Torsades de pointes is a polymorphic form of ventricular tachycardia that is usually associated with QT interval prolongation and may lead to cardiac arrest and sudden death. QT interval prolongation may occur as congenital or acquired manner. Drug utilisation and electrolyte abnormalities are the most common causes of acquired QT interval prolongation. Fluconazole is an anti-fungal drug reported to cause QT interval prolongation and torsades de pointes in adults. Reference Khazan and Mathis1–Reference Wassmann, Nickenig and Bohm4 A medically complicated case of a child who developed ventricular arrhythmia resulting with torsades de pointes, while using multiple drugs including fluconazole has been reported. Reference Esch and Kantoch5 To the best of our knowledge, a torsades de pointes case developed in childhood due to fluconazole and rapidly resolved after drug discontinuation has not been encountered in the literature. We present the case of a 16-year-old girl with pneumonia who developed prolonged QT-associated torsades de pointes on the sixth day of fluconazole treatment.
Case report
A 16-year-old female patient presented to the paediatric emergency service of our hospital with fever, phlegmy cough, and increased respiratory tract secretions. It was learned that she had diffuse axonal injury due to an accident occurred a year ago, using baclofen for spasticity and feeding by gastrostomy. The child had no history of arrhythmia. The family history was negative for arrhythmia, syncope, and sudden cardiac death. Respiratory examination revealed abnormal breathing sounds. Heart sounds were normal and rhythmic, no additional voices and murmurs were detected. She was hospitalised with the diagnosis of complicated pneumonia. Combined antibiotics of vancomycin and ceftriaxone were initiated. Due to persistent fever, on tenth day, fluconazole was added to treatment on an empiric basis.
On day 6 of fluconazole treatment, premature ventricular contractions and a prolonged QT interval (QTc = 560 ms) were observed on the monitor. Several hours later, early afterdepolarizations turned into torsades de pointes ventricular tachycardia on electrocardiogram monitoring (Fig 1). Electrolytes, liver and kidney functions, and troponin values were normal. No signs of myocardial injury were detected. Cardiovascular examination and echocardiogram were normal. Intravenous lidocaine was administered with the loading dose of 1 mg/kg, followed by an infusion of 25 μg/kg/min. Bigeminy ventricular beats and nonsustained ventricular tachycardia were detected in the 24 h Holter monitoring. Therewith, the drugs used by the patient were reviewed. Fluconazole was discontinued. Over the following 24 h, ventricular arrhythmia dissolved and sinus rhythm (QTc interval of 540 ms) was observed in electrocardiogram recording. Lidocaine infusion was ceased and propranolol was started at a dose of 2 mg/kg/day.
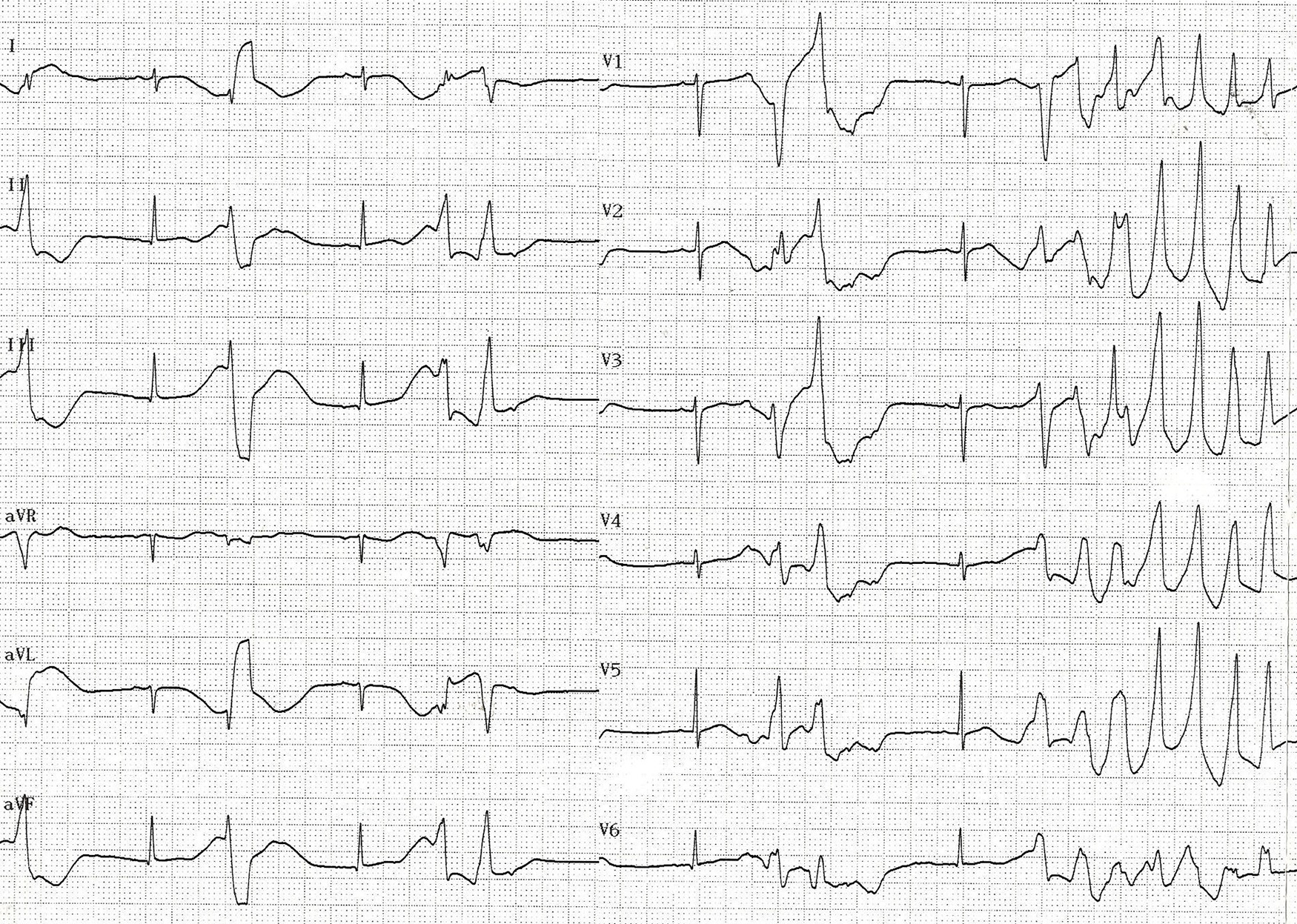
Figure 1. Electrocardiogram showing “torsades de pointes” ventricular tachycardia following early after depolarisations.
Five days later, an electrocardiogram recording showed sinus rhythm with a 402 msQTc (Fig 2) and no arrhythmia was detected in the 24-h Holter monitoring. During this period, the other antimicrobials and baclofen were not discontinued. The patient’s arrhythmia did not recur in the remainder of the hospital admission.

Figure 2. Electrocardiogram record after discontinuation of fluconazole shows disappearance of ventricular arrhythmia.
Discussion
Torsades de pointes was defined by François Dessertenne in 1966 as polymorphic ventricular tachycardia associated with prolonged QT interval. Reference Dessertenne6 Prolongation of the QT interval and development of torsades de pointes are the result of abnormal function of the ion channels and related proteins involved in the repolarization process in ventricular myocytes. Reference Dessertenne6
The risk of torsades de pointes associated with non-cardiac drugs is low. Although mild delays in ventricular repolarization caused by proarrhythmic drugs are often not clinically recognised, risk factors including congenital long QT syndrome, clinically significant bradycardia or heart disease, electrolyte imbalance, impaired liver/kidney function, female gender, advanced age, and concurrent treatment with drugs known to have potential for pharmacokinetic/pharmacodynamic interactions may increase torsades de pointes risk. Reference Owens7 In our patient, there were no risk factors other than female gender. Also, the patient had received baclofen for spasticity. Although baclofen overdose may result in cardiac effects, it has not been reported to cause ventricular arrhythmia.
Triazole antifungal drugs may induce torsades de pointes by affecting cardiac repolarisation, as well as inhibiting hepatic metabolism of other QT prolonging drugs. Reference De Ponti, Poluzzi, Cavalli, Recanatini and Montanaro8 Co-administration of drugs that inhibit CYP3A4 may lead to supratherapeutic drug levels and thus an increased risk of QT prolongation and torsades de pointes. Reference Kao and Furbee9 In our patient, no concomitant drugs with known QT-prolonging potential were received. To the best of our knowledge, pharmacokinetic/pharmacodynamic interactions have not been reported between concomitant drugs and fluconazole. In our case, no possible aetiology was found except for the use of drug that could explain QT prolongation and arrhythmia. After discontinuation of fluconazole, complete elimination of arrhythmia and normalization of the QT interval strongly suggest fluconazole as the aetiology.
Little is also known about the time between starting an azole antifungal drug and the development of torsades de pointes. Salem et al stated that the average time between fluconazole use and the development of torsades de pointes was 4 days. Reference Salem, Reichlin, Fasel and Leuppi-Taegtmeyer10 In our case, there was a temporal relationship between the initiation of fluconazole and torsades de pointes. Torsades de pointes occurred on the sixth day of the treatment and ended a day after fluconazole was discontinued.
In conclusion, this case demonstrates the potential proarrhythmic effect of fluconazole in a child. In case of QT prolongation, drug treatments should be reviewed, drugs with proarrhythmia potential should be discontinued, and drug interactions should be avoided. It should be kept in mind that fluconazole may lead to torsades de pointes by causing prolongation of the QT interval and patients should be monitored by serial electrocardiogram.
Acknowledgements
None.
Financial support
No financial support was received for this submission.
Conflict of interest
None.
Ethical standards
The research does not involve human and/or animal experimentation.





