Introduction
A girl was diagnosed antenatally with transposition of the great arteries, ventricular septal defect, and pulmonary stenosis. She was born at 37 + 5 weeks gestation and weighed 3.2 kg. The patent foramen ovale was restrictive at birth, so a successful percutaneous atrioseptostomy procedure was undergone. The child’s saturation was 80%, the atrial septal defect measured 6 mm, and the bidirectional atrial maximum velocity was 0.6 m/s. At 1.5 months, 4.2 kg, the infant had persistent desaturation at 70% in room air. Heart ultrasound scans (Figure 1) revealed a tiny tortuous arterial duct. Via a 5-French femoral artery access, the arterial duct was stented using two everolimus-eluting coronary stents (4*18 mm and 4*15 mm Xience Skypoint, Abbott), allowing the oxygen saturation to increase to 90% in room air (Figure 2). The child was discharged home 4 days later with aspirin and clopidogrel. At 5 months, 6 kg, the oxygen saturation progressively decreased again to 75% with ultrasound progressive atrial restriction and stent fracture suspicion on chest X-ray. Catheterisation confirmed the type III stent fracture. The stents were repeatedly dilated with coronary balloons (2*8 mm and 5*20 mm NC Emerge Monorail, Boston Scientific), and the proximal duct was stented again using an everolimus-eluting coronary stent (4.5*18 mm Synergy Megatron, Boston Scientific). The invasive mean trans-atrial gradient was 5 mmHg so a 6-mm M Atrial Flow Regulator over an 8-French delivery sheath (Occlutech) was deployed under transoesophageal echography guidance, and the gradient decreased to 3 mmHg. The child was discharged home 2 days later, saturation was 85%, and the bi-antiplatelet therapy was continued. Repeated ultrasound scans showed a patent Atrial Flow Regulator without gradient increase. At 9 months of age, saturation rapidly dropped to 75%. Chest X-ray confirmed the recurrence of a proximal ductal stent fracture. Diagnostic catheterisation showed a patent arterial duct despite the fracture and a low mean trans-atrial gradient of 2 mmHg. The lower gradient was explained by the stent fracture due to a decreased pulmonary flow. One month after the catheterisation, the infant underwent a successful “réparation à l’étage ventriculaire” surgical repair with ductal and atrial shunt closure at a weight of 8 kg. The Atrial Flow Regulator was easily retrieved peroperatively (total cardiopulmonary bypass time 133 min, aortic cross-clamp time 90 min). The post-operative course was uneventful. At 1 year of age, the child was doing well at home with normal saturation. We report for the first time the implantation of an Atrial Flow Regulator device to improve blood mixing in an infant with transposition of the great arteries, ventricular septal defect, and pulmonary stenosis.

Figure 1. Heart ultrasound scans showing an unrestricted left-to-right atrial shunt after atrioseptostomy at birth (a), a restricted left-to-right atrial shunt at 5 months of age (b) with increased Doppler maximal velocity to 1.8 m/s (c), a left-to-right shunt and seldom right-to-left shunt on the day following the implantation of a 6-mm M Atrial Flow Regulator (AFR, Occlutech) on a bicaval view (d), the AFR device barely touching the aortic wall on a parasternal short axis view (e), a reasonable left-to-right Doppler maximal velocity of 1.5 m/s (f), and a colour diameter of the left-to-right shunt across the AFR of 5.4 mm on a 4-chamber subcostal view (g).
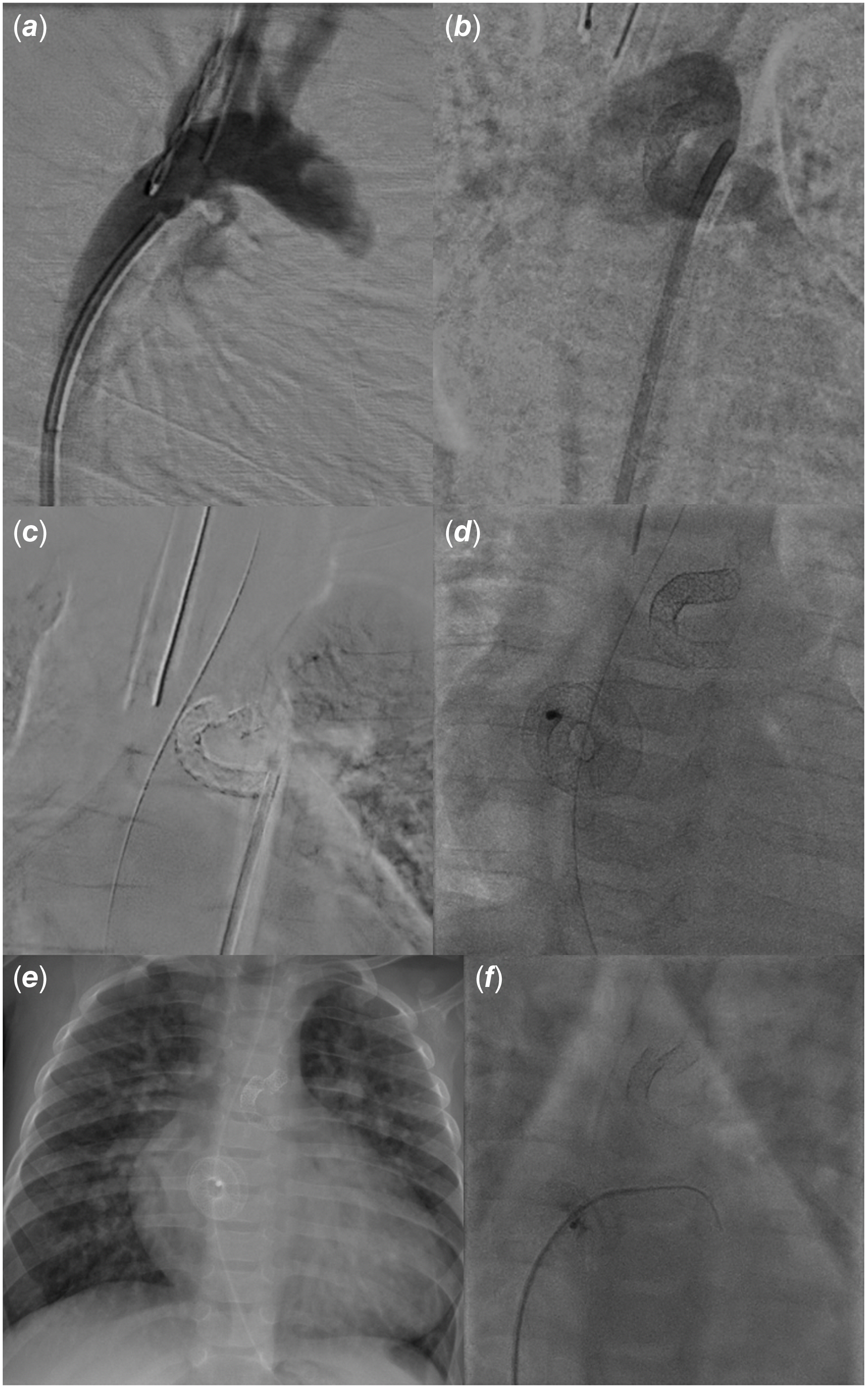
Figure 2. Ninety-degree right anterior oblique angiography revealing a millimetric rounded arterial duct (a), anteroposterior angiography showing the patent stented arterial duct with left-to-right-shunt from the aorta to the pulmonary arteries at the time of stent implantation at 1.5 months of age (b), 10-degree left anterior oblique projection with subtraction showing a stent fracture at the aortic end with separation of stent fragments (c), anteroposterior view after ductal stent-in-stent and Atrial Flow Regulator (AFR, Occlutech) device implantation at 5 months of age (d), en-face chest X-ray at 9 months of age revealing a recurrent stent fracture (e), and 10-degree cranial projection with a right coronary catheter crossing the patent AFR on a hydrophilic-coated (Terumo) guidewire with 2-mmHg trans-atrial gradient at 10 months of age (f).
Discussion
The Atrial Flow Regulator device has been used to relieve increased left atrial pressures in cardiomyopathies Reference Hansmann, Sabiniewicz and Sabiniewicz1 and CHDs, Reference Piccinelli, Testa and Butera2 or right atrial pressures in isolated pulmonary hypertension Reference Youssef, Averin and Richards3 and CHDs. Reference Castaldi, Cuppini, Sirico and etal4 It has also been used to create a calibrated atrial shunt in Fontan circulation to limit cyanosis while unloading the pulmonary circulation. Reference Aregullin, Samuel and Vettukattil5,Reference Pascall, Jones, Savis, Rosenthal and Qureshi6 However, this case is the first to describe its use for blood mixing in transposition of the great arteries physiology.
When left ventricular filling pressures are high, balance should be found between left heart decompression and pulmonary overload. When right ventricular filling pressures are increased, balance should be established between right heart decompression and cyanosis. In these cases, the diameter of the Atrial Flow Regulator device may be selected according to the level of ventricular filling pressures. However, unrestricted atrial flow is preferable in transposition of the great arteries physiology, as in hypoplastic left heart syndrome. Reference Bautista-Rodriguez, Hascoët and Fraisse7 The largest diameter possible should then be chosen. A limit is the risk of compression of surrounding structures according to the child’s age and weight and the length of the atrial septum. In the present case, the infant’s weight was only 6 kg, so a 6-mm M Atrial Flow Regulator was selected. Furthermore, although balloon dilatation of the atrial septal defect before Atrial Flow Regulator implantation has been suggested Reference Bautista-Rodriguez, Hascoët and Fraisse7 , it was not deemed necessary to provide better device support and avoid complications in the present case. Pre-dilatation could have excessively enlarged the atrial septal defect leading to an increased risk of embolisation. The Atrial Flow Regulator inner diameter did not significantly decrease after implantation, probably because of the relative softness of the atrial septum in infants. We hence suggest avoiding pre-dilatation in young children.
Selection of the largest diameter possible according to the child’s age may also prevent device thrombosis. Clots have been described with the 4-mm Atrial Flow Regulator which shall then be avoided. Reference Butera, Piccinelli and Kolesnik8 In the present case, no sign of thrombosis was found. Nevertheless, the AFR was used here for a limited period of 6 months before complete surgical repair. A case of thrombosis has been reported after a longer follow-up period of 14 months in the literature with a 4-mm Atrial Flow Regulator device. Reference Butera, Piccinelli and Kolesnik8 Selecting a larger device suggests using a larger sheath associated with a higher risk of venous access thrombosis as reported when a 10-French delivery sheath was used in a 5-kg 10-month child. Reference Bautista-Rodriguez, Hascoët and Fraisse7 However, we managed using an 8-French sheath which was 2 French lower than the recommended manufacturer size for a 6-mm M Atrial Flow Regulator. Only the use of a 9-French sheath has been reported in the literature so far. Reference Bautista-Rodriguez, Hascoët and Fraisse7
The first procedure realised in the present case was the atrioseptostomy. The complete repair is often delayed in transposition of the great arteries, ventricular septal defect, and pulmonary stenosis. Reference An, Li, Yan, Wang and Hua9 Therefore, the atrial shunt frequently becomes restrictive with growing age and the atrial septum more rigid making the atrioseptostomy procedure more challenging. Stenting has been associated with erosion, migration, and thrombus formation. Reference Cinteza and Carminati10 The Atrial Flow Regulator device can then become an alternative. Its implantation is simple and similar to atrial septal defect closure. Moreover, the Atrial Flow Regulator device was associated with arterial duct stenting in the present case due to the severity of the pulmonary stenosis. The shunt at the arterial level is essential, but maintaining the atrial shunt patency may have played a key role in maintaining a correct child’s saturation until surgical repair 6 months later, despite recurrent ductal stent fracture.
Conclusion
We described for the first time the use of an Atrial Flow Regulator in an infant with transposition of the great arteries, ventricular septal defect, and pulmonary stenosis as a bridge to surgery. This case report highlights the value of this device in improving blood mixing in CHDs. It may pave the way for a new indication of the Atrial Flow Regulator device in selected patients with transposition of the great arteries physiology.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Acknowledgements
The authors acknowledge the patient and the teams who participated in her care.
Authors’ contributions
Saïd Bichali wrote the initial draft of the article. All authors took part in the article revision. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Financial support
Occlutech graciously offered the Atrial Flow Regulator device used for the present patient. This research received no other specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
Ali Houeijeh is a proctor for Abbott and Occlutech. The other authors have no relevant financial or non-financial interests to disclose.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and the Helsinki Declaration of 1975, as revised in 2008. Informed written consent was obtained from the patient’s legal guardians.





