Introduction
The family Lecideaceae Chevall. (Chevallier Reference Chevallier1826; as ‘Lecideae’) was originally erected for all crustose lecideoid genera but now includes only those genera with simple hyaline ascospores and a Lecidea or Porpidia-type ascus structure. The genera having an ascus with an amyloid tube structure (Porpidia-type), halonate ascospores, and branched and anastomosing paraphyses were formerly included in the family Porpidiaceae Hertel & Hafellner (Hafellner Reference Hafellner1984). However, Buschbom & Mueller (Reference Buschbom and Mueller2004) showed that ‘Porpidiaceae’ was not monophyletic unless Lecideaceae was also included and, as Lecideaceae is the earlier name, they included Porpidiaceae in the synonymy of Lecideaceae. This synonymy was confirmed by Miadlikowska et al. (Reference Miadlikowska, Kauff, Hofstetter, Fraker, Grube, Hafellner, Reeb, Hodkinson, Kukwa and Lücking2006), who also demonstrated that the family should be removed from Lecanorales and included it in Lecanoromycetidae without being assigned to an order. Schmull et al. (Reference Schmull, Miadlikowska, Pelzer, Stocker-Wörgötter, Hofstetter, Fraker, Hodkinson, Reeb, Kukwa and Lumbsch2011) resurrected the order Lecideales Vain. within the Lecanoromycetidae for the family and this has been accepted by subsequent authors (Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molná, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017; Wijayawardene et al. Reference Wijayawardene, Hyde, Al-Ani, Tedersoo, Haelewaters, Rajeshkumar, Zhao, Aptroot, Leontyev and Saxena2020).
Lecideaceae currently includes c. 30 genera. Lücking et al. (Reference Lücking, Hodkinson and Leavitt2017) list 28 but omit Porpidinia Timdal and include Mycobilimbia Rehm, which belongs in Ramalinaceae C. Agardh., whereas Wijayawardene et al. (Reference Wijayawardene, Hyde, Al-Ani, Tedersoo, Haelewaters, Rajeshkumar, Zhao, Aptroot, Leontyev and Saxena2020) list 29 but include Eremastrella Vogel, which belongs in Psoraceae Zahlbr. In addition, the recently described Cyclohymenia McCune & M. J. Curtis also belongs in the family (McCune et al. Reference McCune, Curtis and Di2017). Most of these genera, however, contain only a small number of species, with 10 genera being monotypic, and only Lecidea Ach. and Porpidia Körb. containing more than 15 species. Several of the monotypic genera were established by Hertel (Reference Hertel1984) for species known only from the Southern Hemisphere (e.g. Rhizolecia Hertel, Stephanocyclos Hertel, Notolecidea Hertel) and the distinctness of some of these has been questioned (e.g. Fryday & Hertel Reference Fryday and Hertel2014). Conversely, there is little doubt that Lecidea and Porpidia, and possibly other genera, are not monophyletic and some infrageneric groups within them should be recognized as distinct genera (e.g. the L. auriculata/L. tessellata group; Ruprecht et al. Reference Ruprecht, Fernández-Mendoza, Türk and Fryday2020). In addition, several genera were shown to lie outside the Lecideaceae by Schmull et al. (Reference Schmull, Miadlikowska, Pelzer, Stocker-Wörgötter, Hofstetter, Fraker, Hodkinson, Reeb, Kukwa and Lumbsch2011) or Miadlikowska et al. (Reference Miadlikowska, Kauff, Högnabba, Oliver, Molná, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014) (e.g. Bryobilimbia Fryday et al., Clauzadea Hafellner & Bellem., Lecidoma Gotth. Schneid. & Hertel, Romjularia Timdal, etc.) but are retained in the family pending further work; others display characters that do not confirm to the circumscription of the family (e.g. Catarrhospora Brusse, with submuriform ascospores, and Poeltidea Hertel, with pigmented ascospores) and it is possible that these genera also do not belong in Lecideaceae.
Here we describe another monotypic genus (Imsharria) from the southern subpolar region with a suite of characters that does not coincide with those of any known genus, and the distinctness of which was supported by molecular data. We also describe a new species of Porpida, make a new combination in Poeltiaria and include newly generated sequences of the genera Amygdalaria Norman, Farnoldia Hertel, Poeltiaria Hertel, Schizodiscus Brusse and Xenolecia Hertel.
Materials and Methods
This study is based upon material collected by Henry Imshaug and Richard Harris on the Falkland Islands in the austral summer of 1968–1969 and supplemented by collections made by the first author in 2015, along with additional specimens from various herbaria (Table 1).
Table 1. Voucher information of taxa in Lecideaceae for the newly generated sequences of the markers nrITS and mtSSU.

Morphological analyses
Gross morphology was examined with a Leica MZ125 dissecting microscope and apothecial characteristics using a Leica DMLB compound microscope with a polarizing light filter on hand-cut sections mounted in water, 10% KOH (K), 50% HNO3 (N), 50% HNO3 with the subsequent addition of Indian ink (N/ink), or Lugol's reagent (1.5% aqueous IKI). The presence/absence of birefringent crystals is noted as POL+/POL−, respectively. Thallus sections were investigated in water, K and Lugol's reagent. Ascospore measurements of the new species are given as (minimum–)mean ± standard deviation(–maximum), where n is the number of measurements. Photomicrographs were taken with a Sony Cyber-shot DSL HX100V camera attached to the phototube of either the dissecting or compound microscope. Thalline chemistry was investigated by standard spot tests and by thin-layer chromatography following the methods of Orange et al. (Reference Orange, James and White2001). Nomenclature of apothecial pigments follows Meyer & Printzen (Reference Meyer and Printzen2000).
DNA-amplification, sequencing and phylogenetic analyses
Total DNA was extracted from individual thalli using the DNeasy Plant Mini Kit (Qiagen) following the manufacturer's instructions. The lichen material (c. 2–3 mm2) was scraped off with a sterilized scalpel from the centre of the thallus and included apothecia.
The internal transcribed spacer regions of mycobiont nuclear ribosomal DNA (nrITS) and the mitochondrial small subunit (mtSSU) were amplified and sequenced using the following primers: ITS1F (Gardes & Bruns Reference Gardes and Bruns1993), ITS1 and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) for nrITS, and CU6 (https://nature.berkeley.edu/brunslab/tour/primers.html), mrSSU1 (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999), mtSSU for2 and mtSSU rev2 (Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010) for mtSSU. PCR conditions followed Ruprecht et al. (Reference Ruprecht, Fernández-Mendoza, Türk and Fryday2020). The PCR mix contained 0.5 units of GoTaq DNA polymerase, 0.2 nM of each of the four dNTPs, 0.3 μM of each primer and c. 1 ng genomic DNA. The unpurified PCR products were sent to Eurofins Genomics/Germany for sequencing (single direction).
In order to be able to phylogenetically distinguish the new genus Imsharria, sequences of the closest related genera based on the phylogeny of Ruprecht et al. (Reference Ruprecht, Fernández-Mendoza, Türk and Fryday2020), Amygdalaria, Cyclohymenia, Farnoldia, Poeltiaria, Poeltidea, Porpidia and Xenolecia, were downloaded from GenBank or obtained from other researchers (see Table 1, Supplementary Material Table S1 (available online) and Acknowledgements). The genus Lecidea was reduced to species necessary to distinguish the main infrageneric groups. Two members of the Lecanorales, Carbonea vorticosa (Flörke) Hertel and Rhizoplaca macleanii (C. W. Dodge) Castello, were chosen as outgroup.
The sequences of both regions were edited using Geneious Pro v. 6.1.8 (www.geneious.com), aligned both before and after concatenation with MAFFT v. 7.017 (Katoh et al. Reference Katoh, Misawa, Kuma and Miyata2002) using preset settings (algorithm, auto select, scoring matrix, 200PAM/k = 2; gap open penalty, 1.34–0.123) on the alignment used in Ruprecht et al. (Reference Ruprecht, Fernández-Mendoza, Türk and Fryday2020). The single nrITS and mtSSU trees were visually checked for incongruency using a bootstrap value of > 85%.
The final data matrix of the phylogeny comprised 54 concatenated sequences of the markers nrITS (54) and mtSSU (37) with a length of 1269 characters. The phylogenetic tree inferences were carried out in two partitions (nrITS: 1–570, mtSSU: 571–1269) using a maximum likelihood (ML) approach on the IQ-TREE web server (Trifinopoulos et al. Reference Trifinopoulos, Nguyen, von Haeseler and Minh2016) with default settings (ultrafast bootstrap analyses, 1000 BT alignments, 1000 max. iterations, min. correlation coefficient: 0.99, SH-aLRT branch test with 1000 replicates). The best-fit models for each partition were selected with the implemented model finder (Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) of the program IQ-TREE according to BIC. The best models were TIM2e + I + G4 for nrITS and TPM2 + F + I + G4 for mtSSU. Phylogenetic relationships were also inferred using a Bayesian approach as implemented in the software MrBayes v. 3.2. (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003). The analysis was also performed in two partitions assuming the general time reversible model of nucleotide substitution, including estimation of invariant sites and a discrete gamma distribution with six rate categories (GTR + I + Γ; Rodriguez et al. Reference Rodriguez, Oliver, Marin and Medina1990). Two runs with 2 million generations (standard deviation of split frequencies: 0.0082), each starting with a random tree and employing four simultaneous chains, were executed. Every 1000th tree was saved into a file. Subsequently, the first 25% of trees was deleted as the ‘burn-in’ of the chain. A consensus topology with posterior probabilities for each clade was calculated from the remaining 1501 trees.
Both phylogenetic approaches retrieved similar topologies, therefore only the Bayesian tree was visualized with the program FigTree v. 1.4.3 (Rambaut Reference Rambaut2014).
Results
Phylogenetic analyses
The backbone of the phylogeny is not supported, but several main groups/branches can be recognized (Fig. 1). Most of the species of the genus Lecidea form one well-supported group, whereas another well-supported group is formed by the genus Xenolecia, represented by the type species Xenolecia spadicomma (Nyl.) Hertel, together with newly generated sequences of the genus Poeltiaria (P. coromandelica (Zahlbr.) Rambold & Hertel, P. navarina (U. Rupr. & Türk) U. Rupr. & Fryday and Poeltiaria sp., an undescribed species from Tasmania). Poeltiaria tasmanica Fryday, which was described in Poeltiaria because of its hyaline hypothecium but which differs from the other three species of the genus included here in having innate, ±gyrose apothecia, is separated in an unresolved position. Unfortunately, fresh material of the type species of Poeltiaria (P. turgescens (Körb.) Hertel) was not available for sequencing. Another well-supported and heterogeneous group is dominated by species of the genus Porpidia. This includes the Porpidia speirea group, which is presumed to represent Porpidia s. str. (see ‘Discussion’ below) but also includes the species Lecidea auriculata Th. Fr, L. tessellata Flörke and Cyclohymenia epilithica McCune & M. J. Curtis, as well as Immersaria fuliginosa Fryday. The specimen from which the I. fuliginosa sequence was obtained was previously erroneously identified as Lecidea kalbii Hertel by Ruprecht et al. (Reference Ruprecht, Fernández-Mendoza, Türk and Fryday2020). Also highly supported is another heterogeneous group formed by the remaining species currently included in Porpidia, with two species of the genus Amygdalaria nested within them. The new genus Imsharria forms a distinct lineage basal to the aforementioned groups, with the genera Farnoldia and Poeltidea Hertel & Hafellner at the base of the phylogeny.
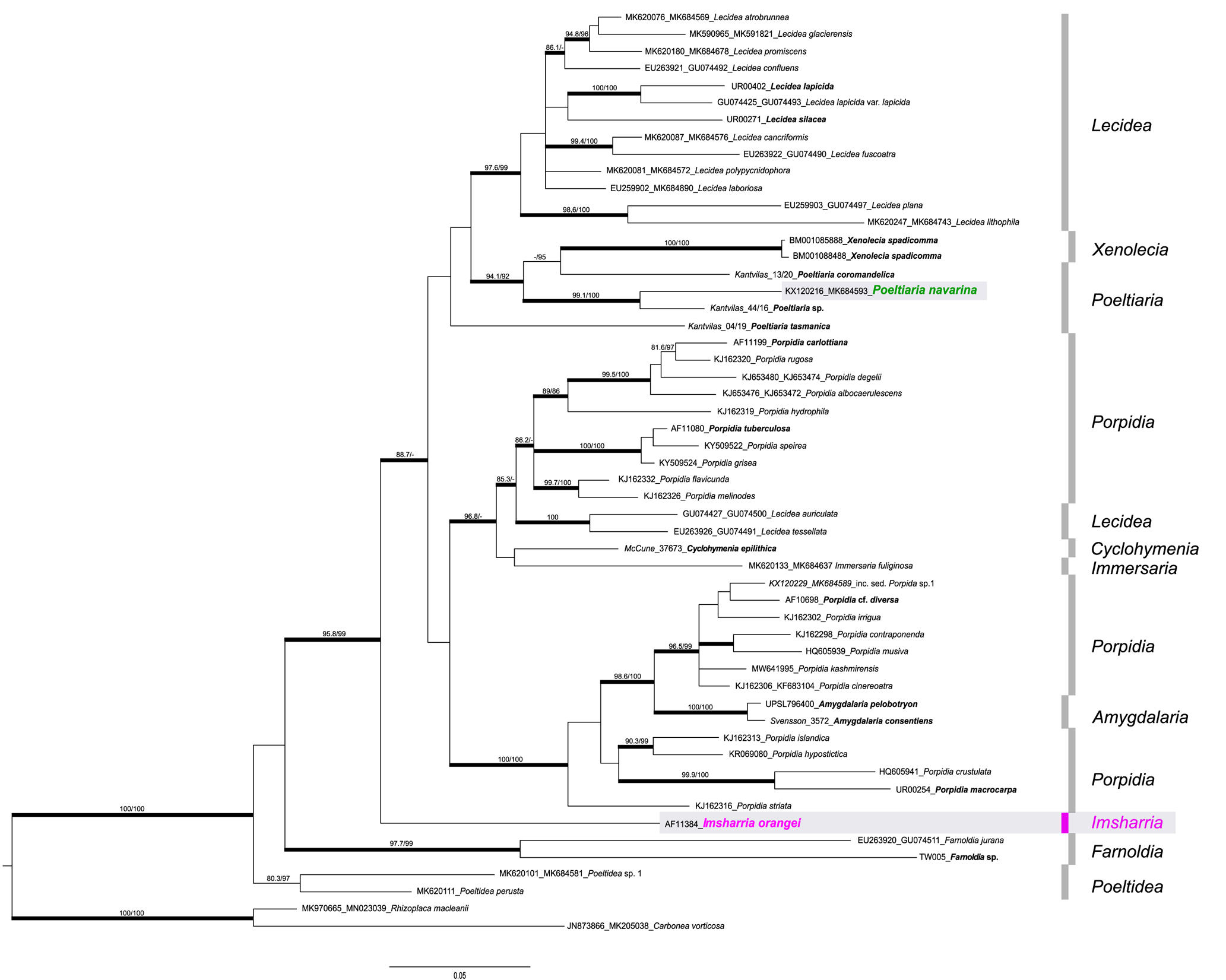
Figure 1. Phylogeny of concatenated nrITS and mtSSU sequences including the genera Amygdalaria, Cyclohymenia, Farnoldia, Immersaria, Lecidea, Poeltiaria, Poeltidea, Porpidia and Xenolecia (Lecideaceae), with the newly described genus/species Imsharria orangei (shaded box, marked in pink). Poeltiaria navarina (formerly Porpidia navarina) is marked in green (shaded box). The labels of the newly added sequences are in bold. The bootstrap values (ML analyses: SH-aLRT ≥ 80%/ UFboot ≥ 95%) were directly mapped onto the Bayesian tree; branches with posterior probability values ≥ 0.95 are depicted in bold. In colour online.
Taxonomy
Imsharria Fryday & U. Rupr. gen. nov.
MycoBank No.: MB 852049
Distinguished from other genera of Lecideaceae by its Porpidia-type asci, hyaline hypothecium, halonate, thick-walled ascospores and its distinct, isolated phylogenetic position (nrITS and mtSSU).
Type species: Imsharria orangei Fryday & U. Rupr.
As this is a monotypic genus, the description below constitutes the generic description.
Etymology
The name commemorates Henry Imshaug and Richard Harris who, in the austral summer of 1968–1969, made the largest ever collection of lichens from the Falkland Islands, including several specimens of the new genus described here. Although all the collections were given Imshaug collection numbers, Imshaug's collection books, which are preserved at MSC, indicate that both Imshaug and Harris each collected several specimens of the new genus.
Imsharria orangei Fryday & U. Rupr. sp. nov.
MycoBank No.: MB 852050
Similar to Lecidea lygomma Nyl. in having innate apothecia, a thallus containing norstictic acid, and simple ascospores, but differing in the Porpidia-type asci, branched and anastomosing paraphyses, hyaline hypothecium, thick-walled halonate ascospores, and an amyloid (I+ violet) medulla.
Type: Falkland Islands, West Falkland, Hill Cove, Mt Adam, 51.5752°S, 60.0750°W, 620 m, stone run above tarn in SW cirque, 7 November 2015, Fryday (11384) & Orange (MSC—holotype; E—isotype).

Figure 2. Habitat and thallus characters of Imsharria orangei (A–C, E & F, holotype; D, Imshaug 39924). A, locality of the holotype collection; Imsharria orangei was collected from rocks to the right of the lake. B, thallus and apothecia. C, round apothecia. D, elongate apothecia (Imshaug 39924). E, thallus margin showing paler zone and black prothallus. F, thallus section showing interrupted algal layer. Scales: B, D & E = 1 mm; C = 0.5 mm; F = 100 μm. In colour online.
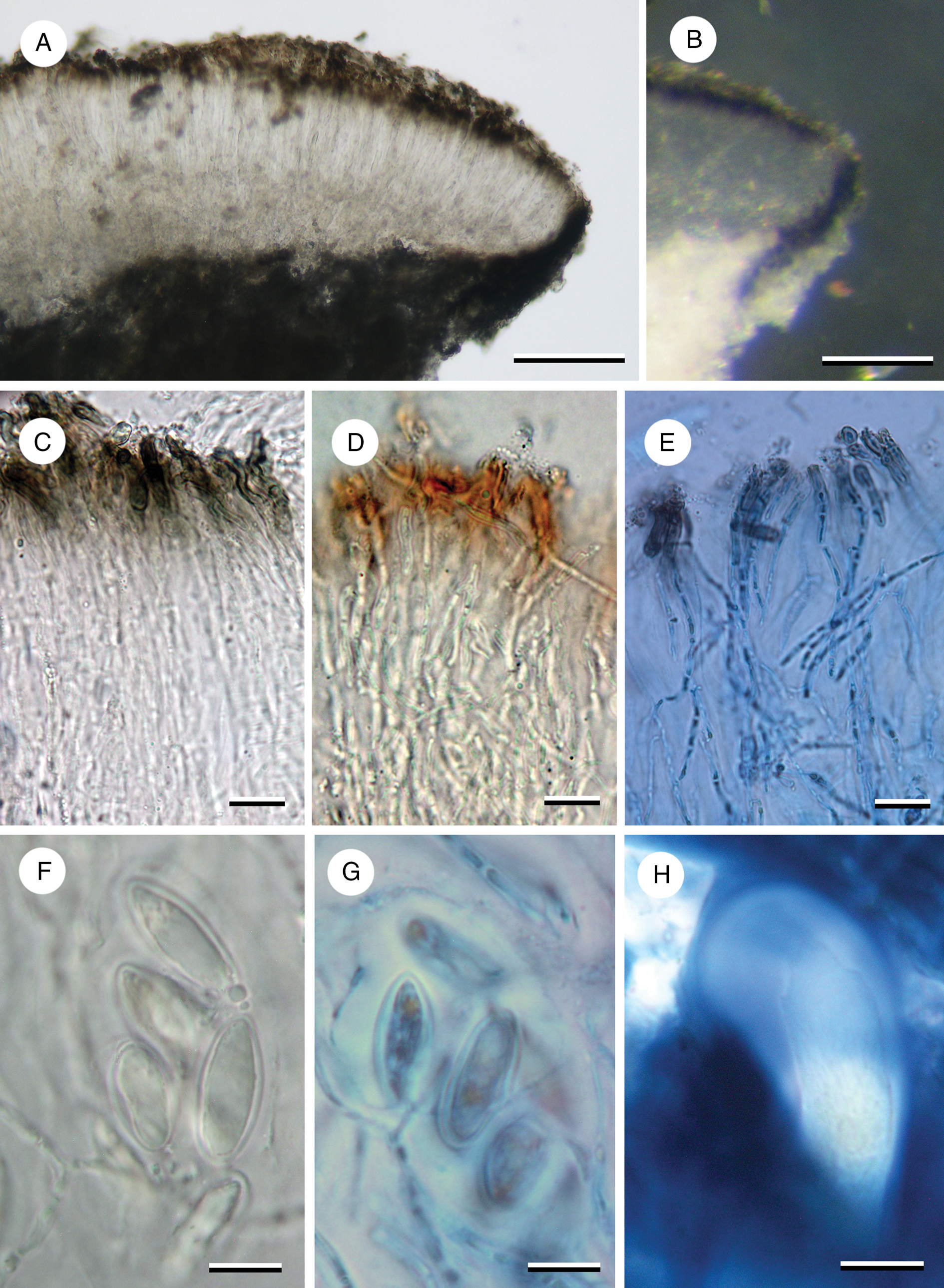
Figure 3. Apothecial characters of Imsharria orangei (A & B, D–H, Imshaug 40008; C, holotype). A, apothecium section. B, apothecium section under incident light showing annular exciple. C–E, paraphyses (C in water; D in N; E in N/ink). F & G, ascospores (F in water; G in N/ink showing perispore). H, ascus in IKI. Scales: A & B = 100 μm; C–H = 10 μm. In colour online.
Thallus effuse, thin (0.1–0.2 mm thick), white to blue-grey, the peripheral 0.5–1.0 mm usually paler than the rest of the thallus, areolate on a black prothallus, marginal prothallus distinct, black, 0.1–0.2 mm wide; areoles 0.1–0.3 mm across, flat to slightly concave; upper cortex c. 50–60 μm thick, hyaline except for the upper 12–20 μm which is pigmented blue-black (N+ red, Cinereorufa-green), composed of vertically aligned septate hyphae 2.0–2.5 μm wide, swelling at the surface to 4–5 μm wide with the upper c. 5 μm of each hypha pigmented blue-black (N+ red, Cinereorufa-green); all parts POL+; medulla composed of loosely interwoven hyaline hyphae, 25–100 µm deep, extending up into the photobiont layer, I+ violet. Photobiont chlorococcoid, cells 9–15 μm diam. with thick hyaline walls, not forming a continuous layer, arranged in clumps 25–50 μm across.
Apothecia lecideine, deeply immersed (below the thallus surface) with a concave brown disc usually surrounded by a crack 0.5–0.8 mm wide separating it from the thallus, occasionally the area adjacent to the apothecium also including some thallus, initially ±orbicular (0.4–0.6 mm diam.) becoming irregular to elongate in outline and sometimes even slit-like (0.6–0.7 × 0.1–0.3 mm); proper margin thin and raised, 0.03–0.05 mm wide, black or grey, becoming slightly inrolled over the disc with a white inner edge. In section, proper exciple very thin, 20–30 μm wide, composed of swollen, vertically aligned hyphae 4–5 μm wide, inner part (adjacent to the hymenium) hyaline, outer part and surface cells dark blue-back (N+ red, Cinereorufa-green), the amount of pigment directly correlated with the degree of exposure, upper cells overlain by a thin epinecral layer of dead hyaline cells 5–10 μm thick, annular, extending vertically down into the medulla. Hymenium 100–125 μm tall, merging into the hypothecium; paraphyses c. 2.0–2.5 μm wide, sparingly branched and anastomosing, septate, sometimes constricted at the septum, slightly swollen at the apex to 3.0 μm wide with a brown cap, occasionally moniliform; epihymenium brown (K−, N+ red-brown), 15–20 μm tall; subhymenium absent. Hypothecium hyaline, 70–150 μm tall, composed of randomly aligned hyphae, not easily distinguishable from the hymenium; POL−. Ascus Porpidia-type, cylindrical, c. 70–90 × 15–20 μm becoming clavate (25 μm wide) when mature; ascospores simple, ellipsoid, thick walled (c. 1 μm), hyaline, with an inconspicuous perispore in water or K but swelling to up to 5 μm in N, (11–)14.25 ± 2.379(–20) × (6–)8.50 ± 1.314(–10) μm, l/w ratio 1.70 ± 0.273 (n = 12).
Conidiomata not observed.
Chemistry
K+ red (needle-shaped crystals in section), C−, Pd+ yellow; norstictic acid by TLC.
Etymology
The specific epithet commemorates the British lichenologist Alan Orange, who visited the Falkland Islands on three occasions, describing several new species and making many other important contributions to our knowledge of the lichen biota of the islands. Alan loved the wild, untamed solitude of the islands and his premature death in early 2023 was a tragic loss.
Distribution and ecology
The new genus is so far known only from the Falkland Islands, where it occurs on siliceous rock stone runs and feldmark, usually at or near mountain summits (Fig. 4).

Figure 4. Distribution of Imsharria orangei (black filled circles). In colour online.
Remarks
The new species is characterized by its thallus containing norstictic acid and with an amyloid (I+ violet) medulla, innate, brown apothecia, Porpidia-type asci and a hyaline hypothecium. Macroscopically, it resembles Lecidea lygomma but that species usually has a paler thallus and black apothecia. In the field it is readily separated from other crustose species by its sunken apothecia with a brown disc and the grey thallus having a conspicuous paler zone at the margin (Fig. 2D).
Additional specimens examined (all MSC)
Falkland Islands: East Falkland: Darwin, Mt Usborne, 1968, Imshaug (39924, 39936, 39938, 39939, 40008, 40097) & Harris; Stanley, Mt Kent, 1968, Imshaug (40438) & Harris. West Falkland: Port Howard, Mt Maria, 1969, Imshaug (41371, 41415, 41423) & Harris; Hill Cove, Mt Adam, 2015, Fryday (11383) & Orange (topotype).
Porpidia imshaugii Fryday sp. nov.
MycoBank No.: MB 852051
Similar to P. skottsbergiana but with larger (c. 20 ×10 μm) ascospores.
Type: Falkland Islands, West Falkland, Port Howard, outcrops on pass SW of Mt Maria summit, 1968, Imshaug (41289) & Harris (MSC0015300—holotype).
(Fig. 5)

Figure 5. Porpidia imshaugii (holotype). A, thallus with apothecia. B, ascospores. C, exciple and hypothecium. Scales: A = 1 mm (insert = 0.5 mm); B = 20 μm; C = 50 μm. In colour online.
Thallus effuse, thick, 0.1–0.2 mm, white, often oxidated orange, areolate; areoles contiguous, irregular, 0.3–0.5 mm across, flat to slightly convex; medulla I−. Photobiont chlorococcoid, cells 9–12 μm diam.
Apothecia black lecideine, sessile, not or only very slightly constricted below in mature apothecia, 0.7–0.9 mm diam.; proper margin smooth, persistent and slightly raised, 0.1 mm wide; disc grey pruinose in young apothecia with inner edge of margin remaining slightly pruinose in mature apothecia. Proper exciple cupular, c. 100 μm wide, but poorly developed below the hypothecium, composed of radiating cellular hyphae c. 4–5 μm wide; cortex c. 10–15 μm thick, orange-brown pigmented (N+ red-brown); cortical cells 5 μm diam.; medulla pale brown to almost hyaline, becoming darker brown towards the hypothecium. Hymenium 110–120 μm; paraphyses slender, c. 1 μm wide, sparingly branched and anastomosing, distinctly swollen at the apex, 5–7 μm wide, conglutinate at epihymenium; epihymenium diffuse, dilute brown (N+ orange-brown) with minute granules, 10–15 μm tall; subhymenium hyaline, 25–35 μm tall. Hypothecium dark orange-brown, 75–100 μm tall, composed of randomly orientated hyphae, merging into the cupular exciple below. Ascus Porpidia-type, cylindrical, c. 80–90 × 10–25 μm, becoming clavate and 25–35 μm wide when mature; ascospores simple, hyaline, distinctly halonate, perispore swelling in K to 5 μm thick (16–)19.33 ± 2.146(–23) × (9–)9.67 ± 0.778(–11) μm, l/w ratio 1.71 ± 1.12.
Conidiomata not observed.
Chemistry
K−, C−, KC−, Pd−, UV+ dull white; no substances detected by TLC.
Etymology
Named in honour of Dr Henry Imshaug, who collected lichens extensively on the Falkland Islands.
Distribution and ecology
Known only from the Falkland Islands, where it is reported only from near the summit of Mt Maria on West Falkland. No other lichens are present on the single collection of the new species but collected from the same locality were Cladia aggregata (Sw.) Nyl., Lithographa graphidioides (Cromb.) Imshaug ex Coppins & Fryday, Pertusaria salacinifera Messuti & A. W. Archer, Thamnolia vermicularis (Sw.) Schaer. and Topeliopsis macrocarpa (C. W. Dodge) Mangold.
Remarks
Closely related to P. skottsbergiana Hertel, which has smaller ascospores (13–)15.0 ± 1.13(–17) × (6–)7.16 ± 1.03(–9) μm. The new species and P. skottsbergiana are anomalous within Porpidia for their ascospores with a thick perispore and the orange-brown hypothecium. They possibly represent a distinct genus but unfortunately molecular data are not available because of the age of the specimens.
A lichenicolous fungus with abundant paraphyses and (1–)3-septate, hyaline ascospores c. (12–)15 × 4–5 μm is present on the thallus of the holotype. It probably represents an undescribed species of Sagediopsis close to S. dissimilis Triebel, which was described growing on Paraporpidia leptocarpa (Nyl.) Rambold & Hertel in Australasia (Triebel Reference Triebel1993) and has 0–1-septate ascospores, (7.5–)8–10.5(–12) × (4–)4.5–6(–6.5) μm.
Comparative collections of P. skottsbergiana examined
South Georgia: Cumberland Bay, 500 m a.s.l, 1902, C. Skottsberg 92 (S—holotype).—Falkland Islands: East Falkland: Mt Usborne, on windward side of Mt Usborne 1 summit, [−51.694, −58.83467], 2300 ft, 1968, Imshaug 39957 (MSC0111547).
Poeltiaria navarina (U. Rupr. & Türk) U. Rupr. & Fryday comb. nov.
MycoBank No.: MB 852051
Basionym: Porpidia navarina U. Rupr. & Türk, in Ruprecht et al., Herzogia 29(2/1), 606 (2016); type: Chile, Tierra del Fuego, Isla Navarino, Cerro Bandera, 54.973165°S, 67.642288°W, 671 m a.s.l., 1 February 2015, U. Ruprecht UR00020 (SZU—holotype).
Remarks
As mentioned above, Porpidia navarina was included in the Poeltiaria clade of our phylogeny and so it is transferred here to Poeltiaria. In fact, P. navarina is morphologically very similar to Poeltiaria corralensis (Räsänen) Hertel, differing primarily in the secondary metabolites produced: stictic acid chemosyndrome in P. navarina (Ruprecht et al. Reference Ruprecht, Søchting and Türk2016), no substances or porphyrilic acid in P. corralensis (Rambold Reference Rambold1989).
Discussion
The newly described genus is clearly distinguished from other genera of the heterogeneous family Lecideaceae by both morphological and phylogenetic characters (Figs 1–3). Specimens of this genus were initially provisionally assigned to the monotypic South African genus Schizodiscus (Brusse Reference Brusse1988): the two genera are similar in having Porpidia-type asci, an unpigmented hypothecium and, in some specimens of Schizodiscus, ascospores with a very thin or non-existent perispore. Brusse (Reference Brusse1988) originally described Schizodiscus as having non-halonate ascospores but later (Brusse Reference Brusse1991) amended his description of the genus to include specimens with halonate ascospores. The only collections of this genus available for molecular study were isotypes of the type species, S. afroalpinus Brusse, which were collected in 1986 (Brusse Reference Brusse1988). Fortunately, our colleague Björn Owe-Larsson was able to obtain an ITS sequence from the isotype held in the herbarium of the Uppsala Museum of Evolution (UPS). The two sequences (ITS) of the genera Imsharria and Schizodiscus have a sequence similarity of 75% and are therefore not closely related. However, important morphological characters such as the Porpidia-type ascus and a hyaline hypothecium are shared not only by Imsharria and Schizodiscus but also by species of the genus Poeltiaria, although species of this genus can be distinguished morphologically by their sessile apothecia and ascospores with a well-developed, conspicuous perispore. Several recent collections of this genus from Tasmania were made available to us by Gintaras Kantvilas and the inclusion of sequences from these specimens in our phylogeny showed that our new species was unrelated to Poeltiaria (Fig. 1).
As mentioned above, previous phylogenies (e.g. Buschbom & Mueller Reference Buschbom and Mueller2004; Miadlikowska et al. Reference Miadlikowska, Kauff, Hofstetter, Fraker, Grube, Hafellner, Reeb, Hodkinson, Kukwa and Lücking2006) have often shown species of Lecidea nested within Porpidia, or the two genera intermixed (e.g. Schmull et al. Reference Schmull, Miadlikowska, Pelzer, Stocker-Wörgötter, Hofstetter, Fraker, Hodkinson, Reeb, Kukwa and Lumbsch2011; Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molná, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014), resulting in Porpidiaceae being reduced to synonymy with the Lecideaceae. However, our phylogeny indicates that the vast majority of Lecidea species (including the type species, Lecidea fuscoatra (L.) Ach.) form a strongly supported clade distinct from species of Porpidia and other genera with a Porpidia-type ascus (e.g. Amygdalaria, Immersaria Rambold & Pietschm., Poeltiaria, Xenolecia), with only two Lecidea species (L. auriculata Th. Fr. and L. tessellata Flörke) resolving with a group of Porpidia species that includes the clade that presumably represents Porpidia s. str. The type species of Porpidia is P. trullisata (Kremp.) Körb., a rare species for which sequence data are unavailable but which is morphologically very similar to P. speirea. Our phylogeny also indicates that Porpidia is not monophyletic. There are two large clades containing Porpidia species that are separated in the phylogeny with at least two other genera, Cyclohymenia and Immersaria, included in one clade and Amygdalaria in the other, making this clade paraphyletic.
Backbone support for our phylogeny is currently low and loci are absent for several important genera and species. We are continuing our investigation of Lecideaceae but are conscious of the first section of the preamble to the International Code of Nomenclature for Algae, Fungi and Plants (ICNafp), which states: ‘This Code aims at the provision of a stable method of naming taxonomic groups, avoiding and rejecting the use of names that may cause error or ambiguity or throw science into confusion. Next in importance is the avoidance of the useless creation of names’. Consequently, to propose any further taxonomic changes on the basis of the current work would be irresponsible, almost certainly damaging to nomenclatural stability and contrary to the expressed purpose of the code.
Key to Lecideaceae on the Falkland Islands
Lecidea s. str. and Porpidia s. lat. are not keyed out to species because there are several, apparently undescribed species in these genera that will be treated elsewhere.

Acknowledgements
Fieldwork on the Falkland Islands by the first author was funded by the UK Government through DEFRA and the Darwin Initiative as part of the project Lower Plants Inventory and Conservation in the Falkland Islands (Reference number DPLUS017). Support for fieldwork and advice regarding landowners was provided by Falklands Conservation. We thank Björn Owe-Larsson (UPS) for obtaining the ITS sequence from an isotype of Schizodiscus afroalpinus, Måns Svensson (UPS), Tim Wheeler and Holger Thüs for sequences of Amygdalaria, Farnoldia and Xenolecia species respectively, and Gintaras Kantvilas (HO) and Bruce McCune (OSC) for the loan of Poeltiaria and Cyclohymenia species respectively. Molecular analyses were financially supported in whole or in part by the Austrian Sciences Fund (FWF) 10.55776/P26638 and 10.55776/P35512. For open access purposes, the author has applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission.
Author ORCIDs
Alan Fryday, 0000-0002-5310-9232; Ulrike Ruprecht, 0000-0002-0898-7677.
Competing Interests
The authors declare none.
Supplementary Material
The Supplementary Material for this article can be found at https://doi.org/10.1017/S0024282924000148.










