INTRODUCTION
Panton–Valentine leukocidin (PVL) has been extensively studied to better understand its contribution to the pathogenic potential of Staphylococcus aureus in humans [Reference Alonzo and Torres1]. The importance of necrotizing pneumonia caused by PVL-producing strains of S. aureus was first pointed out by Gillet et al. in 2002, who described a group of 16 patients presenting with this disease [Reference Gillet2]. To date about 100 cases of necrotizing pneumonia caused by S. aureus have been reported in the literature mostly in individual case reports or in small series. Meta-analyses comprising several cases from such sources [Reference Kreienbuehl, Charbonney and Eggimann3–Reference Vardakas, Matthaiou and Falagas6] as well as a few larger studies [Reference Gillet7, Reference Lopez-Aguilar8] have recently been published. The present study provides unique information of this disease in Central Europe through the uniform and detailed processing of the collected data.
MATERIALS AND METHODS
Data source and collection
The Czech National Reference Laboratory for Staphylococci (NRLS) permanently collects staphylococcal strains isolated from patients with severe infections across the country, together with clinical data on the disease course. According to the sustained surveillance programme, local microbiological laboratories in the Czech Republic are requested to send S. aureus strains from patients with particularly severe and/or atypical forms of infections to the NRLS for detailed analysis. All strains that were sent to the NRLS were included in the study.
PVL-producing strains from patients hospitalized for pneumonia were prospectively collected in the period from December 2007 to December 2013. In the Czech Republic, the National Reference Laboratories may request patients’ medical records including discharge and/or autopsy reports. Additional clinical data were collected from attending clinicians and general practitioners through a standardized telephone interview.
Phenotyping of bacterial strains
Conventional biochemical tests, commercial test kits API Staph (bioMérieux, France), and MALDI-TOF mass spectrometry (Bruker Daltonics, Germany) were used for identification and phenotypic characterization of staphylococcal strains. Strains were assayed by reversed passive latex agglutination (Denka Seiken, Japan) for the production of staphylococcal toxic shock syndrome toxin (TSST-1), exfoliative toxins A and B, and enterotoxins A (E), B, C, and D. Antibiotic susceptibility was tested for 14 antibiotics using the disc diffusion method (Antimicrobial Discs, Oxoid, UK) and inhibition zones were interpreted according to the EUCAST breakpoints [9]. Phage-typing was performed by the standard method using the international typing set of phages (Public Health England, London, UK).
Genotyping of bacterial strains
All isolates were genotyped by pulsed-field gel electrophoresis (PFGE) after SmaI digestion, staphylococcal protein A gene (spa) typing using StaphType software v. 2.2.1 (Ridom, Germany), multilocus sequence typing (MLST) using BioNumerics v. 6.6 (Applied Maths, Belgium) MLST online plugin, agr group determination [Reference Tenover10], SCCmec typing [Reference Milheirico, Oliveira and de Lencastre11], plasmid content analysis [Reference Kuntová12], and prophage typing [Reference Kahánková13]. The genes for PVL [Reference Lina14], leukocidin lukED [Reference Yamada15], staphylococcal enterotoxins [Reference Lovseth, Loncarevic and Berdal16], enterotoxin gene cluster (EGC) [Reference Jarraud17], exfoliative toxin genes [Reference Růžičková18], immune evasion cluster (IEC) [Reference van Wamel19], and arginine catabolic mobile element (ACME) [Reference Diep20] were screened using polymerase chain reaction (PCR) or multiplex PCR.
Statistical analysis
The strength of association between risk factors and patients’ survival was assessed by relative risk (RR) and the corresponding 95% confidence interval (CI). Fisher's exact test was used to test differences in the proportion of deaths between subgroups. All statistical tests were evaluated as two-sided at a significance level of 0·05. To compare the occurrence of disease during the seasons, an exact conditional test of the hypothesis that the ratio of two Poisson rates equal to one was used. Statistical analyses were performed by Stata software, release 9.2 (Stata Corp LP, USA).
Ethical standards
The study was not interventional. It was approved by the Ethic Committee at the National Institute of Public Health, Prague (3986/2014) who judged that there was no need for informed consent to be obtained for this study.
RESULTS
Patients’ data and prospective study of cases
Twelve cases of necrotizing pneumonia caused by PVL-producing S. aureus were collected from throughout the Czech Republic during the 6-year period. The reports came from different cities and no epidemiological link was found between them. The patients’ characteristics and course of the disease are given in Tables 1 and 2. The male/female ratio was 5/7 (age range 4 months to 59 years, median 25 years). Most cases occurred in the winter season, mainly in December and January which was statistically significant (P = 0·0002). The diagnosis of necrotizing pneumonia was confirmed by computed tomography imaging in surviving individuals and by autopsy in seven patients who died. In all patients, pneumonia affecting both lungs and abscess formation/necrosis/tissue breakdown was noted; pleural effusion occurred in 10 patients.
Table 1. Necrotizing pneumonia patients' characteristics and medical histories

* Stress is considered as a possible cause of temporary immunosuppression.
† Flu-like symptoms: fever, chills, myalgia, headache, dry cough.
Table 2. Course of necrotizing pneumonia and outcome

MOF, Multi-organ failure; MODS, multi-organ dysfunction syndrome; CMVI, cytomegalovirus infection; TA, tracheal aspirate; CSF, cerebrospinal fluid; BAL, bronchoalveolar lavage; n.a., not applicable.
* Patients with primary pneumonia.
† Marked left shift.
None of the patients had severe underlying diseases. The early clinical symptoms were diverse and non-specific in most patients: fever with myalgia, headache, back pain, or gastrointestinal disorder were present in 10/12 patients 1–2 days before admission. Two patients (nos. 3 and 9) developed diarrhoea and yielded S. aureus isolates producing enterotoxin A. The course of pneumonia was always very rapid, leading to early sepsis and/or septic shock in all but one patient (no. 7).
Two subgroups of patients could be distinguished with respect to clinical presentation: (i) seven patients (nos. 1–5, 11, 12) developed primary severe pneumonia without pre-existing staphylococcal or pyogenic infection elsewhere in the body; (ii) in four patients (nos. 6, 7, 8, 10), skin and soft tissue infection preceded the pneumonia and PVL-producing S. aureus was isolated from the primary site of infection in three of these. The remaining patient (no. 9) was an intravenous drug user with right-sided endocarditis and haematogenous abscesses in both lungs. S. aureus invaded the bloodstream in all study patients as evidenced at autopsy in patient no. 11 and by positive blood culture in the others.
The source of S. aureus remained unknown in 11/12 patients. Only patient no. 3 was reported to have a history of close contact with a purulent infection (his mother had otitis). With the exception of patient no. 1, all cases were clearly community-acquired infections.
Therapy
For all but one patient, antibiotic therapy was started with various β-lactams, in some cases in combination with other antibiotics. The exception was patient no. 1 who worked in a hospital as a microbiologist. Staphylococci were identified by microscopic examination of her sputum immediately after she was hospitalized. In view of the possibility of methicillin-resistant S. aureus (MRSA) sepsis, she was given intravenous vancomycin and gentamicin on the first day. On the next day, she was diagnosed with severe pneumonia and therapy was switched to linezolid + gentamicin + rifampicin. No other patient was treated with linezolid or clindamycin within the first 2 days after admission because the aetiology had not yet been determined and the therapy was targeted at sepsis and/or community-acquired pneumonia caused by common pathogens. No patient received intravenous immunoglobulin.
Patients who survived recovered slowly, partly because of polymyoneuropathy after prolonged intensive care, and partly due to severe lung disease. Nevertheless, even patients who had severe lung damage eventually recovered, and the survivors returned to a good quality of life.
Prognostic factors
The mortality of necrotizing pneumonia in this study was high (7/12, 58%). However, six of the seven patients who died had primary pneumonia compared with one of the remaining five patients who presented with secondary pneumonia as a complication of staphylococcal/pyogenic infection elsewhere in the body. This difference was of borderline statistical significance (RR 4·29, 95% CI 0·72–25·39, P = 0·072) (Table 2).
The Czech NRLS has been monitoring systematically the prevalence of genes for PVL in S. aureus strains since 2004, and such genes were found in 6·1% of 7027 strains examined up to the end of 2013. Notably, of the PVL-positive strains, 25·3% were classified as methicillin resistant.
Bacterial isolates and their characteristics
The phenotypic and molecular characteristics of the 12 study strains are shown in Table 3. Four strains produced enterotoxin A, and one each enterotoxins B and C. Seven strains were susceptible to all antimicrobials tested and four were MRSA. Phage-typing revealed diverse patterns with four non-typable strains. Genotyping showed substantial variability among the strains and assigned the majority to five clonal complexes (CC8, CC15, CC30, CC80, CC121). Eight strains were unassigned by SCCmec typing, and in seven different MLST types, three each were of ST8 or ST121, and two of ST15. All strains isolates carried PVL-encoding genes but lacked the TSST-1 gene and exfoliative toxin A or B genes.
Table 3. Phenotypic and genotypic characteristics of PVL-positive S. aureus strains from patients with necrotizing pneumonia
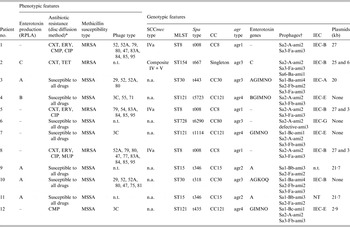
RPLA, Reversed passive latex agglutination; MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; CA-MRSA, community-acquired methicillin-resistant S. aureus; MLST, multilocus sequence type; CC, clonal complex; IEC, immune evasion cluster [Reference van Wamel19]; n.t., non-typable; n.a., not applicable.
* Tested antibiotics: CXT, cefoxitin; ERY, erythromycin; COT, cotrimoxazole; CMP, chloramphenicol; TET, tetracycline; CLI, clindamycin; GEN, gentamicin; VAN, vancomycin; CIP, ciprofloxacin; RIF, rifampicin; LZD, linezolid; FUS, fusidic acid; TGC, tigecycline; MUP, mupirocin.
† Integrase type-head-tail type-amidase type [Reference Kahánková13].
Three (33%) of the MRSA strains from patient nos. 1, 5, and 8 carried the ACME-related arcA gene, exhibited the USA300 PFGE banding patterns, and the latter two strains were similar in plasmid profile to the USA300-HOU-MR clone [Reference Highlander21]. The fourth MRSA strain was of the very rare sequence type ST154 in MRSA and also harboured a unique composite SCCmec element carrying both the ccrA2B2 and ccrC gene complexes and class B mec gene complex.
One methicillin-susceptible S. aureus (MSSA) strain (patient no. 6) belonged to the CC80 group which also includes the European PVL-positive MRSA ST80 clone. The affiliation with this group was also confirmed by the presence of the exfoliative toxin D gene (not shown). This clone has been reported to be highly associated with skin infections [Reference Witte22] which correlates with patient no. 6′s recollection of a chronic furuncle in the past. Three MSSA strains from patient nos. 4, 7, and 12 belonging to CC121 were genetically similar but not identical. These strains harboured the Sa3 phage known to be associated with IEC type E and a complete cluster of enterotoxin genes (G, I, M, N, O). Two other MSSA strains of CC30 group (patient nos. 3 and 10) harboured three prophages, which were probably associated with the presence of PVL and IEC coding genes. These strains were the only ones to lack the lukED genes. The remaining two strains of CC15 (patient nos. 9 and 11) were genetically identical by all the parameters tested and were the only ones undistinguishable by PFGE.
DISCUSSION
Necrotizing pneumonia caused by PVL-producing strains of S. aureus is a rare disease. Its course is unexpectedly rapid and severe, and as a consequence it is expected that the majority of cases are examined for aetiology and reported to the health authorities. As the population of the Czech Republic is about 10 million, the annual incidence of the disease appears to be close to 0·02 cases/100 000 inhabitants based on surveillance data but this cannot be confirmed due to the lack other data sources.
The case mortality in this study is similar to other reports [Reference Löffler5, Reference Hageman23]. The predictors of poor prognosis in our patients seem to be: age <25 years, primary pneumonia, leukopenia, and thrombocytopenia. However, the small number of patients makes it difficult to assess the strength of association of these factors with a poor prognosis. S. aureus necrotizing pneumonia affects mainly young and previously healthy individuals including children and infants [Reference Kreienbuehl, Charbonney and Eggimann3, Reference Li4, Reference Gillet7, Reference Francis24–Reference Montagnani26]. It occurs mainly in the winter season as observed here and by other studies [Reference Francis24, Reference Dabrera27–Reference Reed29], but to our best knowledge none of these associations have been supported by statistical evidence. Synergy between S. aureus and influenza virus has been suggested [Reference Löffler5, Reference Hageman23] and pneumonia caused by PVL-producing S. aureus is assumed to follow influenza infection but often only in a minority of cases [Reference Li4, Reference Rouzic30, Reference Denison31]. Unfortunately, the study patients were not examined to prove/exclude a viral aetiology because their clinical course was highly suggestive for severe sepsis. However, a direct association with influenza may be coincidental because the staphylococcal pneumonia cases described here peaked in December and January while the influenza season peaks in February.
Clinically, necrotizing pneumonia is very difficult disease to diagnose early. Although haemoptysis has been noted by others to be a frequent and relatively specific manifestation of the infection [Reference Löffler5, Reference Hageman23], we found only one patient presenting with this symptom although one other complained of pink-stained sputum. By contrast, one patient developed haematemesis due to extensive vomiting on admission and was diagnosed with Mallory–Weiss syndrome at autopsy. Only one patient with rapid primary staphylococcal pneumonia survived and we believe that the early detection of staphylococci in the tracheal aspirate by direct microscopy was crucial in saving this patient's life. It is therefore very important to develop diagnostic methods to detect the presence of S. aureus in such samples more rapidly than conventional culture.
Several authors have noted that primary pneumonia (spread of infection through the respiratory tract) is associated with a poorer prognosis than secondary pneumonia (haematogenous spread of infection from a focus in another organ) [Reference Kreienbuehl, Charbonney and Eggimann3, Reference Li4, Reference Gillet7, Reference Khanafer32]. It is suggested that in the latter group, patients whose illness began with a skin and soft tissue infection should be separated from those whose first manifestation was infective endocarditis. This classification provides a better clue for the optimal treatment. In this study, the patient who suffered from right-sided endocarditis and secondary pneumonia was the only one who was successfully treated with β-lactams only (oxacillin and meropenem).
Linezolid or clindamycin are recommended for therapy of primary necrotizing pneumonia, both for their ability to inhibit synthesis of PVL and good lung penetration including alveolar lining fluid, bronchial exudate, and pleural effusion [Reference Li4, Reference Morgan25, Reference Dumitrescu33]. Clindamycin appears to be inferior to linezolid for initial/empirical therapy because resistance develops more frequently. Another theoretical argument against clindamycin results from its pharmacokinetics as the concentration of clindamycin at the site of inflammation depends partly on leukocyte transport. In necrotizing pneumonia, the number of incoming leukocytes is often significantly reduced because of their destruction by PVL, and thus a therapeutic concentration of clindamycin may not always be attained in the affected tissue.
Necrotizing pneumonia affects especially young and healthy people. It might therefore be expected that elderly and/or individuals with more than one comorbid condition would be more susceptible to infection due to poor immune status. A possible explanation for the rarity of this infection in such groups may be that older people who have previously been exposed to the pathogen have developed anti-PVL antibodies. This concept correlates well with the fact that intravenous globulin obtained from unimmunized donors can inactivate PVL [Reference Morgan25, Reference Gauduchon34]. A protective role of anti-PVL antibodies has also been suggested [Reference Rasigade35].
The prevalence of PVL-producing strains of S. aureus has been reported to be strongly linked to the prevalence of CA-MRSA strains with varied genetic backgrounds in different geographical regions [Reference David and Daum36]. PVL is encoded by phages of the Siphoviridae family exhibiting a highly mosaic structure of the genome [Reference Kahánková13], but the luk-PV genes are always located in a 6·4-kb conserved region consisting of the host lysis module (ami2 type), luk-PV, and the Sa2 type integrase gene [Reference Goerke37] shown in all strains in this study. Previous studies have shown no relationship between PVL production that depends on the phage's life-cycle or the host's background [Reference Wirtz38], and clinical presentation of the infection [Reference Boakes39].
The present study demonstrated that the Czech cases of necrotizing pneumonia were caused by strains with different genetic backgrounds that can be classified into six lineages. We speculate that MSSA strains assigned to the CC15, CC30, and CC121 groups that are frequently found in asymptomatic carriers, may represent novel invasive clones that have acquired not only PVL, but also other virulence genes by horizontal gene transfer and/or have upregulated their inherent virulence factors. Relatively few PVL-positive ST154 strains have been described in the literature, originating mainly from Mongolia and other Central Asian countries [Reference Monecke40]. Patient no. 2 was from neighbouring Slovakia, but whether he came into contact with Asian immigrants is unknown. Interestingly, those isolates that underwent MLST and spa-typing were t667 [Reference Nair41, Reference Shore42], as was the ST154 strain in the present report.
In conclusion, this study documents that although highly fatal, necrotizing staphylococcal pneumonia is so rare that its specific therapy by linezolid and/or clindamycin cannot be included in the guidelines for initial therapy of patients with community-acquired pneumonia. Rapid diagnostic methods for S. aureus should be developed and implemented to allow early detection of this disease.
ACKNOWLEDGEMENTS
We thank all colleagues involved in this study. This work was supported by the Czech Science Foundation (grant no. GP13-05069P) and by the Internal Grant Agency of the Ministry of Health of the Czech Republic (grant no. NT/12395-5).
DECLARATION OF INTEREST
None.






