–12); norovirus negative 4% (95% CI 3–5); prevalence difference 4% (95% CI −0·5 to 8)]. Older children and adults with asymptomatic norovirus infection also reported loss of appetite more often than norovirus-negative participants in this age group [asymptomatic norovirus infection 9% (95% CI 4–13); norovirus negative 3% (95% CI 2–4); prevalence difference 6% (95% CI 1–11)].
The prevalence of asymptomatic norovirus infection in our study is higher than that reported in previous studies conducted in other high-income countries, which had comparable samples of asymptomatic individuals [Reference de Wit2, Reference Karsten3]. Real-time RT–PCR is known to have slightly higher sensitivity than gel-based RT–PCR [Reference Houde7]. However, this is unlikely to account for the difference of 7% between the prevalence of asymptomatic norovirus infection in the current study and the prevalence in a previous study in The Netherlands [Reference de Wit2], which used gel-based RT–PCR. A previous study conducted in Germany used nested gel-based RT–PCR [Reference Karsten3]; the use of nested PCR primers increases the sensitivity of the gel-based assay [Reference Medici8], meaning that the assay used in the study in Germany is likely to have comparable sensitivity to the real-time RT–PCR used in the current study. It is possible that the differences in asymptomatic norovirus prevalence between the studies are due to differences in the genetic strains of norovirus circulating at the time that the studies were performed. Periodic emergence of new norovirus strains has been associated with increases in the incidence of infection and a new strain emerged in 1995–1996, during recruitment of participants into the Study of Infectious Intestinal Disease [Reference Koopmans9, Reference Noel10].
Diagnostic evaluation studies using panels of stool specimens containing other enteric viruses have demonstrated that current norovirus RT–PCR assays have 100% analytical specificity, including the assay used in the current study [Reference Ando11–Reference Kageyama13]. Therefore, very few, if any, of the asymptomatic norovirus infections reported here are likely to be false positives. Some asymptomatic participants in this study may have been shedding norovirus at levels not detectable by the RT–PCR assay used, which has a detection limit of ∼104 norovirus particles/g stool [Reference Medici8, Reference Kageyama13]; it is therefore possible that the true prevalence of asymptomatic norovirus infection is higher than reported.
Asymptomatic norovirus infection showed wintertime seasonality. Outbreaks of norovirus-associated IID in healthcare settings in England and Wales show strong wintertime seasonality, but, in contrast, there is little seasonality in norovirus outbreaks reported from community settings [Reference Lopman14]. The seasonality of norovirus-associated IID incidence at the community level in England has not been described.
Gastrointestinal and cold-like symptoms were more common in asymptomatic norovirus infections than norovirus-negative participants. The original study was not designed or powered to examine differences in symptom prevalence between these groups, and we had no a priori hypotheses about the relative frequency of symptoms. Therefore, while the 95% confidence intervals for the majority of symptom-prevalence differences did include zero, potential reasons for the observed excess prevalence in asymptomatic norovirus infections are discussed below.
Even after adjustment for season, cold-like symptoms were at higher prevalence in participants with asymptomatic norovirus infection; this may be due to a co-infection with a respiratory virus, because viruses causing the common cold and influenza are transmitted via similar routes to norovirus, e.g. through direct person-to-person contact or from contaminated environmental surfaces [Reference Dennehy15, Reference Goldmann16]. In previous studies, experimentally inoculated volunteers have reported non-specific symptoms such as headache, fever and muscle ache during norovirus infection [Reference Atmar4]; details of fever were not collected from asymptomatic participants in the Study of Infectious Intestinal Disease, so it is also possible that the excess of cold-like symptoms may represent non-specific symptoms associated with norovirus infection. The prevalence of headache and muscle ache in individuals with asymptomatic norovirus infections was slightly lower than that in norovirus-negative participants; while these symptoms have been reported in experimentally inoculated volunteers, symptoms may have been more accurately reported over the shorter clinical observation period in the inoculation studies, compared to the 3-week recall period used for self-reporting of symptoms in the current study.
Participants in the current study were recruited because they had been free of diarrhoea and/or vomiting for at least 10 days; the aetiology of any recent IID symptoms prior to this period was not established. Therefore, we do not know how many of the norovirus infections detected were truly asymptomatic rather than post-symptomatic shedding. Post-symptomatic shedding after experimental inoculation has been demonstrated, lasting up to 8 weeks [Reference Atmar4], so it is likely that some of the asymptomatic infections reported here are the result of prolonged post-symptomatic shedding. This is consistent with the small excess of diarrhoea and vomiting symptoms in participants with asymptomatic norovirus infection. It is also possible that some asymptomatic norovirus infections were due to pre-symptomatic shedding, although the short incubation period of 24–48 h for norovirus disease [Reference Gotz17] means that only a small number of the infections in the current study are likely to be pre-symptomatic shedding.
Irrespective of the source of asymptomatic norovirus infection, further work is needed to understand whether these infections contribute substantially to norovirus transmission leading to sporadic illness or outbreaks. A few published foodborne norovirus outbreak investigations have attributed illness to food contamination by asymptomatically infected food handlers [Reference Lo18]. However, the importance of asymptomatic infections for norovirus transmission outside of food catering settings has not been investigated. While norovirus is shed at much lower concentrations by asymptomatically infected individuals compared to those with disease [Reference Phillips19], the estimated infectious dose is exceptionally small [Reference Teunis20], so norovirus shedding at low concentrations could still potentially lead to transmission. Only studies identifying incident asymptomatic infections, with follow-up of contacts during infection, will reveal the importance of asymptomatic infections for continued norovirus transmission.
ACKNOWLEDGEMENTS
The authors acknowledge the help of the following people: Jim Gray, Miren Iturriza-Gomara, Corrine Amar, Fenella Halstead, Dalia Choudhury and Mihaela Cirdei for completing the laboratory work.
DECLARATION OF INTEREST
None.




