Non-technical Summary
Large, soft-bodied fronds were among the first large and complex creatures that evolved on our planet, and these fronds dominated the seas during the latter half of the Ediacaran Period (575–538 million years ago), which immediately preceded the Cambrian. Some of the first Ediacaran fronds described were found in the Flinders Ranges of Australia. A frond named Rangea longa by Martin Glaessner and Mary Wade in 1966, which was nearly half a meter long, is unique in almost always appearing on the top of the bed that contains it. The morphologies of the specimens they described were variable, which hindered global understanding of how many types were present and the time range that each morphology exhibited. Our study of all specimens ever discovered shows that Glaessner and Wade were correct in concluding that all these specimens belonged to the same species, and superb preservation of these fronds shows that the variation we see among these fossils reflects which side of the frond faced up and what angle it lay on the sea floor when it died. These fronds represent a new genus called Akrophyllas (literally “the frond on the top” in ancient Greek). Akrophyllas lived as an erect frond that was firmly anchored to the shallow sea floor by a bulbous holdfast, and died when it was buried by sand during a storm.
Introduction
The Ediacara biota represents the first abundant and globally distributed, morphologically, architecturally, and physiologically complex macroscopic organisms on Earth (Vickers-Rich et al., Reference Vickers-Rich, Fedonkin, Gehling, Leonov, Ivantsov, Komarower, Fuller, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007; Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Grazhdankin, Reference Grazhdankin2014; Droser et al., Reference Droser, Tarhan and Gehling2017; Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021). The Ediacara biota thrived 575–538 million years ago (Matthews et al., Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2021; Nelson et al., Reference Nelson, Ramezani, Almond, Darroch, Taylor, Brenner, Furey, Turner and Smith2022) and immediately preceded the Cambrian explosion of shelly and burrowing animals (Xiao et al., Reference Xiao, Narbonne, Zhou, Laflamme, Grazhdankin, Moczydlowska-Vidal and Cui2016; Xiao and Narbonne, Reference Xiao, Narbonne, Gradstein, Ogg, Schmitz and Ogg2020).
The Ediacara biota comprises a diverse group of originally soft-bodied taxa that typically are preserved as impressions under event beds of sandstone or volcanic ash (Wade, Reference Wade1968; Gehling, Reference Gehling1999; Narbonne, Reference Narbonne2005). Fronds dominate many Ediacaran assemblages and were the first globally widespread, large (decimeter- to meter-scale) eukaryotes with a construction that allowed them to be vertical and elevated, permitting feeding and respiration in the water column, although the nature and function of Ediacaran tiering remains controversial (compare Clapham and Narbonne, Reference Clapham and Narbonne2002; Ghisalberti et al., Reference Ghisalberti, Gold, Laflamme, Clapham, Narbonne, Summons, Johnston and Jacobs2014; Mitchell and Kenchington, Reference Mitchell and Kenchington2018; Darroch et al., Reference Darroch, Gutarra, Masaki, Olaru and Gibson2023; Pérez-Pinedo et al., Reference Pérez-Pinedo, Neville, Pasinetti, McKean, Taylor and McIlroy2023).
Traditional Ediacaran taxonomy emphasized unity of fronds as a high-level taxon (Glaessner, Reference Glaessner, Robison and Teichert1979), but more recent studies have suggested that frond morphology more likely represents convergent evolution due to competition for nutrients, oxygen, or gamete dispersal in the water column (Laflamme and Narbonne, Reference Laflamme and Narbonne2008; Dececchi et al., Reference Dececchi, Narbonne, Greentree and Laflamme2017). Differences in branching architecture provide a key to subdividing Ediacaran fronds into three robust clades (Laflamme and Narbonne, Reference Laflamme and Narbonne2008; Xiao and Laflamme, Reference Xiao and Laflamme2009: Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Brasier et al., Reference Brasier, Antcliffe and Liu2012; Dececchi et al., Reference Dececchi, Narbonne, Greentree and Laflamme2017; Dunn et al., Reference Dunn, Liu and Gehling2019a, Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liub). Arboreomorpha exhibit parallel first-order branches, and in the best-preserved specimens also exhibit second-order and rarely third-order branches perpendicular to the previous order of branching (commonly forming a structure resembling a pea-pod; Jenkins and Gehling, Reference Jenkins and Gehling1978; Laflamme et al., Reference Laflamme, Gehling and Droser2018; Dunn et al., Reference Dunn, Liu and Gehling2019a). Rangeomorpha consist of branches that are self-similar, fractal over at least three orders of magnitude, with all subsequent branching orders invariably at an acute angle to the previous branching order (Narbonne, Reference Narbonne2004; Laflamme et al., Reference Laflamme, Narbonne, Greentree and Anderson2007; Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al., Reference Brasier, Antcliffe and Liu2012; Vickers-Rich et al., Reference Vickers-Rich, Ivantsov, Trusler, Narbonne and Hall2013; Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019b). Erniettomorpha consist of petaloids composed of unornamented tube-like first-order branches that are not divided into smaller-scale branches (Narbonne et al., Reference Narbonne, Saylor and Grotzinger1997; Grazhdankin and Seilacher, Reference Grazhdankin and Seilacher2002; Ivantsov et al., Reference Ivantsov, Narbonne, Trusler, Greentree and Vickers-Rich2016; Darroch et al., Reference Darroch, Gibson, Syversen, Rahman and Racicot2022). First-order branches in the Arboreomorpha invariably emanate from a central stalk, but this is only true of some genera in the Rangeomorpha and one genus (Swartpuntia) in the Erniettomorpha.
Glaessner and Daily (Reference Glaessner and Daily1959) first reported and illustrated abundant Ediacaran fronds from Australia, some of which have become iconic images of the Ediacara biota worldwide. Most Ediacaran fronds in Australia are specimens of Arborea, first described as Rangea arborea Glaessner and Daily, Reference Glaessner and Daily1959, a taxon that has been studied by numerous subsequent workers (Glaessner and Wade, Reference Glaessner and Wade1966; Jenkins and Gehling, Reference Jenkins and Gehling1978; Laflamme and Narbonne, Reference Laflamme and Narbonne2008; Laflamme et al., Reference Laflamme, Gehling and Droser2018; Dunn et al., Reference Dunn, Liu and Gehling2019a). Glaessner's original collection from Ediacara Range contained 15 slabs collected by V.H. Mincham and B. Flounders in September 1957 and/or September 1958, which is referred to as the “Mincham-Flounders collection” throughout this paper, supplemented by the holotype, which was collected in October 1958 from a bed elsewhere in Ediacara Range (Jenkins and Gehling, Reference Jenkins and Gehling1978). The tops of these slabs include at least 20 specimens (12 of them well preserved) of an elongate frond that subsequently was named Rangea longa Glaessner and Wade, Reference Glaessner and Wade1966. Ten additional specimens of this taxon subsequently were collected from mostly unknown localities elsewhere in the Flinders Ranges (e.g., Sun, Reference Sun1986, fig. 3). No detailed studies of this taxon have been carried out in the more than 50 years since it was defined, only three slabs with specimens of ‘Rangea’ longa have been published previously, and almost all the taxonomic reassignments of this species have been based solely on features in the holotype. This lack of a complete description and modern analysis has led to taxonomic confusion and has reduced the importance of ‘Rangea’ longa in global compilations and analyses of Ediacaran fossils (Fedonkin et al., Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007; Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Dececchi et al., Reference Dececchi, Narbonne, Greentree and Laflamme2017). The present study evaluates these fronds in light of recent developments in our understanding of Ediacaran paleobiology.
Geologic setting
The fossils reported in this study occur in the Ediacara Member, the most significant fossiliferous unit within the Pound Subgroup (Gehling, Reference Gehling2000; Gehling and Vickers-Rich, Reference Gehling, Vickers-Rich, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007; Tarhan et al., Reference Tarhan, Droser, Gehling and Dzaugis2015; McMahon et al., Reference McMahon, Liu, Tindal and Kleinhans2020; Reid et al., Reference Reid, Payne, García-Bellido and Jago2020), from the Flinders Ranges in the Neoproterozoic to middle Cambrian succession of the Adelaide Fold Belt of South Australia. No radiometric dates are available from this succession, but the Ediacara Member occurs above the global Shuram-Wonoka-EN3 C-isotope anomaly (Xiao et al., Reference Xiao, Narbonne, Zhou, Laflamme, Grazhdankin, Moczydlowska-Vidal and Cui2016; Xiao and Narbonne, Reference Xiao, Narbonne, Gradstein, Ogg, Schmitz and Ogg2020), which elsewhere in the world ended at ca. 567 Ma (Canfield et al., Reference Canfield, Knoll, Poulton, Narbonne and Dunning2020; Rooney et al., Reference Rooney, Cantine, Bergmann, Gómez-Pérez, Al Baloushi, Boag, Busch, Sperling and Strauss2020), and occurs below the top of the Ediacaran, which elsewhere is dated at ca. 538 Ma (Nelson et al., Reference Nelson, Ramezani, Almond, Darroch, Taylor, Brenner, Furey, Turner and Smith2022).
Abundant and extraordinarily preserved fossil assemblages occur in the Ediacara Member of the Rawnsley depositional sequence and are limited by the stratigraphic distribution of facies suited to the preservation of non-skeletal organisms (Gehling, Reference Gehling2000). The Ediacara Member is a siliciclastic sequence of shallow marine and potentially delta-front environments deposited between storm- and fair-weather wave base (Gehling, Reference Gehling2000) or alternating non-marine and near-shore environments (McMahon et al., Reference McMahon, Liu, Tindal and Kleinhans2020). The Ediacara Member occurs on an erosional surface that cuts into the underlying Chace Quartzite Member of the Rawnsley Quartzite and Bonney Sandstone (Tarhan et al., Reference Tarhan, Droser, Gehling and Dzaugis2015) with a relief of 10–260 m (Reid et al., Reference Reid, Payne, García-Bellido and Jago2020). The fossiliferous, ripple-laminated sandstone beds of the Ediacara Member are interpreted to represent storm sands (Gehling, Reference Gehling2000; McMahon et al., Reference McMahon, Liu, Tindal and Kleinhans2020). Preservation of Ediacara-type fossil impressions on these sandstone surfaces can been attributed to microbial casting of the organisms immediately following their death (Gehling, Reference Gehling1999; Liu et al., Reference Liu, McMahon, Matthews, Still and Brasier2019a, Reference Liu, McMahon, Matthews, Still, Brasier and Marosib) and early silica diagenesis (Tarhan et al., Reference Tarhan, Hood, Droser, Gehling and Briggs2016, Reference Tarhan, Hood, Droser, Gehling, Briggs, Gaines, Robbins and Planavsky2019); formation of biofilms and authigenic clays also may have played important roles in preservation of these soft-bodied organisms (Laflamme et al., Reference Laflamme, Schiffbauer, Narbonne and Briggs2011; Slagter et al., Reference Slagter, Hao, Planavsky, Konhauser and Tarhan2022). Typical Flinders-style preservation of Ediacara-type fossil impressions exhibits complex textured organic surfaces with impressions of the bases of holdfasts, the tops of resistant epifaunal organisms (e.g., Dickinsonia), and non-resistant epifaunal organisms such as the petalodia of other frondose taxa typically preserved as positive impressions on bedding soles (Gehling, Reference Gehling1999; Narbonne, Reference Narbonne2005; Droser et al., Reference Droser, Tarhan and Gehling2017).
Frondose fossils of the Ediacara Member typically are preserved as impressions on the lower surfaces (soles) of sandstone beds (Glaessner and Wade, Reference Glaessner and Wade1966; Wade, Reference Wade1968; Gehling, Reference Gehling1999, Reference Gehling2000; Tarhan et al., Reference Tarhan, Droser and Gehling2010) with rarer occurrences on the tops of beds. However, all specimens of ‘Rangea’ longa in the Mincham-Flounders collection and almost all specimens collected elsewhere in the Flinders Ranges are preserved on the tops of the beds (Figs. 1.2, 1.3, 2.1, 3.1, 4, 5). Two specimens that are not associated with the Mincham-Flounders collection and do not match its lithology occur as part and counterpart cleavage reliefs within laminated sandstone event beds (Fig. 1.1) and a single confirmed specimen occurs on the sole of a sandstone event bed (Fig. 1.4).

Figure 1. Taphonomic variation in preservation of Akrophyllas longa n. comb. on bedding surfaces. (1) SAM P24593, the largest-known specimen of Akrophyllas n. gen., preserved in part and counterpart as a cleavage relief within a thick bed of laminated sandstone from Nilpena, with preservation of the marginal rim (mr), central stalk (cs), and two orders of branching (br) through composite molding. (2) SAM P12716 showing preservation of current-aligned and locally overlapping specimens of Akrophyllas n. gen. on an epirelief (top) surface in the Mincham-Flounders collection from Ediacara Range. Fronds (A) and (B) preserved in reverse view; frond (D) preserved in obverse view. Frond (B) partly overlies (A); frond (D) partly overlies (C), which may represent a separate frond, a shrinkage rim, or an earlier touchdown of frond (D). Note the short stem or naked stalk at the base of frond (B) and the prominent circular holdfast crater at (E). (3) SAM P12721a. Reverse side of the petalodium of Akrophyllas longa n. comb. showing a zigzag axial trace (zz) preserved on an epirelief (top) surface in the Mincham-Flounders collection from Ediacara Range. (4) SAM P40757, the only known specimen of Akrophyllas n. gen. preserved on the sole of a bed (negative hyporelief). This frond shows obverse-side preservation. Prominent holdfast disc to the right of the frond. All scale bars = 1 cm.
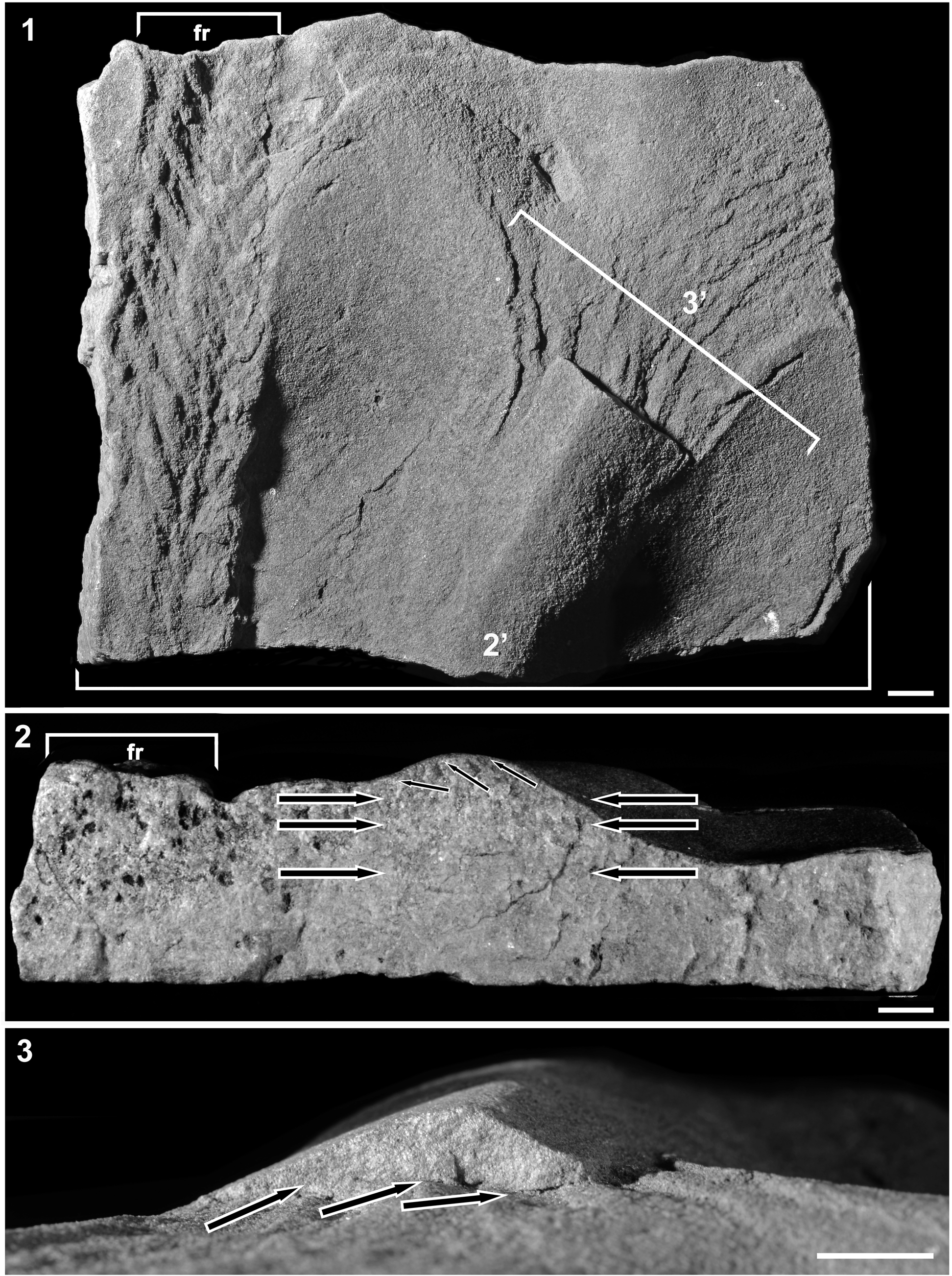
Figure 2. Top surface (1) and cross-sectional (2, 3) views of SAM P40445 from the Mincham-Flounders collection from Ediacara Range. (1) Top surface showing the reverse side of the frond Akrophyllas n. gen. (“fr”) on a surface marked by scours and a ripple. (2) Bed cross-section along the line marked 2’ in (1), showing a sharp-based event bed with structureless sandstone passing upward into planar-laminated sandstone (bold arrows), scoured top, and current-ripples (cross-lamination indicated with fine arrows). (3) Close-up of the current ripple along the line marked 3’ in (1) showing its sharp scoured base (arrows) and internal cross-lamination dipping to the left. All scale bars = 1 cm.

Figure 3. Tops and soles of beds in the Mincham-Founders collection from Ediacara Range. (1–5) SAM P12736. (1, 3) Photograph and map of the positions of Akrophyllas n. gen. fronds and frond stems/stalks on the top surface of the bed. (2, 4) Photograph and map of the positions of Aspidella holdfast discs on the bottom (sole) surface of the bed. (5) Superposition of maps (3) and (4) shows the connection of Akrophyllas n. gen. fronds preserved on the top of the bed with their holdfast discs preserved on its sole. (6) SAM P12730, sole showing microbial textures and a specimen of Dickinsonia. All scale bars = 1 cm.

Figure 4. SAM P13777. Holotype of Akrophyllas longa n. comb. illuminated from multiple directions; large white arrows in the upper right of each image denotes the direction of lighting. (1, 2) Complete specimen of bifoliate frond. From bottom (proximal) to top (distal) the specimen progressively changes from low positive epirelief with a well-developed proximal central stalk (cs) to low negative epirelief with branches meeting directly under the trace of the stalk. The marginal rim (mr) is especially visible on the lower left margin of the frond but becomes discontinuous distally. Specimen whitened with ammonium chloride. (3) Oblique view of a cast of the distal end of the frond, illustrating imbrication of the first-order branches (br) with an architecture of three orders of strictly orthogonal branches and marginal rim (mr). Zigzag axial trace (zz) in distal preservation of the frond. (4) Close up of two first-order branches (br) from the upper left of (1) showing details of second- and third-order branching architecture. All scale bars = 1 cm.

Figure 5. Distal tips and branching intersections in specimens of Akrophyllas longa n. comb. from the Flinders Ranges. All specimens coated with ammonium chloride. (1) SAM P12730a, juvenile specimen from the Mincham-Flounders collection preserved in obverse view exhibiting the distal tip of petalodium. (2) SAM BT-D226, latex of the tip of an uncollectible juvenile specimen from Bathtub Gorge preserved in obverse view showing a central stalk continuing to the distal end of the frond. (3) SAM P12736B, partial petalodium from the Mincham-Flounders collection from Ediacara Range displaying proximal stalk that passes into a zigzag pattern created by insertion of primary branches into a cylindrical stalk that is lower than the plane of view. (4) SAM P12743, multiple aligned and partly overlapping Akrophyllas n. gen. fronds from the Mincham-Flounders collection, with (B) preserved in reverse view, (C) preserved in obverse view, and a large holdfast disc (D) that postdates deposition of this bed. All scale bars = 1 cm.
The Mincham-Flounders collection
The Mincham-Flounders collection comprises 15 slabs that originally may have constituted a single broken bed (Glaessner and Daily, Reference Glaessner and Daily1959; Wade, Reference Wade1968; Jenkins and Gehling, Reference Jenkins and Gehling1978). The precise geographic and stratigraphic position of the Mincham-Flounders bed within the Ediacara Member in the Ediacara Range is unknown. The beds that underlie and overlie the Mincham-Flounders bed are also unknown, but the smooth surfaces on the sole and top of this bed and the superb preservation of Ediacara-type impression fossils on these surfaces suggest that the underlying and overlying beds most likely were mud.
Slabs of the Mincham-Flounders collection are typically well-sorted, fine- to medium-grained quartzose to subarkosic arenites with abundant fronds, scours, and current ripple-marks on the tops of the bed (Figs. 1.2, 1.3, 2, 3). Basal surfaces of the fossiliferous sandstone slabs are sharp (Fig. 2.2) and commonly exhibit microbial textures (Fig. 3.6) typical of those previously described from the Ediacara Member (Gehling, Reference Gehling1999, Reference Gehling2000; Tarhan et al., Reference Tarhan, Droser and Gehling2010), along with typical Flinders-style sole preservation of Ediacaran megafossils including Dickinsonia (Fig. 3.6) and Tribrachidium (sole of SAM P12716). Aspidella, interpreted by most modern workers as holdfast discs for fronds (Gehling et al., Reference Gehling2000; Tarhan et al., Reference Tarhan, Droser and Gehling2010, Reference Tarhan, Droser, Gehling and Dzaugis2015; Burzynski and Narbonne, Reference Burzynski and Narbonne2015; Droser et al., Reference Droser, Tarhan, Evans, Surprenant and Gehling2020), occurs as discoid impressions on the soles of sandstone beds (Fig. 3.2), hemispherical craters extending downwards from the tops of beds (Fig. 1.2E), and the impression of the base of a disc on the top of the fossil surface (Fig. 5.4D). Detailed mapping of the sole and top of a slab from the Mincham-Flounders collection (SAM P12736) shows that discs preserved on the sole of this bed precisely match the positions of the proximal ends of stems/stalks and fronds preserved on the top the top of the same bed (Fig. 3.1–3.5), confirming the connection between the buried disc and its attached frond via a short stem or a naked stalk. All specimens of Aspidella in the Mincham-Flounders bed are interpreted as partly buried, bulbous holdfasts that were variably flattened during sediment compaction.
Specimens of ‘Rangea’ longa in the Mincham-Flounders collection vary considerably in size and preservation (Figs. 1–5). Wade (Reference Wade1968) and Jenkins and Gehling (Reference Jenkins and Gehling1978) postulated that the fronds in the Mincham-Flounders collection were gregarious, living in proximity to one another, and included individuals of multiple sizes indicating multiple stages of growth, a lifestyle also observed in other Ediacaran fronds such as the erniettomorph Ernietta (Gibson et al., Reference Gibson, Rahman, Maloney, Racicot, Mocke, Laflamme and Darroch2019). Wade (Reference Wade1968) reported that slabs of the Mincham-Flounders collection show a surface strongly scoured by current action. Every slab containing multiple fronds in the Mincham-Flounders collection shows fronds with a consistent orientation relative to each other (Figs. 1.2, 3.1, 5.4), a relationship that is typical of current-aligned fronds attached to the sea bottom by discoidal holdfasts (Seilacher, Reference Seilacher1999; Wood et al., Reference Wood, Dalrymple, Narbonne, Gehling and Clapham2003; Brasier et al., Reference Brasier, Liu, Menon, Matthews, McIlroy and Wacey2013; Tarhan et al., Reference Tarhan, Droser, Gehling and Dzaugis2015). Scour pits, which formed alongside and in the lee of fronds (Fig. 2.1), and some fronds partly overlie adjacent fronds on the same bed top (Figs. 1.2, 5.4).
These features collectively imply that the fronds of the Mincham-Flounders collection were erect in life and subsequently fell into the positions in which they are now preserved. The connection of discs on the bottom of the Mincham-Flounders event bed with fronds on its top (Fig. 3) implies that the fronds were living attached to the sea bottom before deposition of the sand bed that buried holdfasts under its base and preserved the fronds attached to them on its top.
Most specimens lie flat on the upper surface of the bed, but at the end of the storm event some were buried at a gentle angle to the upper bedding surface with some parts buried while other parts protruded above the sediment-water interface (Fig. 6). Parts of the soft-bodied organisms that protruded above the surface (e.g., right-hand side of the frond in Fig. 6.2) would have decomposed before they could be preserved (Ivantsov et al., Reference Ivantsov, Narbonne, Trusler, Greentree and Vickers-Rich2016), but where the frond passed diagonally through the top of the sandstone bed it preserved an oblique cross-section through the frond (Fig. 6.3, 6.4). This can be seen most clearly in the holotype (SAM P13777; Fig. 4), which grades from a proximal region with mainly positive epirelief (bottom of Fig. 4.1 and 4.2) that reflects preservation of the stratigraphically uppermost part of the felled frond (labeled “Positive epirelief to flat” in Fig. 6.4), to a middle region of mainly negative epirelief (top of Fig. 4.1 and 4.2) that reflects preservation of the stratigraphically lowermost part of the felled frond (labeled “Negative epirelief” in Fig. 6.4), to a distal region exhibiting increasingly poor epirelief preservation passing towards the distal end of the frond (approaching the scale bar in the SE corner of Fig. 4.3) that reflects where the frond was fully above the sediment-water interface and thus could not be preserved (right-hand side of Fig. 6.2–6.4). This change from positive to negative epirelief distally through the frond cannot simply represent collapse of a hollow central stalk distally because it is shown equally in both the stalk and in the branches that flank it and because branching features can be seen under the trace of the stalk distally that are not visible proximally, but these attributes are fully compatible with exposure of lower stratigraphic sections through a felled frond inclined at an angle to the bedding surface that preserves it. Preservation of inclined Ediacaran fossils in the tops of fluidized event beds can provide unique insights into their morphology (Narbonne et al., Reference Narbonne, Saylor and Grotzinger1997; Vickers-Rich et al., Reference Vickers-Rich, Ivantsov, Trusler, Narbonne and Hall2013; Ivantsov et al., Reference Ivantsov, Narbonne, Trusler, Greentree and Vickers-Rich2016; see also Laflamme et al., Reference Laflamme, Gehling and Droser2018), and in the case of the fronds in the Mincham-Flounders collection, provide tangential cross-sections through key specimens that reveal their three-dimensional structure.

Figure 6. Diagrammatic reconstruction of fossil preservation at an oblique angle on the top of an event bed. (1) Akrophyllas longa n. comb. felled during burial. (2) Burial of the frond at an oblique angle, with the left side of the specimen buried and the right side still above the sediment-water interface after the end of the burial event. (3) Exposed part of the frond decomposes while the buried portion is preserved either as an impression or cast. Deposition of mud. (4) Modern weathering of the shale to expose a tangential cross-section through the frond longitudinally.
Unlike the carbonaceous compressions and early-mineralized tissues that characterize most fossil Lagerstätten, Ediacara-type fossils are typically preserved as impressions on sandstone beds or less commonly under beds of volcanic ash (Narbonne, Reference Narbonne2005). Ediacaran fossil preservation is therefore critically dependent on the grain-size of the casting bed, in which the individual sand grains or volcanic crystals represent the ‘pixels’ that dictate the resolution of the finest biological features that can be preserved on the fossil (Laflamme et al., Reference Laflamme, Narbonne, Greentree and Anderson2007; Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009). The finest resolution among specimens of ‘Rangea’ longa is seen in the holotype, which is preserved in very fine-grained sandstone that superbly casts three orders of branching down to the submillimeter-scale (Fig. 4), which is in marked contrast with the coarse-grained sandstone casting (e.g., specimen in Fig. 1.1) that preserves only broad-scale features of the branches, central stalk, and marginal rim. The fine- to medium-grained sandstone slabs of the Mincham-Flounders collection that constitute the bulk of the specimens in this study are intermediate in their grain size and in their preservational quality, with clear first-order branches adorned with faint to excellent second-order branches, but with only sporadic preservation of remnants of the third-order branches. All these specimens provide useful information, but the grain size strongly affects the type of information that can be obtained from different specimens. All photographic images in this paper are presented at the sizes that permit both the overall shape and the smallest-scale biological features on the fossil to be seen and are interpreted with this caveat in our text and reconstructions.
Materials and methods
Repository and institutional abbreviation
All collected specimens of Akrophyllas n. gen. are housed in the paleontological collections of South Australian Museum (SAM P) in Adelaide, South Australia. Holotype SAM P13777 from Ediacara, Flinders Ranges, South Australia (Glaessner and Wade, Reference Glaessner and Wade1966, pl. 100, fig. 4). Other well-preserved specimens are illustrated in Figures 1–3, 5, and 7 under the SAM numbers listed in the figure captions.
Systematic paleontology
Clade Arboreomorpha
Remarks
There is currently no universally accepted taxonomic hierarchy for Ediacaran biota above the rank of genus. The use of “clade” to indicate closely related Ediacaran taxa follows Erwin et al. (Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011), Laflamme et al. (Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013, Reference Laflamme, Gehling and Droser2018), and Dececchi et al. (Reference Dececchi, Narbonne, Greentree and Laflamme2017). This paper follows the frond terminology proposed by Laflamme and Narbonne (Reference Laflamme and Narbonne2008) and the suggestion of Dunn et al. (Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021) regarding the use of branch terminology for developmental discussions and for description of morphology. “Proximal” refers to the part of the frond nearest its base and “distal” refers to the part of the frond nearest its tip.
Genus Akrophyllas new genus
- Reference Glaessner and Daily1959
Rangea arborea (part) Glaessner in Glaessner and Daily, p. 383, pl. XLV, fig. 1 (only).
- Reference Glaessner1962
Charnia sp.; Glaessner, p. 483, pl. 1, fig 5.
- Reference Glaessner and Wade1966
Rangea longa; Glaessner and Wade, p. 614, pl. 100, fig 4, text-fig. 1.
- Reference Germs1973
Glaessnerina longa; Germs, p. 5.
- Reference Jenkins and Gehling1978
Charniodiscus longus; Jenkins and Gehling, p. 351.
- Reference Sun1986
Charniodiscus longus; Sun, p. 367, fig. 3.
- Reference Vickers-Rich, Fedonkin, Gehling, Leonov, Ivantsov, Komarower, Fuller, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007
Charniodiscus longus; Vickers-Rich et al., p. 266
- Reference Vickers-Rich, Fedonkin, Gehling, Leonov, Ivantsov, Komarower, Fuller, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007
Glaessnerina longa; Vickers-Rich et al., p. 273.
- Reference Vickers-Rich, Fedonkin, Gehling, Leonov, Ivantsov, Komarower, Fuller, Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007
Rangea longa; Vickers-Rich et al., p. 285.
- Reference Dunn, Liu and Gehling2019a
Arborea; Dunn et al., fig. S2.
- Reference Wang, Pang, Chen, Wan, Xiao, Zhou and Yuan2020
Charniodiscus longus; Wang et al., table 1.
- Reference Pérez-Pinedo, McKean., Taylor, Nicholls and McIlroy2022
Charniodiscus longus; Pérez-Pinedo et al., p. 12, fig. 2D.
Type species
Rangea longa Glaessner and Wade, Reference Glaessner and Wade1966, from the Ediacaran of Ediacara Range, Australia, by monotypy.
Diagnosis
New. Elongate, gently tapering, bifoliate petalodium attached to a discoid to spheroidal holdfast via a short stem or a naked stalk. Petalodium architecture consisting of three orders of strictly orthogonal branching, with mm-scale, second-order rectangular branches at right angles to the first-order branches and submillimetric third-order rectangular branches at right angles to the second-order branches. Frond petalodium consisting of a highly constrained array of cm-scale, rectangular to sigmoidal first-order branches emanating at an acute angle from both sides of an axial stalk or a zigzag axial crease in an alternate pattern to form two facing petaloids. Akrophyllas n. gen. differing from all other Ediacaran fronds in exhibiting a stalk that is visible only on the obverse side of the frond and is internal to the reverse side where the first-order branches instead meet at a zigzag axial trace. Both sides of the frond exhibiting similar size, shape, arboreomorph architecture, and branch sizes. No evidence of a backing sheet.
Occurrence
Akrophyllas n. gen. is presently known only from the Ediacara Member of the Rawnsley Quartzite in South Australia. The holotype and the specimens in the Mincham-Flounders collection were collected from two unknown levels in the Ediacara Member in the Ediacara Range, formerly designated as the Ediacara Range Fossil Reserve in 1958 and the Ediacara Conservation Park in 2007, and now part of Nilpena Ediacara National Park. Other specimens are from unknown localities in the Flinders Ranges. Locations for all specific fossil occurrences in the Flinders Ranges listed in this paper can be found in Gehling (Reference Gehling2000, fig. 1).
Description
The holotype (SAM P13777, Fig. 4) is a bifoliate petalodium fragment that exhibits a prominent central stalk flanked on either side by first-order branches with well-preserved microstructure that includes second-order and third-order branching. The holotype is incomplete at both its proximal and distal ends, with the preserved portion 150 mm long with a width that tapers from 61 mm at its proximal end to 45 mm at its distal end. Preservation in very fine-grained sand resulted in the holotype having the highest resolution of any specimen of Akrophyllas n. gen. All features on the left-hand side of the holotype are better preserved than their counterparts on the right-hand side.
On both sides of the fossil, first-order branches pass from the central axis at an acute angle in parallel rows, forming a petaloid on each side of the frond midline (Fig. 4). First-order branches ~10 mm wide and 30 mm long are adorned with 2-mm-wide linear second-order branches oriented perpendicular to the primary branches. Submillimeter-scale third-order branches are oriented perpendicular to the second-order branches, parallel with the first-order branches (Fig. 4.1, 4.3). Each first-order branch overlaps the distal end of the next branch to form an imbricate array, with each branch in the series gently dipping towards the proximal end of the frond (Fig. 4). Composite molding shows that the branches originally were subrectangular but that partial overlap of imbricated branches has made the exposed surface of each branch appear sigmoidal. All first-order branches emanate from the central axis and terminate at a marginal rim that marks the outer perimeter of the petalodium. This margin appears as a continuous, beaded ridge on the lower left side of the frond and appears more discontinuous distally (Figs. 4.1, 4.3).
Illumination of the holotype from different directions (Fig. 4) shows the central stalk in the proximal half of the holotype as a smooth rounded ridge preserved in positive epirelief, which is consistent with its preservation on the outside of the exposed surface of the felled frond. Distally, the stalk is represented only by a shallow trough (negative epirelief; Fig 4.1, 4.2), and composite molding in this trough shows branches from both sides of the frond meeting in an alternate arrangement (Fig. 4.1, 4.3) stratigraphically below the stalk of the felled frond.
Other specimens support and refine the description based on the holotype. All are incomplete specimens on broken slabs, but several specimens preserve a distally tapering tip (Figs. 1.1, 1.2A, 5.1, 5.2) and some fronds are directly attached to a disc on the sole of the same bed on which the fronds are preserved on its top (Fig. 3). All frond fragments are strongly elongate with moderate distal taper throughout the preserved portion of the petalodium. Extrapolations of the broken specimens that constitute Akrophyllas n. gen. suggest that most complete specimens were centimeters to decimeters in length. The largest-known frond fragment is 32 cm long as measured from the tip of the frond to the broken base of the slab where the frond is 7.7 cm wide, which represents a minimum length since the specimen shows no evidence of an attachment disc or proximal taper at the broken surface (Fig. 1.1). Although there is abundant evidence for overlapping specimens in accumulations of this gregarious frond (Figs. 1.2, 5.4), no evidence of a third petaloid was found in any specimen.
All specimens exhibit centimeter-scale, sigmoidal to rectangular, first-order branches that pass off the midline structure of the frond at an acute angle in both directions to form a petaloid on each side of the frond. All branches are strictly parallel with adjacent branches, with no evidence of branch rotation or dislocation. Imbrication of first-order branches is clearly shown (Fig. 1.2 specimens B and D) where distal edges of first-order branches partly overlie proximal edges on the right-hand side of both specimens.
Several specimens exhibit a sharp marginal rim formed by turning upwards and linking of the distal tips of adjacent first-order branches (Figs. 1.1, 1.2D, 1.4, 4.1–4.3, 5.1, 5.2). Millimeter-scale second-order branches at right angles to the first-order branches are visible at least locally on most specimens. Submillimetric third-order branches at right angles to the second-order branches are well preserved on the holotype (Fig. 4) and are sporadically preserved on other specimens due to the resolution limits of grain size.
Specimens of the Mincham-Flounders collection, which comprised all available slabs and specimens from a single bed, are represented by two nearly equally common variants of Akrophyllas longa n. comb., both about the same size and shape, with identically orthogonal branching architecture, and all oriented in the same direction on the same bedding plane. Variant 1 exhibits first-order branches that meet at a central stalk 1–17 mm in diameter (mean: 6.7 mm in diameter; ~15% of the width of the petalodium) that runs the entire visible length of the frond (Figs. 1.2D, 3.1, 5.1, 5.4C). Variant 2 exhibits first-order branches on either side of the petalodium that meet at a centrally located zigzag axial trace (Figs. 1.2A, 1.2B, 1.3, 2.1).
Glaessner and Wade (Reference Glaessner and Wade1966, text fig. 1) suggested that the two sides of this taxon differed in preserving the stalk on one side and a zigzag axial trace on the other. In addition to the holotype (described above), two specimens preserved obliquely to the bed in the Mincham-Flounders collection (Fig. 5.3, 5.4B) support their view in showing direct relationships between the central stalk visible on half of the specimens and the zigzag axial trace visible at a different stratigraphic level on the other half of the specimens. Further illustration of this is provided by the specimen illustrated in Figure 7—a full-relief cast showing the first-order branches meeting at a zigzag axial trace that directly backs onto a short, cylindrical stem or naked stalk that passes upward into the central stalk running along the other side of the petalodium. The nearly equal abundance of these two variants among fronds that occur on the same bedding plane and that are otherwise similar in size, shape, arboreomorph architecture, and in the size of each order of branches in their structure, coupled with four specimens including the holotype that show hints of both a zigzag axial trace and a central stalk on different levels in the same specimen, implies that these two variants represent opposite sides of the same frond species, with the central stalk external on one side of the frond (herein termed the ‘obverse’ side) and a zigzag axial trace visible on its ‘reverse’ side.
Etymology
Akros meaning “at the top” in Greek, in reference to its unusual preservation on the tops of sandstone beds. Phyllas meaning “leaf” in Greek, in reference to the overall lanceolate to linear, leaf-like shape of its petalodium. In combination with its original species name “longa”, it is the “long leaf at the top”.
Remarks
This material previously has been referred to at least five different genera in two different major groupings of Ediacaran fronds. Its architecture indicates that it is an arboreomorph whose construction differs from all previously described fronds.
Reconstructing Akrophyllas longa n. comb.
Understanding of the morphology of Akrophyllas longa n. comb. was hindered for more than 50 years by the disparate material in the collection made by Mincham and Flounders, which raised questions about the range of morphology and how many taxa are present in this collection. Our recognition that all the specimens on the top of this bed and similar finds throughout the Flinders Ranges constitute taphonomic variants of a single frondose taxon permits resolution of these questions and formulation of a three-dimensional reconstruction of Akrophyllas n. gen. (Fig. 8).

Figure 7. Detail of SAM P12716 (specimen B in Fig. 1.2) showing features of both the reverse and obverse sides of the frond. Reverse-side features are preserved in positive epirelief on the cast and consist of cm-scale first-order branches (br 1) emanating from a zigzag axial trace (zz), whereas branches beyond the edge of the cast are preserved in negative epirelief as impressions of the obverse side of the branch (br 2). A short stem or blind central stalk (cs) is present on the obverse side at the base of the frond but is completely covered distally by branches on the reverse side of the frond. Specimen coated with ammonium chloride. Scale bar = 1 cm.

Figure 8. Morphological reconstruction of Akrophyllas longa n., comb. (1) Complete erect frond attached to the sea bottom. The twist midway through the frond is diagrammatic to show both sides of the frond. (2) Close-up of the obverse side of the frond, which shows the first-order branches passing off a prominent central stalk. (3) Close-up of the reverse side of the frond, which shows the first-order branches meeting at a zigzag axial trace running the length of the frond.
Akrophyllas n. gen. is reconstructed as a bifoliate frond with a bulbous holdfast (Fig. 8). The holdfast is connected to the petalodium by a short (<1 cm) stem or naked stalk (Figs. 1.2B, 3.5, 7, 8.1) that continues through the petalodium as the central stalk. This internal stalk was likely fluid-filled and constructed of strong but flexible material that was collapsible, as shown by its variable degree of fill among different specimens and in different parts of the same specimen (Fig. 1.1, 1.2D, 1.4), attributes that are similar to stalks in other Ediacaran taxa (see Seilacher, Reference Seilacher1999; Narbonne and Gehling, Reference Narbonne and Gehling2003; Laflamme et al., Reference Laflamme, Narbonne, Greentree and Anderson2007; Laflamme and Narbonne, Reference Laflamme and Narbonne2008; Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009; Laflamme et al., Reference Laflamme, Gehling and Droser2018; Dunn et al., Reference Dunn, Liu and Gehling2019a, Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021). The stalk is evident only on the obverse side of the frond (Fig. 8.1, 8.2) where it is flanked on either side by a parallel array of first-order branches that are attached directly to the stalk and pass out from it at an acute angle. The stalk is internal to the reverse side of the frond and thus is not visible on this side; instead, first-order branches appear to meet at a zigzag axial trace (Fig. 8.3). The branches are firmly bound together at both the stalk and at the marginal rim along the edge of the petalodium, but contrary to the views of Pérez-Pinedo et al. (Reference Pérez-Pinedo, McKean., Taylor, Nicholls and McIlroy2022) there is no evidence of a “backing sheet” on either side of the frond. The branches alternate attachment on either side of the stalk (Fig. 8), demonstrating monopodial attachment similar to rangeomorphs such as Avalofractus (Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009) and Rangea (Vickers-Rich et al., Reference Vickers-Rich, Ivantsov, Trusler, Narbonne and Hall2013) and the arboreomorph Arborea (Laflamme et al., Reference Laflamme, Gehling and Droser2018, Dunn et al., Reference Dunn, Liu and Gehling2019a).
Most bifoliate Ediacaran fronds are bilaterally symmetrical about their central axis (Laflamme and Narbonne, Reference Laflamme and Narbonne2008), and many Akrophyllas n. gen. fronds are similarly flat and symmetrical on either side of their central axis (e.g., Figs. 1.1, 1.2D, 1.4, 5.1–5.3, 5.4C). Minor differences are shown between the two petalodia of the holotype (Fig. 4.1, 4.2), with the left-hand side wider and better preserved than the right-hand side. More extreme examples of asymmetry between the petalodia can be seen in some Akrophyllas n. gen. specimens that exhibit the reverse side of the frond (e.g., Figs. 1.2B, 1.3, 2.1, 5.4B, 7), in which one or both petalodia can stick upwards to form a V-shaped cross-section with the opening of the “V” facing upwards (when viewed distally along the length of the frond). The petalodium that is tilted out of the horizontal plane is consistently narrower, presumably due to telescoping of features during compaction or modern erosion of high points. In part, this upraised petalodium reflects draping of fronds on the scoured surface of the Mincham-Flounders bed, but in part it may also reflect an oblique angle between the two petaloids that comprise the petalodium on the reverse side of Akrophyllas n. gen. and a reflex angle between the petaloids on the obverse side in life. The different shapes of fronds have different hydrodynamic properties (Singer et al., Reference Singer, Plotnick and Laflamme2012; Ghisalberti et al., Reference Ghisalberti, Gold, Laflamme, Clapham, Narbonne, Summons, Johnston and Jacobs2014; Gibson et al., Reference Gibson, Rahman, Maloney, Racicot, Mocke, Laflamme and Darroch2019, Reference Gibson, Furbish, Rahman, Schmeeckle, Laflamme and Darroch2021; Darroch et al., Reference Darroch, Gutarra, Masaki, Olaru and Gibson2023), and if this oblique angle between the two folia of Akrophyllas n. gen. was primary, the obverse side would have been anhedral and the reverse side would have been dihedral with significantly different hydrodynamic responses to moving water in the shallow-water, storm-influenced environment in which Akrophyllas n. gen. lived.
Ediacaran fronds can be preserved at the base, within, or on top of event beds of sandstone or volcanic ash, but for taphonomic reasons the vast majority worldwide are preserved at the base of event beds (Gehling, Reference Gehling1999; Narbonne, Reference Narbonne2005). The preponderance of Akrophyllas n. gen. specimens that occur on the tops of sandstone event beds may be fortuitous, or may reflect taphonomic conditions particular to the Flinders Ranges or some difference in the hydrodynamic or buoyancy properties of this frond.
Comparisons and affinities
Over the more than 60 years since it was first reported, Akrophyllas longa n. comb. has had a confused nomenclatural history that has seen it referred to four different rangeomorph genera and two arboreomorph genera.
Rangeomorph comparisons
Rangea Gürich, Reference Gürich1930, was the first Ediacaran frond described anywhere and remained the only described Ediacaran frond genus until Ford (Reference Ford1958) named Charnia and Charniodiscus, so it is not surprising that these fossils were originally compared with Rangea (Glaessner and Daily, Reference Glaessner and Daily1959) and were later formally defined as Rangea longa Glaessner and Wade, Reference Glaessner and Wade1966. Similarities with other rangeomorph genera have also led to the fossils of the Mincham-Flounders collection also being referred to Charnia (Glaessner, Reference Glaessner1962, pl. 1, fig. 5), a species of Glaessnerina Germs, Reference Germs1973, and an unnamed frond illustrated by Anderson (Reference Anderson and Lapedes1978) (Sun, Reference Sun1986) that subsequently became the type specimen of Trepassia Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009. All of these designations were made on the basis of similarities in the overall frondose shape that now are regarded as convergent (Laflamme and Narbonne, Reference Laflamme and Narbonne2008), and predated recognition of the significance of frond architecture as a high-level taxonomic criterion (Laflamme and Narbonne, Reference Laflamme and Narbonne2008; Erwin et al., Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011; Dececchi et al., Reference Dececchi, Narbonne, Greentree and Laflamme2017). Akrophyllas n. gen. does not exhibit rangeomorph architecture, in which the fronds consist entirely of elements commonly termed frondlets that exhibit self-similar branching over several fractal scales and are used as modules to construct larger structures (Narbonne, Reference Narbonne2004; Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al., Reference Brasier, Antcliffe and Liu2012; Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021), and thus cannot be regarded as a rangeomorph.
Other significant morphological differences further serve to distinguish Akrophyllas n. gen. from the specific rangeomorph genera with which it was formerly compared. Rangea is a multifoliate frond that consists of a hexaradial axial bulb that passes into a conical axial stalk extending the length of the fossil with six vanes arranged radially around the stalk; each vane consists of a bilaminar sheet composed of a repetitive pattern of unfurled rangeomorph elements (Vickers-Rich et al., 2011). Essentially all of these features are absent from Akrophyllas n. gen. Charnia is superficially similar to the reverse side of Akrophyllas n. gen in exhibiting rectangular to sigmoidal first-order branches that are further subdivided into second- and third-order divisions, but the architecture of Akrophyllas n. gen. is strictly orthogonal with first-order branches at right angles to second-order branches and second-order branches at right angles to the third-order branches, whereas in Charnia second- and especially third-order branches are at an acute angle to higher-order branches (Laflamme et al., Reference Laflamme, Narbonne, Greentree and Anderson2007; Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019b, Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021). In addition, Charnia does not exhibit either a marginal rim or a visible stalk (Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021), both of which are readily evident in Akrophyllas n. gen. The two species that comprise Glaessnerina show Charnia-style architecture but differ sufficiently in their construction that they would no longer be regarded as congeneric. The type species of Glaessnerina, G. grandis Glaessner and Wade, Reference Glaessner and Wade1966, is represented by a single specimen that Runnegar (Reference Runnegar, Schopf and Klein1992) regarded as a junior synonym of Charnia—a view with which we concur.
In addition to the architectural and constructional differences above, all these taxa are broadly elliptic whereas Akrophyllas n. gen. is highly elongate in shape. Sun (Reference Sun1986) compared his newly discovered specimen of Akrophyllas n. gen. (herein illustrated in Fig. 1.1) with a specimen subsequently designated as the holotype of Trepassia, which is a highly elongate rangeomorph frond from Mistaken Point that exhibits an internal central stalk (Narbonne and Gehling, Reference Narbonne and Gehling2003; Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009). However, Trepassia is strictly parallel-sided and lacks a marginal rim whereas the petalodium of Akrophyllas n. gen. is gently tapering with a well-developed marginal rim, features that may reflect differences in both construction and development between Akrophyllas n. gen. and Trepassia. Architecturally, branching at different scales is strictly orthogonal in Akrophyllas n. gen., with the second-order branches at right angles to the first-order branches and the third-order branches strictly at right angles to the second-order branches, whereas both of these angles are considerably more acute in the rangeomorph Trepassia.
Sun (Reference Sun1986) also compared Akrophyllas n. gen. with his new genus Paracharnia, which is an elongate frond from the Dengying Formation of China. Both of these taxa are elongate but that is where the similarity ends. Paracharnia is parallel-sided, like Trepassia, whereas Akrophyllas n. gen. tapers distally. Paracharnia has an enormous central stalk flanked by tiny branches whereas the reverse is true in Akrophyllas n. gen. Paracharnia is probably a valid genus, but the poor preservation of its architecture complicates meaningful comparisons of Paracharnia with other frondose taxa. Thus, we contend that the development of significant elongation (in terms of high frond length to width ratios and reduced stem lengths) is more likely a convergent feature rather than a signal of phylogenetic affinities.
Arboreomorph comparisons
The first Ediacaran frond named in Australia was Rangea arborea Glaessner in Glaessner and Daily, Reference Glaessner and Daily1959, a taxon that was later designated as the type species of Arborea Glaessner and Wade, Reference Glaessner and Wade1966. Arborea-type architecture is typified by Arborea arborea (Glaessner in Glaessner and Daily, Reference Glaessner and Daily1959) and consists of a parallel series of sigmoidal to rectangular first-order branches passing off a central stalk, with prominent second-order branches at right angles to the first-order branches and rarely third-order branches that are subtransverse to the second-order branches (Laflamme and Narbonne, Reference Laflamme and Narbonne2008; Laflamme et al., Reference Laflamme, Gehling and Droser2018; Dunn et al., Reference Dunn, Liu and Gehling2019a). Second-order branches visible on the front side of Arborea arborea are commonly oval, giving rise to the classic ‘peapod structure’ described from the best-preserved specimens of Arborea, but preservational factors can result in second-order branches being rectangular, or represented by closely spaced parallel ridges transverse to the first-order elements, or entirely absent in different parts of the same specimen or different specimens on the same or other beds (Jenkins and Gehling, Reference Jenkins and Gehling1978; Laflamme et al., Reference Laflamme, Gehling and Droser2018; Dunn et al., Reference Dunn, Liu and Gehling2019a). Some previously defined species of Arborea and Charniodiscus apparently lack second-order divisions (e.g., Wang et al., Reference Wang, Pang, Chen, Wan, Xiao, Zhou and Yuan2020) and should only tentatively be referred to the Arboreomorpha on the basis of constructional features, but where second- and third-order branches are preserved in arboreomorphs they invariably are transverse to the immediately higher-order branches (Jenkins and Gehling, Reference Jenkins and Gehling1978; Laflamme et al., Reference Laflamme, Narbonne and Anderson2004; Ivantsov, Reference Ivantsov2016; Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021; Pérez-Pinedo et al., Reference Pérez-Pinedo, McKean., Taylor, Nicholls and McIlroy2022). Arboreomorph architecture is clear in Akrophyllas n. gen., which exhibits branching that is strictly orthogonal with first-order branches at right angles to second-order branches and third-order branches orthogonal to the second-order branches (Figs. 4, 8). First-order branching in arboreomorphs ranges from being perpendicular to acute relative to the central stalk and can be either alternate or opposite in arrangement. All known specimens of Akrophyllas n. gen. exhibit exclusively acute branching in an alternate arrangement.
The two main arboreomorph taxa, Charniodiscus Ford, Reference Ford1958, and Arborea Glaessner and Wade, Reference Glaessner and Wade1966, have had a confused nomenclatural history. Jenkins and Gehling (Reference Jenkins and Gehling1978) regarded Arborea as a subjective junior synonym of Charniodiscus Ford, Reference Ford1958, a decision later reversed by Laflamme et al. (Reference Laflamme, Gehling and Droser2018) based on evidence presented by Brasier and Antcliffe (Reference Brasier and Antcliffe2009) that the holotype of the type species of Charniodiscus, C. concentricus Ford, Reference Ford1958, is multifoliate with possible fractal architecture. Both of the points presented by Brasier and Antcliffe (Reference Brasier and Antcliffe2009) were challenged in a recent analysis of C. concentricus by Pérez-Pinedo et al. (Reference Pérez-Pinedo, McKean., Taylor, Nicholls and McIlroy2022), who nonetheless concluded that Charniodiscus and Arborea should be regarded as separate genera within the Arboreomorpha, and proposed that they could be distinguished by two entirely constructional features: a backing sheet on a planar-foliate petalodium in Arborea (see Dunn et al., Reference Dunn, Liu and Gehling2019a) and the lack of a backing sheet on a petalodium which they inferred to be conical based on their new reconstruction of C. concentricus. The global applicability of these criteria awaits testing, but Akrophyllas n. gen. does not exhibit the backing sheet Pérez-Pinedo et al. (Reference Pérez-Pinedo, McKean., Taylor, Nicholls and McIlroy2022) regarded as diagnostic of Arborea and does not exhibit the inferred conical petalodium that they regarded as diagnostic of Charniodiscus. No previously named genus of the Arboreomorpha has a highly elongate shape or exhibits a stalk on only one side of its petalodium.
The strictly orthogonal pattern of branching on both sides of Akrophyllas n. gen. is diagnostic of arboreomorph architecture, but Akrophyllas n. gen. blurs several of the constructional features that formerly served as secondary criteria for the recognition of Arboreomorpha. All arboreomorph fronds worldwide exhibit a stalk that is at least partly visible on both sides of the frond (Laflamme et al., Reference Laflamme, Narbonne and Anderson2004; Ivantsov, Reference Ivantsov2016; Dunn et al., Reference Dunn, Liu and Gehling2019a; Pérez-Pinedo et al., Reference Pérez-Pinedo, McKean., Taylor, Nicholls and McIlroy2022). Some rangeomorph fronds lack a visible stalk, but others exhibit stalks that can be either internal or external (Brasier et al., Reference Brasier, Antcliffe and Liu2012; Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021). Akrophyllas n. gen. differs from all previously described fronds in exhibiting a stalk that is visible only on the obverse side of the frond and is internal to the reverse side. Consequently, the construction visible on the obverse side of Akrophyllas n. gen. is typical of the arboreomorphs in exhibiting a central stalk with primary branches passing off it and rebranching to form the frondose structure, but the reverse side of Akrophyllas n. gen. is superficially similar in construction to rangeomorphs like Charnia in which primary branches on both sides meet in an alternate arrangement at a zigzag axial trace rather than a visible stalk. The Akrophyllas n. gen. branching pattern, in which each first-order branch overlaps with the next branch to form an imbricate array (Figs. 1.2, 4, 8), is a construction that is common in rangeomorphs such as Charnia, Avalofractus, and Trepassia (Laflamme et al., Reference Laflamme, Narbonne, Greentree and Anderson2007; Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009) but is only very sparingly reported from arboreomorphs (Laflamme et al., Reference Laflamme, Gehling and Droser2018). This anatomical dichotomy may have contributed to the half-century-long debate about the affinities of Akrophyllas n. gen., with different authors comparing different architectural and constructional aspects of this single species of fossil frond with a variety of rangeomorph and arboreomorph genera.
In summary, the orthogonal branching architecture on both sides of Akrophyllas n. gen. clearly relates it to the Arboreomorpha as presently defined, but its construction shows attributes of both the Arboreomorpha and the Rangeomorpha. This might suggest either that the Arboreomorpha and Rangeomorpha are more closely related than currently considered, or alternatively that some constructional aspects in arboreomorph, rangeomorph, and possibly erniettomorph fronds may have converged through time while maintaining the diagnostic architecture in each of these clades. Present understanding provides equivocal support for both models, and further emphasizes the need for new discoveries and analyses to resolve evolutionary relationships and patterns among the large, morphologically complex, soft-bodied fronds that dominated Ediacaran seas.
Conclusions
A gregarious assemblage of elongate fossil fronds from Ediacara, originally named Rangea longa Glaessner and Wade, Reference Glaessner and Wade1966, has had a confused taxonomic history due to the rather disparate morphology of the specimens that constitute the type specimen and the material collected by Mincham and Flounders in the late 1950s. Our detailed studies confirm the view of Glaessner and Wade (Reference Glaessner and Wade1966) that all these specimens are conspecific, and further suggest that they represent different preservational modes that collectively elucidate the three-dimensional morphology of this fossil frond. These specimens show typical arboreomorph architecture of multiple orders of branches that are strictly orthogonal with respect to higher and lower orders of branching but differ in construction from all described Ediacaran fronds and are herein designated as the new genus Akrophyllas, which is an elongate, bifoliate frond with highly constrained orthogonal (arboreomorph) architecture and a prominent stalk that was external on the obverse side of the frond but internal to its reverse side where the first-order branches meet at a zigzag axial trace. Akrophyllas n. gen. fronds are strongly aligned with each other, partly overlie adjacent individuals, are directly connected to holdfast discs on the bottom of the same bed, and formed loci for down-current scours, implying that these fronds lived erect on the shallow sea floor.
Acknowledgments
Narbonne acknowledges funding from the Natural Sciences and Engineering Research Council of Canada (NSERC 2014-05561/2020-06741) and a Queen's University Research Chair. Grimes acknowledges scholarship funding from a Queen's University Graduate Entrance Tuition Award (GETA) and the R. Samuel McLaughlin Fellowship. We thank R. McIntosh who guided us during our visit to Ediacara and R. and J. Fargher for access to the National Heritage Nilpena fossil site on their property, acknowledging that these sites lie within the Adnyamathanha Traditional Lands. Special thanks are due to M.-A. Binnie and D. Garcia-Bellido who greatly assisted our efforts in the South Australia Museum. R.W. Dalrymple, C. Greentree, and N. Heikoop provided valuable discussion, and exceptionally useful reviews by M. Laflamme, A. Liu, and an anonymous referee significantly improved this contribution. This paper is dedicated to the memory and accomplishments of H. Mincham and B. Flounders who discovered and collected this key fossil bed at Ediacara, and to M. F. Glaessner and M. Wade whose paleontological contributions substantially enriched our understanding of what is now called the Ediacaran Period.
Declaration of competing interests
The authors declare that they have no conflict of interest.











