INTRODUCTION
Listeria monocytogenes is a pathogen associated with food and is mainly transmitted through ready-to-eat food such as soft cheese, vacuum-packed gravad and cold-smoked salmon, salads, raw milk, and uncooked meat. The clinical picture consists of septicaemia and/or meningitis, and abortion in pregnant women. Listeriosis primarily affects pregnant women, newborns, and individuals with weakened immune systems. The overall case-fatality rate from invasive listeriosis is from 20% to >50% [Reference Schlech1].
In Sweden, the first isolation of L. monocytogenes was from rabbits in 1910 [Reference Hülphers2], and in 1960, human listeriosis became a notifiable disease [Reference Larsson, Cronberg and Winblad3]. Sweden is divided into 21 counties and surveillance is based on these administrative regional units (Fig. 1). During 2009, Sweden had the second highest number of cases reported (n = 73), and had the second highest number of reported cases within the EU (0·78 cases/100 000). During 2013, Sweden had the highest number of cases (n = 93) since 1960, i.e. 0·97 cases/100 000 people [4]. In developed countries where listeriosis is documented, the incidence rate is around 0·3/100 000 population [Reference Todd and Notermans5]. Scandinavian countries have consistently higher rates than other countries, which is possibly due to the consumption of smoked fish [Reference Todd and Notermans5].

Fig. 1 [colour online]. Number of isolates collected from the different counties in Sweden. Götaland region: Skåne (M); Blekinge (K); Halland (N); Västra Götaland (O); Gotland (I); Kronoberg (G); Kalmar (H); Jönköping (F); Östergötland (E). Svealand region: Örebro (T); Södermanland (D); Västmanland (U); Uppsala (C); Stockholm (AB); Värmland (S); Dalarna (W). Norrland region: Gävleborg (X); Västernorrland (Y); Jämtland (Z); Västerbotten (AC); Norrbotten (BD). For four isolates there was no information.
L. monocytogenes isolates from human patients in Sweden suffering from invasive listeriosis during 1986–2007 have been characterized through serotyping and pulsed-field gel electrophoresis (PFGE) [Reference Parihar6]. The aim of the present study was to continue the characterization of human L. monocytogenes isolates for the periods before (1958–1985) and after (2008–2010). A second aim was to relate genotypic results to epidemiological information, and to identify possible clustering of L. monocytogenes genotypes in time, season, location, age, or gender. In the present paper, results from the entire 53-year period (1958–2010) are presented and discussed.
MATERIALS AND METHODS
L. monocytogenes isolates
Isolates of L. monocytogenes (n = 932) from patients with invasive listeriosis were received from clinical laboratories throughout Sweden and represented 64% of the 1460 human listeriosis cases reported during the entire 53-year period: a mother–child pair was considered as one case. The overall case-fatality rate was estimated as 30%. Isolates were from blood (n = 531), cerebrospinal fluid (n = 137) or other sources (n = 68) of sporadic cases, and from one known outbreak in 1994/1995 involving eight patients [Reference Ericsson7]. Data regarding source was lacking for 196 isolates and duplicate isolates from patients were disregarded. The isolates were frozen at −75°C in 80% brain heart infusion (Merck, Germany) and 20% v/v glycerol until analysis by serotyping and PFGE.
Serotyping
All L. monocytogenes isolates were serotyped with Listeria O antiserum types I/II, I, IV, V/VI, VI, VII, VIII, IX and H antiserum A, AB, C, D (Mast Diagnostics, UK) according to the manufacturer's instructions, with some modifications [Reference Parihar6].
PFGE
Each isolate was restricted with the AscI enzyme and subsequently subjected to PFGE, according to Graves & Swaminathan [Reference Graves and Swaminathan8] with some modifications [Reference Parihar6]. Gels with profiles were visualized by short-wave ultraviolet (312 nm) light and photographed with a Polaroid camera. The pictures were analysed visually and the DNA restricted fragments were sized against lambda ladder PFG marker no. 340 S (New England Bio-Labs Inc., USA). Image analysis software was not used for comparison.
PFGE types were established based on the number and distribution of bands in each profile. The profiles were considered distinguishable if there was a difference of one band or more. Each unique PFGE strain was designated in a specific way. For example, serovar, AscI profile, and variant; thus, the designation 1/2a:4A indicated the strain belonged to serovar 1/2a, AscI profile 4, and variant A.
RESULTS AND DISCUSSION
Results from a previous characterization of human Swedish L. monocytogenes isolates from 1986 to 2007 by Parihar et al. [Reference Parihar6], are included in the present study. Thus, the present paper covers the characterization of L. monocytogenes isolates from 932 human patients with invasive listeriosis in Sweden during the period 1958–2010.
Characterization
Of the 932 human L. monocytogenes isolates, 183 different PFGE types were identified, of which 83 PFGE types were each represented by only one isolate. The number of isolates, serovars, number of PFGE types, and the six most common PFGE types of L. monocytogenes isolates per region during 1958–2010 are presented in Table 1. The distribution of L. monocytogenes isolates, serovars, and the six most common PFGE types per year (1958–2010) are presented in Table 2. Thus, 483 serovar 1/2a isolates were distributed in 114 PFGE types (an average of 4·24 isolates per PFGE type); 90 serovar 1/2b isolates gave 32 PFGE types (2·81 isolates per PFGE type); 21 serovar 1/2c isolates gave nine PFGE types (2·33 isolates per PFGE type); three serovar 3b isolates gave one PFGE type; and 335 serovar 4b isolates gave 31 PFGE types (10·80 isolates per PFGE type) (Table 3).
Table 1. Distribution of L. monocytogenes isolates in Sweden by serovar, PFGE type and region during 1958–2010
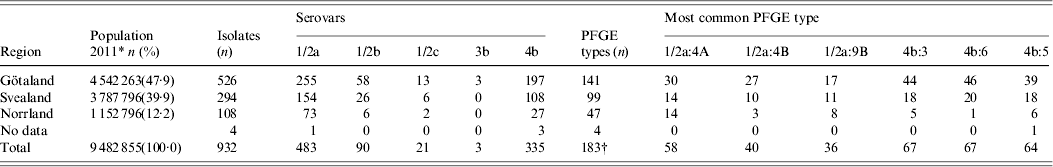
PFGE, Pulsed-field gel electrophoresis.
* Statistics Sweden [54].
† Total number of PFGE types identified.
Table 2. Serovars, PFGE types and the six most common PFGE types of L. monocytogenes isolates in Sweden, per year, during 1958–2010
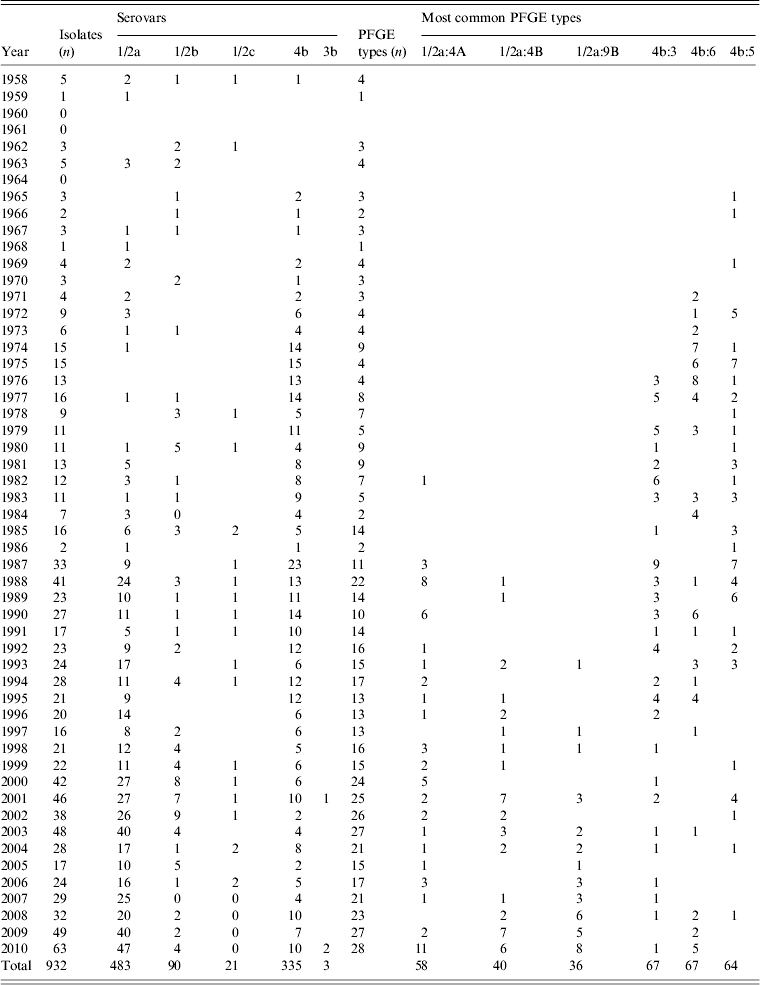
PFGE, Pulsed-field gel electrophoresis.
Table 3. Serovar and PFGE type distribution of L. monocytogenes isolates in Sweden during 1958–2010
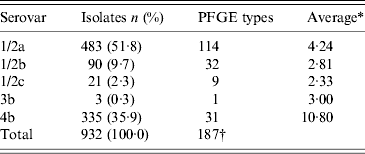
PFGE, Pulsed-field gel electrophoresis.
* Average number of isolates per PFGE type.
† 183 PFGE types were identified. Four PFGE types are shared by serovars 1/2a and 1/2c.
The period studied (1958–2010) was divided into three periods: 1958–1971, when three serovars 1/2a, 1/2b, and 4b were equally common; 1972–1995, when serovar 4b was the main L. monocytogenes serovar in human listeriosis; and 1996–2010, when serovar 1/2a was the main L. monocytogenes serovar (Table 4). As the present study was retrospective, the data about the sources were not supported by Swedish epidemiological investigations of outbreaks or case-control studies of sporadic cases. However, food sampling has been conducted several times in Sweden during different periods [Reference Loncarevic, Danielsson-Tham and Tham9–Reference Kannius and Karlsson13].
Table 4. Serovar and PFGE type distribution of L. monocytogenes isolates in Sweden, by period, during 1958–2010
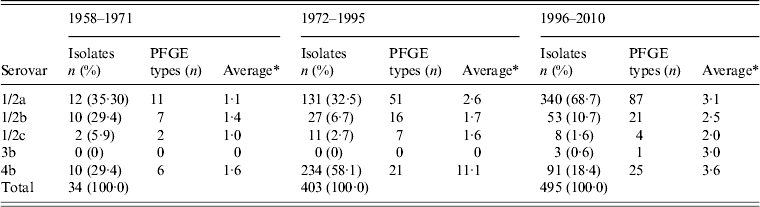
PFGE, Pulsed-field gel electrophoresis.
* Average number of isolates per PFGE type.
L. monocytogenes isolates from 1958 to 1971
From 1958 to 1971, serovars 1/2a, 1/2b, and 4b were equally common, although based on a small number of available human isolates (n = 34). During a possible outbreak of listeriosis in Uppsala at the beginning of the 1960s, bacteriological investigations on eggs and poultry failed to identify Listeria bacteria [Reference Ekelund14]; however, this investigation was conducted two decades before the foodborne route for L. monocytogenes was established.
L. monocytogenes isolates from 1972 to 1995
Epidemic cheese-borne strains
Of the available human isolates (n = 403) from 1972 to 1995, serovar 4b was shared by 234 (58·1%) and serovar 1/2a was only shared by 131 isolates (32·5%) (Table 4). During the 1980s, large outbreaks and sporadic cases of foodborne listeriosis due to closely related strains sharing serovar 4b were reported from both the USA and Europe [Reference Kathariou15]. An indistinguishable PFGE type was identified in a Swiss soft cheese outbreak between 1983 and 1987 [Reference Bille and Glauser16], a Danish outbreak in 1985–1987 [Reference Samuelsson17] and again in 1989–1990 [Reference Jensen, Frederiksen and Gerner-Smidt18], the latter probably due to blue mould cheese and, in California in 1985 due to fresh cheese [Reference Linnan19]. One of the most common PFGE types during 1972–1995 in Sweden, shared the same PFGE type (4b:6) as the epidemic cheese-borne strains from Switzerland, Denmark, and USA [Reference Kathariou15, Reference Buchrieser20, Reference Ericsson21].
Raw milk soft cheeses and L. monocytogenes serovars
One hypothesis was a majority of human L. monocytogenes serovar 4b isolates in Sweden, during the period 1972–1995, came from soft raw milk cheeses imported from European countries. In 1987, Beckers et al. [Reference Beckers, Soentoro and Delfgou-van Asch22] reported nine (65%) of 14 French soft raw milk cheeses purchased in The Netherlands were positive for L. monocytogenes. Eppert et al. [Reference Eppert23] found 15 (46·9%) of 32 French soft raw milk cheeses sampled on the German market between 1992 and 1994 were positive for L. monocytogenes. Of 30 French soft raw milk cheeses purchased during 1989–1993 in Sweden, 13 (43·3%) were positive for L. monocytogenes [Reference Loncarevic, Danielsson-Tham and Tham9].
In 1995, the first documented outbreak of listeriosis in France, which involved 36 patients, was linked to soft raw milk cheese, Brie de Meaux: the epidemic strain belonged to serovar 4b [Reference Goulet24–Reference De26]. In 1997, another 14 people became ill after consuming the soft raw milk cheese Livarot, Pont l’Évêque (serovar 4b) [Reference de Valk, Vaillant and Goulet25, Reference Jacquet27] and in 1999, three more cases were due to the soft raw milk cheese Epoisses (serovar 4b) [Reference De26]. These cheese brands were available on the Swedish market during 1989–1993 [Reference Loncarevic, Danielsson-Tham and Tham9]. In Belgium in 1997, a case of listeriosis in a 73-year-old immunocompromised man was associated with the consumption of a French Camembert cheese bought a few days before he became ill: the isolates from both patient and cheese shared serovar 1/2a [Reference Gilot28]. In 1998, de Valk et al. [Reference de Valk29] stated ‘soft cheese may account for a substantial proportion of sporadic listeriosis’, and in a case-control study in metropolitan France during 1997, 49% of the sporadic cases of listeriosis studied could be attributed to eating soft cheese [Reference de Valk29].
A majority of the decrease in L. monocytogenes in ready-to-eat foods, including cheese, in France during the 1990s is probably related to stricter control measures implemented during food production [Reference Goulet30]. In cheese production, milking hygiene has improved and cows with L. monocytogenes mastitis are not used for milk production: several further precautionary measures have been implemented in raw milk cheese preparation [Reference de Valk, Vaillant and Goulet25, Reference De26, Reference Sanaa, Coroller and Cerf31]. The number of samples from dairy products contaminated at ⩾100 c.f.u./g decreased by 41% between 1993/1994 and 1995/1996 [Reference Jacquet27]. As ready-to-eat products became less contaminated, the number of listeriosis cases in France decreased by 68% between 1987 and 1997 [Reference Jacquet27]. In France, the annual number of listeriosis cases per million population, fell from 5·2 in 1995 to 3·8 in 1996 [Reference Jacquet27]. Since 1996, serovar 4b has been reduced to the second or third most common serovar in human clinical cases in Sweden.
L. monocytogenes isolates from 1996 to 2010
Cold-smoked and gravad fish and L. monocytogenes serovar 1/2a
There has been a shift in human listeriosis in Sweden and L. monocytogenes serovar 1/2a isolates are now more frequent than serovar 4b. In Finland, serovar 1/2a isolates became dominant in 1991 [Reference Lukinmaa32], but 1/2a was also the major serovar in Sweden in 1988 and 1993. In Canada, a predominant L. monocytogenes serovar 1/2a clone caused human listeriosis cases and outbreaks during 1988–2010 [Reference Knabel33].
Serovar 1/2a is the serovar regularly found in cold-smoked and gravad salmon purchased in Sweden and, L. monocytogenes has been found in gravad and cold-smoked fish samples. In a recent study 14% of each group were positive for the presence of L. monocytogenes, and the National Food Administration of Sweden concluded these products ‘constitute the main problem’ [Reference Thisted34]. A European Union (EU)-wide survey on L. monocytogenes during 2010 and 2011 aimed to estimate the prevalence of L. monocytogenes in hot- or cold-smoked or gravad fish within the EU. Packaged (not frozen) hot- or cold-smoked or gravad fish (3053 batches) from 3632 retail outlets in 26 EU member states and one non-EU country were sampled, both on arrival at the laboratory and at the end of their shelf-life. Across the entire EU, the prevalence in fish samples was 10·4% at the time of sampling and 10·3% at the end of shelf-life. The proportion of samples with a L. monocytogenes count exceeding 100 c.f.u./g was 1% at the time of sampling and 1·7% at the end of shelf-life [35].
Gravad and cold-smoked salmons are vacuum-packed ready-to-eat products with a lengthy best-before date of 4–5 weeks: therefore, they constitute an optimal environment for the facultative anaerobic, psychrotrophic organism Listeria. The consumption of gravad and cold-smoked salmons increased markedly in Sweden during the 1990s [Reference Loncarevic36]. In 1996 in Sweden, 20·7% of gravad and 11·5% of cold-smoked vacuum-packed fish purchased, especially salmon, harboured L. monocytogenes [Reference Loncarevic, Tham and Danielsson-Tham10]. In the UK, a survey of retail cold-smoked fish found 236/1344 (17·4%) products were positive for L. monocytogenes, all samples had a level of <100 c.f.u./g [37]. The current PFGE types found in human cases of invasive listeriosis in Sweden are frequently encountered in vacuum-packed cold-smoked and gravad salmon/rainbow trout [Reference Mandorf11–Reference Kannius and Karlsson13]. In Spain, a cluster of L. monocytogenes isolates in smoked salmon share pulsotype 1, a type recently isolated from clinical cases in Spain [Reference Garrido38]. In Brazil, 41% of salmon samples in a gravlax salmon processing line were positive for L. monocytogenes [Reference Cruz39].
A decrease or stabilization in the number of human isolates sharing serovar 4b and an increasing number of serovar 1/2a isolates have also been identified in other countries, e.g. Italy [Reference Gianfranceschi40] and Switzerland [Reference Pak41].
L. monocytogenes serovar 4b
In Sweden, the decrease in the number of listeriosis cases caused by serovar 4b strains since 1996 might be due to precautionary measures taken during cheese production in France and other European countries. In a study from May to December 1999, only four (4·4%) of 91 French soft raw milk cheeses (red smear) were positive for L. monocytogenes [Reference Rudolf and Scherer42]. Although this represented an improvement, the overall results from analyses of 329 European soft, semi-soft, and hard red-smear cheeses made of raw or pasteurized milk were not encouraging, as 6·4% were positive for L. monocytogenes. Thus, the authors concluded that red-smear cheeses should still be regarded as a considerable public health risk. A EU-wide survey in 2010 and 2011 sampled 3452 cheeses at the end of their shelf-life from retail outlets in 26 EU member states and one non-EU country. The prevalence across the entire EU was only 0·47%. The EU-level proportion of cheese samples with a L. monocytogenes count exceeding 100 c.f.u./g was 0·06% at the end of shelf-life [35]. During the 1990s, other food products were carriers of L. monocytogenes serovar 4b and caused outbreaks in France, such as pork tongue in jelly in 1992 [Reference Jacquet43] and rillettes in 1993 [Reference Goulet44]. However, to our knowledge, those products were not generally imported into Sweden. The National Food Administration of Sweden requested two large national samplings of food products for analysis of L. monocytogenes; the first in 2001 [Reference Rosengren45, Reference Rosengren and Lindblad46] and the second in 2010 [Reference Thisted34]. Products such as pig tongue in aspic and rillettes were not tested by the National Food Administration nor sampled by the different Health Boards in these studies, as they are not popular foods and not generally available from retail outlets.
Historical, seasonal, and geographical variation, 1958–2010
In the collection of 932 human L. monocytogenes isolates from 1958 to 2010, the majority (29·5%) of serovar 1/2a isolates were isolated during October–January, with a peak in October, whereas, most (32·6%) of the serovar 4b and 1/2b isolates were isolated during June–October, with a peak in August. In the USA, more serovar 1/2a isolates are found in water during autumn and winter, whereas, serovar 4b are more often isolated during summer [Reference Orsi, den Bakker and Wiedmann47].
In the north of Sweden (Fig. 1), i.e. Jämtland (Z), Västerbotten (AC) and Norrbotten (BD), cases of listeriosis are registered almost entirely during January–March, and in the south of Sweden, i.e. Skåne (M), the majority of cases are registered during summer and autumn (Fig. 1). Of the most common PFGE types, 1/2a:4A was distributed across 17 counties and 4b:5 was distributed across 16 counties. Conversely, PFGE types 4b:6, indistinguishable from the epidemic cheese-borne strains in Switzerland, Denmark, and USA (1983–1990), and PFGE types 4b:3 and 1/2a:4b were concentrated in Götaland (south region of Sweden), possibly due to its proximity to the European continent. In Norrland (north region of Sweden), PFGE type 4b:6 was reported in only one county, type 4b:3 was reported in three counties and 1/2a:4B in two counties (Table 1).
Some PFGE types are present over long periods, such as 1/2b:75A (1970–2010) and 4b:5 (1965–2008), whereas, others appear during limited periods, such as 1/2a:53 (1985–1995) and 1/2a:11B (1990–1994). The two currently dominant PFGE types in Sweden, 1/2a:4A and the closely related 1/2a:4B, were almost non-existent during the 1960s and 1970s (Table 2). Lukinmaa et al. [Reference Lukinmaa32] suggest a PFGE closely related to the Swedish type 1/2a:4A was common in sporadic cases in Finland from 1994 onwards. Similarly, Clark et al. [Reference Clark48] state isolates of one unique type were not seen before 2002 in Canada, but have been detected in each subsequent year. Emerging PFGE types could reflect a change in dietary habits [Reference Lukinmaa32].
Relationship between PFGE type and serovar
Generally, all L. monocytogenes isolates belonging to one PFGE type share the same serovar. However, isolates within four PFGE types were distributed in either serovar 1/2a or 1/2c, i.e. 1/2:12A (six 1/2a, nine 1/2c isolates), 1/2:12B (four 1/2a, two 1/2c), 1/2:93 (two 1/2a, two 1/2c), and PFGE type 1/2:105 (one 1/2a, one 1/2c). Although correlation between ‘pulsotypes and serotypes’ is reported [Reference Gianfranceschi40], few L. monocytogenes isolates with indistinguishable PFGE profiles displaying different serovars (1/2a and 3a [Reference Lukinmaa32], 3b and 1/2b [Reference Revazishvili49]) have been identified.
Age and gender, 1958–2010
Women dominated the 20–49 years age group, even though pregnant women were excluded as they are more sensitive to L. monocytogenes [Reference Okike, Lamont and Heath50]: in the age range 50–89 years, the majority (59·4%) of cases were male (Table 5). Predominance of males has also been reported by Schlech [Reference Schlech1]. Pregnancy-associated cases decreased over time (Table 6) similarly to some other countries, where decreased or constant numbers have been reported [Reference Goulet51]. The decrease in Sweden is possibly due to dietary recommendations from the National Food Administration which has provided advice on food to pregnant women since the early 1990s. According to the dietary advice, pregnant women should avoid gravad and smoked fish at the end of its shelf-life, cheeses made from unpasteurized milk, and even some soft cheeses made from pasteurized milk, and sliced cold cuts and cold foods at the end of their shelf-life. The preponderance of serovar 4b in pregnancy-associated isolates is in agreement with other studies [Reference Goulet51–Reference Doorduyn53]. However, there was an increase in listeriosis in Sweden in the older age group (⩾60 years) which constituted 11·1% of all cases reported during 1958–1969; 33·7% in 1970–1979; 50·3% in 1980–1989; 66·2% in 1990–1999; and 75·5% in 2000–2010 (Table 6). Even in other European countries there is an increased incidence of listeriosis in the elderly population aged >60 years [Reference Goulet51].
Table 5. Distribution of L. monocytogenes isolates in Sweden by gender and age during 1958–2010
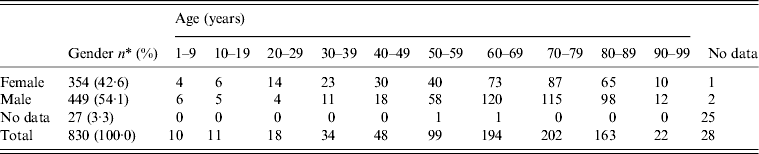
* Pregnant and newborns are excluded.
Table 6. Serovar distribution of L. monocytogenes isolates in pregnant-associated cases and the elderly in Sweden, by decade, during 1958–2010

CONCLUSION
Between 1972 and 1995, human listeriosis in Sweden was mainly caused by serovar 4b, possibly due to the consumption of raw soft cheese imported from European countries. The number of cases caused by serovar 4b has decreased due to improved cheese-production hygiene, but since 1996, serovar 1/2a has been the dominant serovar. Based on the serovars and the PFGE types identified, an association between human cases of listeriosis and the consumption of vacuum-packed gravad and cold-smoked salmon is suggested.
ACKNOWLEDGEMENTS
This study was financially supported by the Research Foundation of Ivar and Elsa Sandberg, to whom we express our gratitude.
DECLARATION OF INTEREST
None.









