Observational studies have shown that a high intake (more than 15 % of daily energy intake) of SFA is associated with high blood concentrations of cholesterol and high CHD mortality(Reference Keys, Menotti and Aravanis1, Reference Kromhout, Bloemberg and Feskens2). However, a recent meta-analysis of prospective epidemiological studies suggested that there is no significant evidence for concluding that dietary saturated fat is associated with an increased risk of CHD or CVD(Reference Siri-Tarino, Sun and Hu3). In addition, there is good evidence that not all dietary SFA are necessarily associated with a negative impact on atherosclerosis biomarkers and that their specific identity, individual intake level and dietary origin must be considered(Reference Rioux and Legrand4–Reference Parodi7). For instance, medium-chain SFA (MCFA) have been shown not to increase blood cholesterol concentrations in human subjects(Reference Hu, Stampfer and Manson8). Furthermore, TAG composed of MCFA (Medium-chain TAG) can be used as a readily available energy source for quick energy, which may lead to a decrease in glucose requirements. Also, because MCFA are not incorporated into chylomicrons, they are less likely to be stored in adipose tissues. Therefore, structured lipids containing both essential fatty acids and MCFA may be useful to target specific diseases and metabolic conditions(Reference Akoh9).
Reactive oxygen species (ROS) can cause cell and tissue damage and lipid peroxidation, leading to impaired cellular function and alterations in the physico-chemical properties of cell membranes, which in turn disrupt vital functions(Reference Drew and Leeuwenburgh10). MCFA are likely to be highly resistant to peroxidation(Reference Bray, Lee and Bray11, Reference Bach and Bababayn12). Antioxidant enzymes provide protection against ROS and, similar to many other biochemical systems, their effectiveness varies with the stage of development and other physiological aspects of the organism(Reference Halliwell and Gutteridge13, Reference Livingstone, O'hara, Frettsome, Atkinson and Thorndyke14). The most important antioxidant enzymes are superoxide dismutase, catalase and glutathione peroxidase(Reference Halliwell and Gutteridge13). Thus, the extent of lipid peroxidation is likely to depend in part on both the activity of antioxidant enzymes and the mix of fatty acids present in a target for peroxidation(Reference Parihar and Dubey15).
In a previous study(Reference Sengupta and Ghosh16), we observed that the ingestion of MCFA along with essential fatty acids improves platelet aggregation and haematological parameters of hypercholesterolaemic rats. In the present study, we investigate the effect of capric acid (10 : 0), a MCFA, added to mustard oil on plasma lipids, antioxidant enzymes and lipid peroxidation in normal and hypercholesterolaemic rats.
Materials and methods
Chemicals and enzymes
Mustard oil was extracted from brown mustard seeds by the solvent extraction method and physically refined and bleached. Capric acid was procured from Sigma Chemical Company, St Louis, MO, USA. Lipase TLIM (Thermomyces lanuginosus) was a gift from Novozymes India Private Limited, Bangalore, Karnataka, India. All other reagents used were of analytical grade and procured from Merck India Limited, Mumbai, India.
Preparation of experimental oil
Capric acid-enriched mustard oil was prepared by the reaction between capric acid and mustard oil in a packed bed bioreactor using lipase TLIM as a catalyst(Reference Sengupta, Pal and SilRoy17). Unreacted fatty acid was separated from the oil by vacuum distillation.
Chromatographic analysis of oils
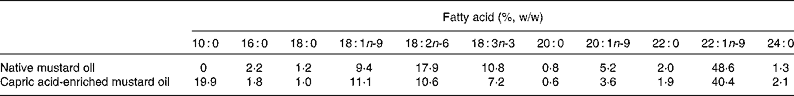
Fatty acid compositions of native and capric acid-enriched mustard oil were analysed by GC. The oils were saponified with 0·5 m-KOH and methylated with boron trifluoride in methanol. The gas chromatograph (Agilent 6890 N; J&W Scientific, Wilmington, DE, USA) was fitted with a DB-Wax capillary column (30 m × 0·32 mm × 0·25 μm) and a flame ionisation detector. N2, H2 and airflow rate were maintained at 1, 30 and 300 ml/min, respectively. Inlet and detector temperatures were kept at 250°C and the oven temperature was programmed to increase from 150 to 190°C at a rate of 15°C/min, then to hold for 5 min, and then to increase to 230°C at a rate of 4°C/min, and then again to hold for 10 min. The fatty acid compositions of the native and capric acid-enriched mustard oils are shown in Table 1. The capric acid-enriched oil contained 19·9 % of fatty acids as capric acid.
Table 1 Fatty acid compositions of native and capric acid-enriched mustard oils
Feeding experiment
The work was done under the supervision of the Animal Ethical Committee of the Department of Chemical Technology (University of Calcutta). Charles Foster male albino rats were housed in individual cages. The rats were acclimatised for 2 weeks while receiving free access to water and to a standard laboratory diet. For the duration of the study, the rats were exposed to a 12 h light–12 h dark cycle. Rats weighed 80–100 g at the start of the feeding experiment. They were divided into four groups, each consisting of six animals. Each group was fed a different diet. Diets contained native mustard oil (200 g/kg diet), native mustard oil (200 g/kg diet) plus cholesterol (10 g/kg diet), capric acid-enriched mustard oil (200 g/kg diet) or capric acid-enriched mustard oil (200 g/kg diet) plus cholesterol (10 g/kg diet). The diets were prepared weekly and stored at − 20°C. The dietary composition is shown in Table 2.
Table 2 Proximate composition of the diets
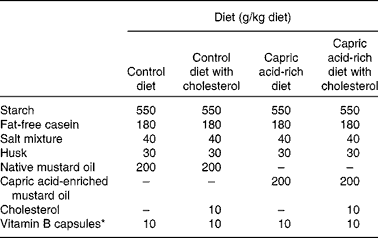
* Number of capsules used per kg diet.
Sample collection
After 30 d of the dietary treatments, rats were fasted for 12 h. Then they were anaesthetised with chloroform and killed. Blood samples were collected from the abdominal aorta into EDTA tubes and centrifuged at 1500 rpm for 15 min to separate the erythrocytes and plasma. Liver and brain were harvested, weighed and stored at − 20°C until further analysis.
Analysis of plasma lipid concentrations
Total cholesterol, HDL-cholesterol and TAG concentrations were determined using enzyme kits supplied by Merck India Limited.
Enzyme assays
Measured amounts of liver and brain were homogenised in phosphate buffer. The samples were then centrifuged and the supernatants were used for the enzyme assay. The activity of catalase was determined spectrometrically by the method of Aebi(Reference Aebi18). Superoxide dismutase activity was assayed by measuring the auto-oxidation of haematoxylin as described by Martin et al. (Reference Martin, Dailey and Sugarman19). Reduced glutathione (GSH) was determined by the method of Ellman(Reference Ellman20). Total activity of glutathione peroxidase (EC.1.11.1.9.) was determined in the tissue homogenates and plasma according to Flohe & Günzler(Reference Flohe and Günzler21). All enzyme activities are expressed as enzyme units per mg protein. Protein content was determined using the method of Lowry et al.(Reference Lowry, Rosebrough and Farr22).
Products of lipid peroxidation
For lipid peroxide measurement, approximately 1 g of liver or 0·4 ml of plasma was placed into a glass centrifuge tube (70 ml) for 2 min in a solvent mixture consisting of 10 ml chloroform and 20 ml methanol, and homogenised on ice. Then, 10 ml of chloroform was added and homogenisation continued for another 30 s. Finally, 10 ml of redistilled water were added and the mixture was homogenised for 30 s. The tubes were then centrifuged for 20 min at 4000 rpm, and the chloroform layer was separated(Reference Bligh and Dyer23). Thiobarbituric acid-reactive substances were measured according to the method described by Schmedes & Hølmer(Reference Schmedes and Hølmer24). Malondialdehyde (MDA) concentration was calculated by taking the extinction coefficient of MDA to be 1·56 × 105/m cm(Reference Dhar, Bhattacharyya and Bhattacharyya25).
Statistical analysis
All the data are presented as means with their standard errors. Statistical comparisons between groups were performed using Student's t test.
Results
Effect of the different diets on plasma lipid concentrations
In the absence of added dietary cholesterol, plasma total cholesterol, non-HDL-cholesterol and TAG concentrations were lower in rats fed the capric acid-enriched mustard oil compared with those fed the native mustard oil (Table 3). Conversely, HDL-cholesterol concentration was higher in rats fed the capric acid-enriched mustard oil (Table 3). Adding cholesterol to the diet increased plasma total cholesterol, non-HDL-cholesterol and TAG concentrations and decreased HDL-cholesterol concentration (Table 3). However, the plasma lipid profile was better when rats received the capric acid-enriched mustard oil plus cholesterol compared with those that received the native mustard oil plus cholesterol (Table 3).
Table 3 Plasma lipid concentrations in rats fed the different diets
(Mean values with their standard errors for six rats per diet group)
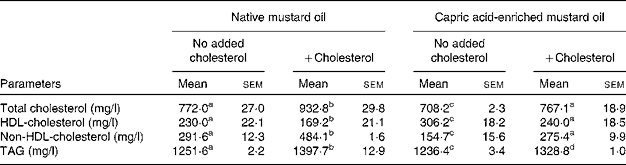
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Antioxidant enzyme activities
The same pattern of effect of the diets was seen on antioxidant enzyme activities in both liver and brain (Tables 4 and 5). In the absence of added dietary cholesterol, antioxidant enzyme activities were much higher in the liver and brain of rats fed the capric acid-enriched mustard oil compared with those fed the native mustard oil (Tables 4 and 5). Adding cholesterol to the diet decreased the activities of all enzymes, although the effect was not significant in all cases. However, all enzyme activities were higher in liver and brain when rats received the capric acid-enriched mustard oil plus cholesterol compared with those that received the native mustard oil plus cholesterol (Tables 4 and 5). The highest enzyme activities were always seen in the group receiving the capric acid-enriched mustard oil. Interestingly, enzyme activities were often higher in tissues of rats fed the capric acid-enriched mustard oil plus cholesterol than in those fed the native mustard oil.
Table 4 Antioxidant activity of liver tissue
(Mean values with their standard errors for six rats per diet group)
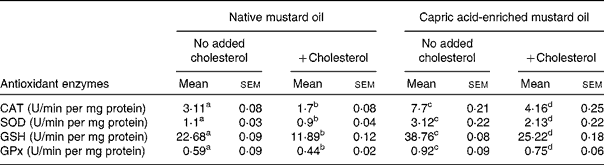
CAT, catalase; SOD, superoxide dismutase; GSH, reduced glutathione; GPx, glutathione peroxidase.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 5 Antioxidant activity of brain tissue
(Mean values with their standard errors for six rats per diet group)
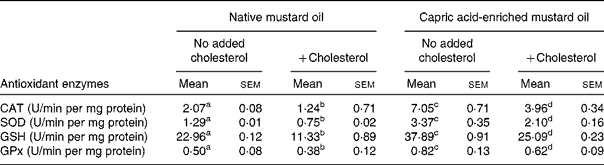
CAT, catalase; SOD, superoxide dismutase; GSH, reduced glutathione; GPx, glutathione peroxidase.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Lipid peroxidation
The same pattern of effect of the diets was seen on MDA concentrations in liver, brain and plasma (Table 6). In the absence of added dietary cholesterol, MDA concentrations were much lower in tissues and plasma of rats fed the capric acid-enriched mustard oil compared with those fed the native mustard oil (Table 6). Adding cholesterol to the diet increased MDA concentrations. However, MDA concentrations were lower when rats received the capric acid-enriched mustard oil plus cholesterol compared with those that received the native mustard oil plus cholesterol (Table 6). The highest MDA concentrations were always seen in the group receiving the native mustard oil plus cholesterol.
Table 6 Lipid peroxidation of liver and brain homogenates and plasma peroxidation
(Mean values with their standard errors for six rats per diet group)
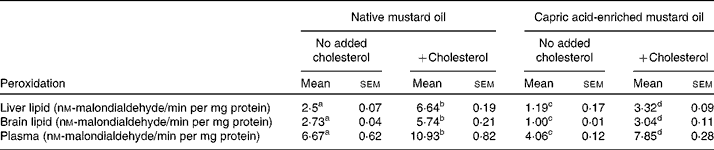
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
The capric acid-enriched mustard oil comprised almost 20 % of fatty acids as capric acid. The increased capric acid content was accommodated by a reduction in the content of several other fatty acids including linoleic, α-linolenic and erucic acids. Thus, the modified oil had a lower content of unsaturated fatty acids and PUFA.
Feeding rats a diet containing cholesterol resulted in the anticipated adverse profile of blood lipids. In human subjects, this adverse profile increases the risk of CHD(Reference Kannel, Castelli and Gordon26–Reference Gotto, Gorry and Thompson29). The present study indicated that oil enriched with capric acid resulted in an improvement in the blood lipid profile even when cholesterol was present in the diet. Thus, capric acid may be useful to lower disease risk.
Hypercholesterolaemia increases the levels of the lipid peroxidation product MDA in the blood and aortic tissue(Reference Prasad and Kalra30, Reference Prasad and Kalra31). This suggests that hypercholesterolaemia induces oxidative stress. Indeed, the ROS-producing activity of polymorphonuclear leucocytes is increased in hypercholesterolaemia(Reference Prasad and Kalra31). Various factors have been implicated in the release of ROS and polymorphonuclear leucocytes during hypercholesterolaemia(Reference Freeman and Crapo32). ROS exert their cytotoxic effects by causing peroxidation of unsaturated fatty acids of membrane phospholipids, which can result in an elevation in membrane fluidity and permeability and loss of cellular integrity(Reference Meerson, Kagon and Kozlov33, Reference Frank and Massaro34). Here, we show that the inclusion of capric acid in the diet decreases MDA concentration even in the presence of hypercholesterolaemia, suggesting that oxidative stress was presumably lower in animals fed that diet.
The activities of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase form the first line of defence against ROS(Reference Devi, Prasad and Saraswathi35), and the decrease in these activities seen in rats fed cholesterol most probably contributes to the observed oxidative stress (i.e. the increased MDA concentration). Since antioxidant enzymes play an important role in controlling lipid peroxidation(Reference Devi, Prasad and Saraswathi35), an increase in the activities of these enzymes can delay the progression of atherosclerosis. In the present study, the antioxidant enzyme activities were increased when capric acid was fed even when hypercholesterolaemia was induced. Restoration of the activities of these enzymes in the liver and brain tissue with capric acid may be due to the increased intracellular concentration of the non-enzymatic antioxidant GSH, whose level was decreased in the liver and brain of rats with hypercholesterolaemia. GSH was measured, expressed as U/min per mg protein. Capric acid increased GSH levels in both liver and brain tissues. GSH is one of the body's most important endogenous antioxidants responsible for free radical scavenging in all cell types(Reference Busse, Zimmer and Schnopohl36, Reference Arivazhagan, Juliet and Panneerselvam37). Thus, capric acid treatment offers increased antioxidant protection to hepatic tissue even during hypercholesterolaemia.
We interpret the present findings to demonstrate that capric acid is responsible for improved blood lipid profile, enhanced antioxidant defences and decreased lipid peroxidation. However, it must also be recognised that the capric acid-rich oil contained less PUFA than the native oil.
On the basis of the data presented here, we propose that dietary supplementation with capric acid might benefit humans, especially leading to improved antioxidant defences in individuals with hypercholesterolaemia and thereby lowering atherosclerosis risk.
Acknowledgements
The authors thank Dr Santinath Ghosh, Department of Chemical Technology, University of Calcutta for his support. M. G. conceived the study and supervised the work; A. S. conducted the experiments. Financial support was obtained from the University of Calcutta (through UPE projects). The authors declare that they have no conflict of interest.








