The prevalence of cancer survivorship is increasing due to innovations in cancer screening (early detection) and treatment, increasing life expectancy and population growth( Reference Bluethmann, Mariotto and Rowland1 ). In Westernised countries, the 5-year survival rate of all cancers is approximately 68 %( Reference Bluethmann, Mariotto and Rowland1 , 2 ), with the expected number of cancer survivors projected to increase to 26·1 million by 2040 in the USA( Reference Bluethmann, Mariotto and Rowland1 ). Across the cancer continuum from diagnosis to end-of-life care, both short- and long-term quality of life is often reduced due to disease- and treatment-related side effects, with cancer-related fatigue (CRF), pain and psychological distress reported most commonly( 3 ). Recent investigations have suggested CRF is one of the most prominently reported side effects of cancer treatments( Reference Wang, Zhao and Fisch4 – Reference Cella, Peterman and Passik7 ), with up to 80 % of cancer patients experiencing CRF during treatment( Reference Hofman, Ryan and Figueroa-Moseley8 – Reference Nelson, Gonzalez and Jim10 ) and for many years after treatment cessation( Reference Wang, Zhao and Fisch4 , Reference Servaes, Verhagen and Schreuder11 – Reference Langston, Armes and Levy13 ). Severe and persistent CRF have shown to inhibit quality of life by considerably reducing functional capacity to fully participate in daily living tasks( Reference Olson, Krawchuk and Quddusi14 ). The distressing impact of CRF not only affects quality of life but has also been shown to be a predictor of cancer recurrence and reduced overall survival( Reference Groenvold, Petersen and Idler15 ). The National Cancer Institute( 16 ) has declared CRF to be a high research priority for future clinical trials to improve the quality of life of cancer survivors and, in turn, be prepared for the increasing demand of supportive care, given the projected cancer survivorship statistics.
The pathophysiology of CRF is relatively unknown and the treatment of CRF is largely symptomatic( Reference Berger, Mooney and Alvarez-Perez17 ). The cause of CRF is thought to be multi-dimensional, including pro-inflammatory responses to cancer treatment (i.e. IL-6, IL-8 and TNF-α) and lean muscle mass degradation( Reference Bower, Ganz and Tao18 – Reference Bower and Lamkin20 ). Clinical practice recommendations suggest pharmaceutical interventions and lifestyle interventions (i.e. exercise, counselling and diet) to be effective treatments of CRF( Reference Berger, Mooney and Alvarez-Perez17 ). Whilst there is substantial evidence for exercise and psychological intervention to reduce CRF( Reference Baguley, Bolam and Wright21 – Reference Mustian, Sprod and Janelsins23 ), to date pharmaceutical interventions have revealed inconsistent effects on CRF( Reference Mustian, Alfano and Heckler22 ), and no systematic investigations have been conducted to inform targeted nutrition care for CRF. The dietary quality in cancer survivors is considerably poor, with only 15·1 % of cancer survivors meeting the diet recommendations for fruit (2 serves/d) and vegetables (5 serves/d)( Reference Blanchard, Courneya and Stein24 ), which may in turn suggest there is significant scope to use nutrition therapy interventions to potentially improve cancer treatment side effects, such as CRF. In breast cancer survivors, a dietary intake high in fibre (>25 g/d)( Reference Guest, Evans and Rogers25 ) and fruits and vegetables( Reference Alfano, Day and Katz26 , Reference Alfano, Imayama and Neuhouser27 ) has been positively associated with low levels of CRF. Anthropometric measures such as high BMI >30 kg/m2 ( Reference Reinertsen, Cvancarova and Loge28 ), high adipose tissue (>34 %)( Reference Winters-Stone, Bennett and Nail29 ) and low skeletal mass index (validated measure of muscle index through tomography scan of L3 or T4 muscle mass)( Reference Neefjes, van den Hurk and Blauwhoff-Buskermolen30 ) are also predictors of CRF in breast cancer. To support the National Cancer Institute research priority, it is imperative that the optimal nutrition care plan (including consult length, frequency, duration and delivery mode) and dietary recommendations (dietary energy, macronutrients and dietary patterns) to improve the outcomes of CRF are investigated to inform dietetic practice.
Existing evidence suggests nutrition therapy is inconsistently associated with improved quality of life for people with cancer( Reference Kassianos, Raats and Gage31 ). However, the inclusion of adjunctive lifestyle interventions (i.e. exercise, meditation, mindfulness) alongside nutritional interventions in the studies comprised within this systematic review confounds the association of nutrition therapy with quality of life. Dietary recommendations aiming to improve quality of life and health outcomes in cancer patients may vary substantially based on the specific cancer population, treatment modality and disease- and treatment-related side effects (e.g. metabolic alterations, deteriorations in body composition, early satiety, nausea)( Reference Arends, Bachmann and Baracos32 , Reference Rock, Doyle and Demark-Wahnefried33 ). Nutrition interventions that improve quality of life can be achieved by targeting and enhancing specific health outcomes (i.e. body mass) which are often compromised by cancer treatment. However, it is unknown whether targeting a health gain through nutrition therapy will also lead to improvements in CRF. Thus, systematically reviewing and meta-analysing the effects of different nutrition therapies aiming to improve CRF or quality of life through target health outcomes warrants exploration to inform future research and clinical practice. The aim of this study was to systematically review and meta-analyse randomised controlled trials (RCT) that investigated the isolated effects of nutrition therapy (i.e. without any adjunct lifestyle intervention such as exercise or psychology), on CRF and quality of life in people living with and beyond cancer.
Methods
Literature search
The systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement( Reference Moher, Liberati and Tetzlaff34 ). Electronic databases searched in September 2017 included PubMed, Scopus, CINAHL and CENTRAL (Cochrane Central Register of Controlled Trials). A full list of the search terms are provided in online Supplementary data (File 1).
Inclusion criteria
The inclusion criteria as specified by the Population, Intervention, Control Outcomes, Study design framework were as follows: (1) population: any histologically confirmed diagnosis of cancer (including all stages and treatments of cancer), (2) intervention: any structured dietary change or modification of dietary patterns (describing quantities, proportions, variety and frequency of food groups), (3) control: comparison group not receiving the intervention at any time point during the trial, comparison to a group receiving a different diet or a wait list control care, (4) outcomes: a measure of CRF and/or quality of life and (5) study design: RCT, randomised comparative trials, controlled trials or single-group cohort studies. Only full-text English articles of human trials, published in peer-reviewed journals, were included in the search process.
Titles and abstracts of articles identified through the search process were first reviewed by B. J. B. to exclude articles out of our study scope. Subsequently, B. J. B., O. R. L. W. and T. L. S. independently screened the full texts to identify eligible articles. Disagreements were discussed and resolved, with all parties agreeing on the exclusion or inclusion of articles. Articles that met the inclusion criteria were examined to ensure the same participants were not reported in more than one article. The data extraction procedure followed the PRISMA statement( Reference Moher, Liberati and Tetzlaff34 ). Reference lists of eligible articles were manually checked for additional references.
Data extraction
Details of (1) participant and study characteristics, (2) the nutrition intervention and (3) study results were independently extracted by one author (B. J. B.). Nutrition interventions were defined as any change in nutritional intake to increase/decrease foods or change in ratio of macronutrients, with the exception of any supplement use( Reference Mahan and Raymond35 ). Studies involving oral feeding tubes were excluded from this review to enable accurate assessment of the efficacy of dietary pattern manipulation on CRF and quality of life. Any intervention incorporating strategies that may have influenced quality of life or CRF, for example, meditation, relaxation, exercise and psychological support were excluded. Measures of CRF included the Functional Assessment of Cancer Therapy (FACT)-fatigue, FACT-general, multidimensional fatigue inventory-short form, Schwartz Cancer Fatigue Scale, Piper Fatigue Scale, Brief Fatigue Inventory (BFI), and measures of quality of life included the Medical Outcomes Study: 36-Item Short-Form Survey (SF-36), European Organisation of Research and Treatment of Cancer (EORTC) quality of life questionnaire, any cancer-specific FACT or EORTC quality-of-life questionnaires or any other measure of CRF or quality of life. Data documented in tables included the raw questionnaire values (including standard deviation or measurements of error) and significance with the associated P value.
Data synthesis
The pooled effect size was calculated for CRF and quality of life for each study including details on sample size, mean, standard deviation, standard error of the mean and/or 95 % CI. The random effect standardised mean difference (SMD), 95 % CI, z value, α value for z and Q statistic for heterogeneity were calculated using Review Manager Software( 36 ). For studies only reporting CI or standard error of the mean, the standard deviation was obtained following the Cochrane Handbook recommendations( Reference Higgins and Green37 ). A two-tailed α value ≤0·05 for z and non-overlapping 95 % CI were considered to represent statistically significant SMD changes in CRF or quality of life when nutrition therapy was compared with a usual care or control group. I 2 statistics for consistency and τ 2 were extracted from Review Manager Software, and a P-value for Cochrane Q P≤0·10 or an I 2 statistic ≥50 % indicated substantial heterogeneity( Reference Higgins and Green37 ). Subgroup analyses were performed for the nutrition therapy, delivery of nutrition therapy and duration of the intervention.
Quality assessment
Methodological quality of the included articles was independently reviewed by two authors (B. J. B. and O. R. L. W.) using the American Dietetic Association (ADA)’s evidence analysis library quality criteria checklist for primary research( 38 ). The criteria are written as ‘yes/no’ questions to examine the validity of the study design and its execution of two categories: relevance and validity of questions. A final rating of positive, neutral or negative was assigned to each study, based on the number of questions answered as yes, neutral or no.
Results
Study design and research quality
The systematic search found 6988 articles identified through the databases (Fig. 1). After duplicate removal through the selected databases, a total of 6699 abstracts were screened for inclusion. Abstracts of the full-text records were assessed for eligibility; forty-eight articles were subsequently included for full-text revision, with one article additionally included through reference lists screening of the included articles. A total of sixteen articles met the inclusion criteria and were included in the review( Reference Baldwin, Spiro and McGough39 – Reference Zick, Colacino and Cornellier54 ), with one intervention described in two separate studies( Reference Isenring, Bauer and Capra43 , Reference Isenring, Capra and Bauer44 ).
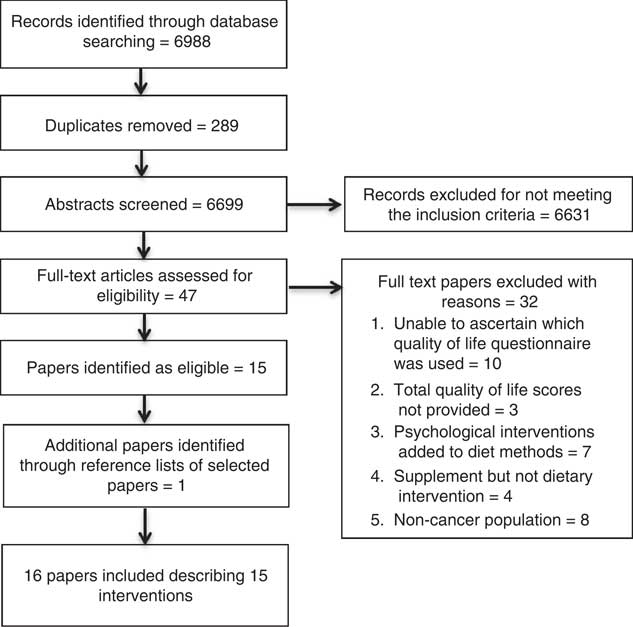
Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta Analyses diagram.
Quality assessment
Results of the methodological quality assessment are presented in online Supplementary material (File 2). Methodological quality scores ranged from 76 %( Reference Pakiz, Flatt and Mills47 ) to 89 %( Reference Isenring, Capra and Bauer44 , Reference Zick, Colacino and Cornellier54 ), with a mean score of 82 %. All fifteen interventions scored a positive score( Reference Baldwin, Spiro and McGough39 – Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 – Reference Zick, Colacino and Cornellier54 ) for methodological quality on the ADA quality assessment of study relevance and validity( 55 ). All studies scored high in the relevance of methodological design; however, only one intervention blinded data collectors from the treatment( Reference Paxton, Garcia-Prieto and Berglund48 ). An intention to treat statistical approach was used in eight studies (53 %)( Reference Baldwin, Spiro and McGough39 , Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 , Reference Persson, Johansson and Sjöden49 – Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 , Reference Zick, Colacino and Cornellier54 ), and clinical significance along with statistical significance was provided in ten studies (67 %)( Reference Baldwin, Spiro and McGough39 – Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Silvers, Savva and Huggins52 – Reference Zick, Colacino and Cornellier54 ).
Study populations
A total of 1290 participants were included across the fifteen interventions. The sample sizes ranged from 18( Reference Brown, Capra and Williams40 ) to 358( Reference Baldwin, Spiro and McGough39 ). The average age of participants ranged from 51·5( Reference Gnagnarella, Misotti and Santoro42 ) to 72·0 years( Reference Silvers, Savva and Huggins52 ). Studies included participants diagnosed with gastrointestinal (n 3)( Reference Baldwin, Spiro and McGough39 , Reference Persson, Johansson and Sjöden49 , Reference Silvers, Savva and Huggins52 ), breast (n 3)( Reference Darga, Magnan and Mood41 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Zick, Colacino and Cornellier54 ), undefined (n 3)( Reference Brown, Capra and Williams40 , Reference Gnagnarella, Misotti and Santoro42 , Reference Uster, Ruefenacht and Ruehlin53 ), head and neck (n 2)( Reference Isenring, Bauer and Capra43 , Reference Isenring, Capra and Bauer44 , Reference Ravasco, Monteiro-Grillo and Marques50 ), lung (n 2)( Reference Kiss, Isenring and Gough45 , Reference Ovesen, Allingstrup and Hannibal46 ), colorectal (n 2)( Reference Persson, Johansson and Sjöden49 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) and ovarian (n 2)( Reference Ovesen, Allingstrup and Hannibal46 , Reference Paxton, Garcia-Prieto and Berglund48 ) cancer. Participants received various cancer treatments, including radiotherapy (n 4)( Reference Isenring, Bauer and Capra43 – Reference Kiss, Isenring and Gough45 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ), chemotherapy (n 3)( Reference Baldwin, Spiro and McGough39 , Reference Brown, Capra and Williams40 , Reference Ovesen, Allingstrup and Hannibal46 ), surgery (n 1)( Reference Pakiz, Flatt and Mills47 ) and surgery with chemotherapy (n 1)( Reference Silvers, Savva and Huggins52 ); the remaining six studies did not disclose the treatment modality( Reference Darga, Magnan and Mood41 , Reference Gnagnarella, Misotti and Santoro42 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Persson, Johansson and Sjöden49 , Reference Uster, Ruefenacht and Ruehlin53 , Reference Zick, Colacino and Cornellier54 ).
Measures of cancer-related fatigue
A total of eight studies reported the effects of nutrition therapy on CRF( Reference Darga, Magnan and Mood41 , Reference Gnagnarella, Misotti and Santoro42 , Reference Paxton, Garcia-Prieto and Berglund48 – Reference Silvers, Savva and Huggins52 , Reference Zick, Colacino and Cornellier54 ). In all, four studies used the EORTC-C30( Reference Gnagnarella, Misotti and Santoro42 , Reference Persson, Johansson and Sjöden49 – Reference Silvers, Savva and Huggins52 ), whilst one study each used the SF-36( Reference Paxton, Garcia-Prieto and Berglund48 ), BFI( Reference Zick, Colacino and Cornellier54 ) and FACT-F( Reference Darga, Magnan and Mood41 ). The efficacy of nutrition therapy on CRF was a primary outcome in only one of eight (13 %) studies( Reference Zick, Colacino and Cornellier54 ).
Measures of quality of life
A total of fourteen studies reported the effects of nutrition therapy on quality of life( Reference Baldwin, Spiro and McGough39 – Reference Uster, Ruefenacht and Ruehlin53 ). In all, nine studies used the EORTC-C30( Reference Baldwin, Spiro and McGough39 , Reference Brown, Capra and Williams40 , Reference Gnagnarella, Misotti and Santoro42 – Reference Isenring, Capra and Bauer44 , Reference Persson, Johansson and Sjöden49 – Reference Uster, Ruefenacht and Ruehlin53 ), two studies used the FACT-G( Reference Baldwin, Spiro and McGough39 , Reference Darga, Magnan and Mood41 ), FACT-L( Reference Kiss, Isenring and Gough45 ), FACT-G, SF-36( Reference Paxton, Garcia-Prieto and Berglund48 ), Quality-of-Life Index( Reference Ovesen, Allingstrup and Hannibal46 ) and Polyp Prevention Trial Quality of Life Factors( Reference Pakiz, Flatt and Mills47 ). The efficacy of nutrition therapy on quality of life was a primary outcome in eight of fifteen (53 %) studies( Reference Baldwin, Spiro and McGough39 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Persson, Johansson and Sjöden49 – Reference Uster, Ruefenacht and Ruehlin53 ).
Study characteristics
Intervention characteristics are displayed in Table 1. A total of nine RCT included a control or waitlist control group( Reference Ovesen, Allingstrup and Hannibal46 – Reference Zick, Colacino and Cornellier54 ), six RCT used an alternative diet as a comparison group( Reference Brown, Capra and Williams40 , Reference Gnagnarella, Misotti and Santoro42 , Reference Kiss, Isenring and Gough45 ), one study used supplements as a comparison group( Reference Isenring, Bauer and Capra43 , Reference Isenring, Capra and Bauer44 ), one three-arm study compared nutrition therapy, an oral supplement and a control group( Reference Baldwin, Spiro and McGough39 ), and one four-arm study compared nutrition therapy, Weight Watchers©, combined nutrition therapy and Weight Watchers and a control group( Reference Darga, Magnan and Mood41 ). A pseudo-RCT (alternate method of group allocation) was seen in only one study where the nutrition therapy intervention group was compared with a group of hospital patients provided standard dietetic practice( Reference Brown, Capra and Williams40 ). Of the fifteen, fourteen (94 %) studies reported that a dietitian or nutritionist delivered the intervention( Reference Baldwin, Spiro and McGough39 – Reference Darga, Magnan and Mood41 , Reference Isenring, Bauer and Capra43 – Reference Zick, Colacino and Cornellier54 ); whilst one study did not indicate the health professional delivering the intervention( Reference Pakiz, Flatt and Mills47 ).
Table 1 Study characteristics
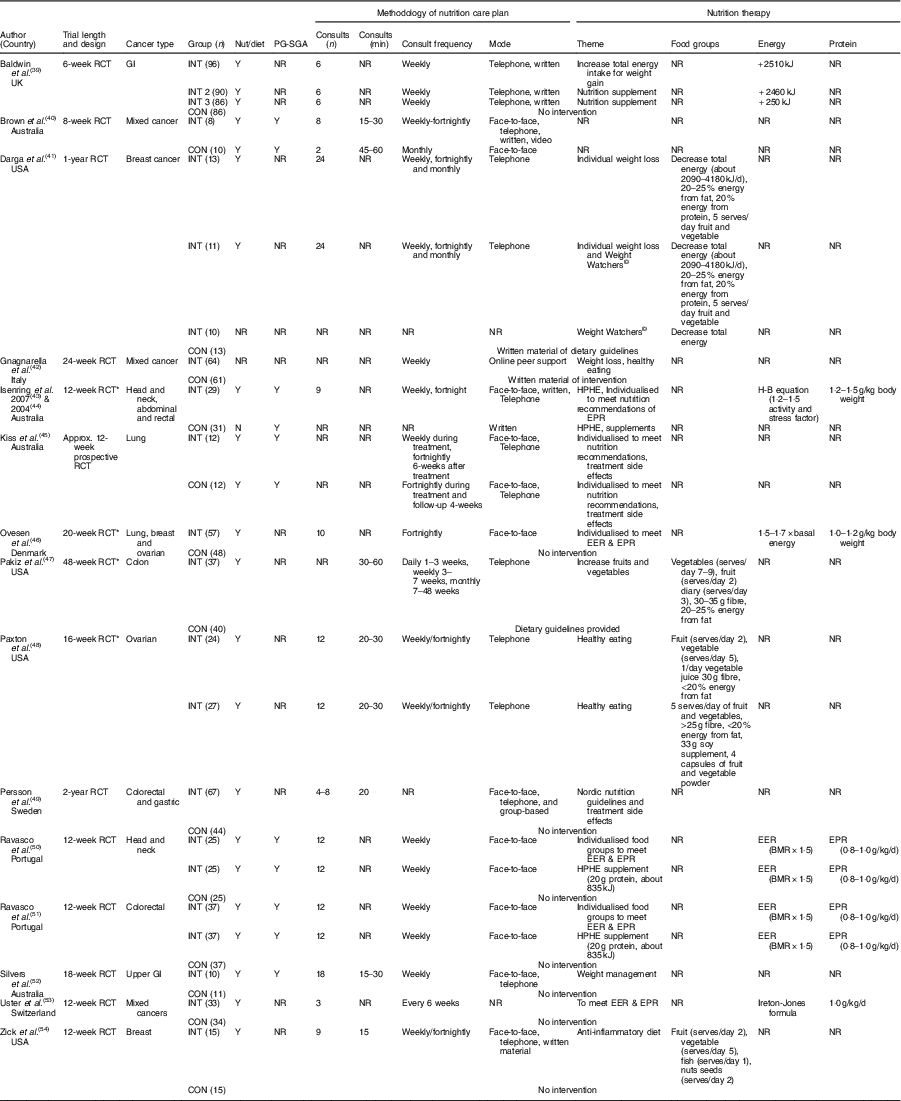
CON, control or comparison group; EER, estimated energy requirements; EPR, estimated protein requirements; GI, gastrointestinal; H-B, Harris–Benedict; HPHE, high protein high energy; INT, intervention; NR, not reported; nut/diet, intervention delivered by a nutritionist or dietitian; PG-SGA, Patient-Generated Subjective Global Assessment; RCT, randomised controlled trial; Y, yes.
* Randomised comparative trial.
Length
All fifteen studies reported the length of the intervention, ranging from 6 weeks( Reference Baldwin, Spiro and McGough39 ) to 24 months( Reference Persson, Johansson and Sjöden49 ). Eight studies were of intervention length of <12 weeks( Reference Baldwin, Spiro and McGough39 , Reference Brown, Capra and Williams40 , Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 , Reference Zick, Colacino and Cornellier54 ). One study each were of 16 weeks( Reference Paxton, Garcia-Prieto and Berglund48 ), 18 weeks( Reference Silvers, Savva and Huggins52 ) and 24 weeks( Reference Pakiz, Flatt and Mills47 ). One study was of 1 year( Reference Darga, Magnan and Mood41 ) and two studies was of 2 years( Reference Paxton, Garcia-Prieto and Berglund48 , Reference Persson, Johansson and Sjöden49 ).
Duration of dietetic consults
Of the fifteen, five studies reported the duration of the prescribed nutrition consults( Reference Brown, Capra and Williams40 , Reference Pakiz, Flatt and Mills47 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Silvers, Savva and Huggins52 , Reference Zick, Colacino and Cornellier54 ). The nutrition consults ranged from 15 to 60 min across the five studies( Reference Brown, Capra and Williams40 , Reference Pakiz, Flatt and Mills47 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Silvers, Savva and Huggins52 , Reference Zick, Colacino and Cornellier54 ). Two studies prescribed 15–30 min consults( Reference Silvers, Savva and Huggins52 , Reference Zick, Colacino and Cornellier54 ), one study prescribed 20–30 min consults( Reference Paxton, Garcia-Prieto and Berglund48 ) and one study prescribed 30–60 min consults( Reference Pakiz, Flatt and Mills47 ). One study compared 15–30 min consults v. 45–60 min nutrition consults( Reference Brown, Capra and Williams40 ).
Frequency
All fifteen studies reported the frequency of nutrition consultations; six reported weekly consultations( Reference Baldwin, Spiro and McGough39 , Reference Brown, Capra and Williams40 , Reference Gnagnarella, Misotti and Santoro42 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 ), one reported weekly or fortnightly( Reference Brown, Capra and Williams40 ), one reported fortnightly consultations( Reference Ovesen, Allingstrup and Hannibal46 ). One study reported daily (1–3 weeks), weekly (3–7 weeks) and monthly consults (7–48 weeks)( Reference Pakiz, Flatt and Mills47 ). Three studies reported the weekly consults were moved to fortnightly after 4 weeks( Reference Zick, Colacino and Cornellier54 ), 6 weeks( Reference Isenring, Capra and Bauer44 ) and 8 weeks( Reference Paxton, Garcia-Prieto and Berglund48 ). One study reported weekly consults were moved to fortnightly (at 12 weeks) and monthly (at 24 weeks)( Reference Darga, Magnan and Mood41 ). One intervention reported a nutrition consult occurred before treatment and then fortnightly during treatment( Reference Kiss, Isenring and Gough45 ). One reported a range of three to seven consults over 24 months( Reference Persson, Johansson and Sjöden49 ).
Mode
Of the fifteen studies, fourteen reported the mode of delivery of the nutrition consult( Reference Baldwin, Spiro and McGough39 – Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 – Reference Zick, Colacino and Cornellier54 ). Four studies utilised combined face-to-face and telephone consults( Reference Isenring, Bauer and Capra43 – Reference Kiss, Isenring and Gough45 , Reference Persson, Johansson and Sjöden49 , Reference Silvers, Savva and Huggins52 ), whilst three studies each examined face-to-face consults( Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) or telephone consults( Reference Darga, Magnan and Mood41 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Persson, Johansson and Sjöden49 ). The remaining studies used face-to-face, telephone and videoconferencing( Reference Brown, Capra and Williams40 ), face-to-face, telephone and written material( Reference Zick, Colacino and Cornellier54 ), written and telephone consultations( Reference Baldwin, Spiro and McGough39 ) and nutrition information delivered through online material, blogs and peer support( Reference Gnagnarella, Misotti and Santoro42 ).
Nutrition therapy
The prescribed nutrition therapy of the intervention and/or control groups was reported in fourteen of the fifteen studies( Reference Baldwin, Spiro and McGough39 , Reference Darga, Magnan and Mood41 – Reference Zick, Colacino and Cornellier54 ). Nutrition therapy to meet or exceed the estimated energy and protein requirements was prescribed in six of fifteen studies( Reference Baldwin, Spiro and McGough39 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 ). One study prescribed an additional 2500 kJ compared with the usual care( Reference Baldwin, Spiro and McGough39 ), while four studies used formulas to estimate the total energy requirements: 1·5 times the BMR( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ), 1·7 times the basal metabolic energy expenditure( Reference Ovesen, Allingstrup and Hannibal46 ) and the Ireton–Jones formula (energy expenditure including age, weight, sex, trauma and burns)( Reference Uster, Ruefenacht and Ruehlin53 ). Estimated protein requirements were used in six of fifteen studies( Reference Isenring, Bauer and Capra43 – Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 ), ranging from 0·8( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) to 1·5( Reference Isenring, Bauer and Capra43 ) g/kg per d of body weight. According to one study, prescribed protein comprised 20 % of total energy intake( Reference Darga, Magnan and Mood41 ), whilst another study prescribed weight management and nutrition advice for the management of treatment-related side effects( Reference Silvers, Savva and Huggins52 ). Of the fourteen studies, two prescribed weight loss interventions, with one prescribing a 2090–4180kJ/d deficit( Reference Gnagnarella, Misotti and Santoro42 ) whilst the other provided no defined deficit( Reference Darga, Magnan and Mood41 ). Dietary pattern interventions was seen in four studies and focused on recommendations for fruit and vegetable intake and obtaining 20–25 % energy from fat( Reference Darga, Magnan and Mood41 , Reference Pakiz, Flatt and Mills47 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Zick, Colacino and Cornellier54 ). Two interventions recommending the same two fruits and five vegetables serves/d( Reference Paxton, Garcia-Prieto and Berglund48 , Reference Zick, Colacino and Cornellier54 ), whilst Zick et al. ( Reference Zick, Colacino and Cornellier54 ) further detailed one fish and one nuts/seeds serves per d to encompass an anti-inflammatory dietary pattern. The Nordic nutritional guidelines( 56 ) were compared with the usual care in one RCT( Reference Persson, Johansson and Sjöden49 ).
Nutrition outcome measurements
Total energy intake was reported in eight of fifteen studies( Reference Isenring, Bauer and Capra43 , Reference Isenring, Capra and Bauer44 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Paxton, Garcia-Prieto and Berglund48 – Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 , Reference Zick, Colacino and Cornellier54 ) and total protein intake was reported in six of fifteen studies( Reference Isenring, Bauer and Capra43 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Persson, Johansson and Sjöden49 – Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 ). The Patient-Generated Subjective Global Assessment( Reference Ottery57 ) (PGSGA; a nutrition assessment tool incorporating diet intake, physical parameters, treatment side effects and physical functioning for oncology patients) was measured in six of fifteen studies( Reference Brown, Capra and Williams40 , Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 , Reference Ravasco, Monteiro-Grillo and Marques50 – Reference Silvers, Savva and Huggins52 ). Of the fifteen studies, twelve reported the effect of nutrition interventions on anthropometrical outcomes of body mass( Reference Baldwin, Spiro and McGough39 , Reference Darga, Magnan and Mood41 , Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 – Reference Persson, Johansson and Sjöden49 , Reference Silvers, Savva and Huggins52 , Reference Uster, Ruefenacht and Ruehlin53 ), BMI( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Zick, Colacino and Cornellier54 ) or fat-free mass( Reference Isenring, Bauer and Capra43 , Reference Kiss, Isenring and Gough45 , Reference Ovesen, Allingstrup and Hannibal46 ).
Dropout, attendance and adverse effects
All studies reported dropout rates ranging from 0 %( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) to 43 %( Reference Uster, Ruefenacht and Ruehlin53 ). Only one of the fifteen (7 %) studies reported on the adverse effects of the nutrition intervention, with this study reporting zero adverse events( Reference Paxton, Garcia-Prieto and Berglund48 ). Four studies (27 %) reported adherence to the nutrition intervention( Reference Baldwin, Spiro and McGough39 , Reference Kiss, Isenring and Gough45 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Zick, Colacino and Cornellier54 ). Three studies used checklists of food groups to measure adherence to the dietary strategies, with adherence reported as 11–47 %( Reference Paxton, Garcia-Prieto and Berglund48 ), 75 %( Reference Kiss, Isenring and Gough45 ) and 73–95 %( Reference Zick, Colacino and Cornellier54 ). One study used a food diary to report adherence( Reference Baldwin, Spiro and McGough39 ), however as only 25 % of these records were completed, the analysis was not documented( Reference Baldwin, Spiro and McGough39 ). Four studies reported nutrition consult attendance (face-to-face and/or telephone), with attendance rates of 75 %( Reference Paxton, Garcia-Prieto and Berglund48 ), 93 %( Reference Zick, Colacino and Cornellier54 ) and 100 %( Reference Kiss, Isenring and Gough45 , Reference Silvers, Savva and Huggins52 ).
Effects on cancer-related fatigue
The effects of the interventions on CRF are displayed in Table 2. The pooled effects (SMD 0·18 (95 % CI –0·02, 0·39)) of nutrition therapy compared with usual care showed no apparent improvements in CRF (Fig. 2). There was no heterogeneity I 2=28 %, P=0·23. One (13 %) of eight nutrition interventions showed between-group improvements in CRF compared with the usual care( Reference Zick, Colacino and Cornellier54 ). Three (38 %) of eight nutrition interventions showed within-group improvements in CRF from the nutrition intervention( Reference Ravasco, Monteiro-Grillo and Marques50 – Reference Silvers, Savva and Huggins52 ), whilst no significant within-group changes were seen in the comparative or control groups (Fig. 3).
Table 2 Cancer-related fatigue outcomes
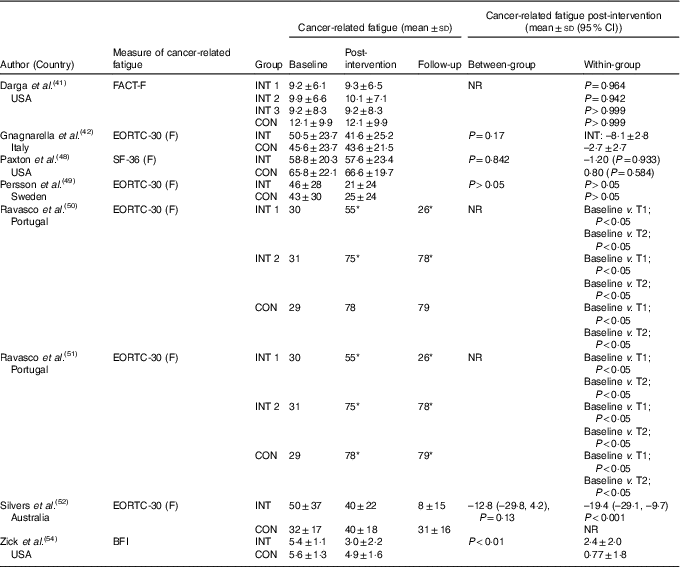
BFI, Brief Fatigue Inventory; CON, control; EORTC-30, European Organisation for Research and Treatment of Cancer-30 (questions); F, fatigue; FACT, Functional Assessment of Cancer Therapy; INT, intervention; NR, not reported; SF-36, Medical Outcome Study 36-Item Short-Form Health Survey; T, time point.
* Significance <0·05.
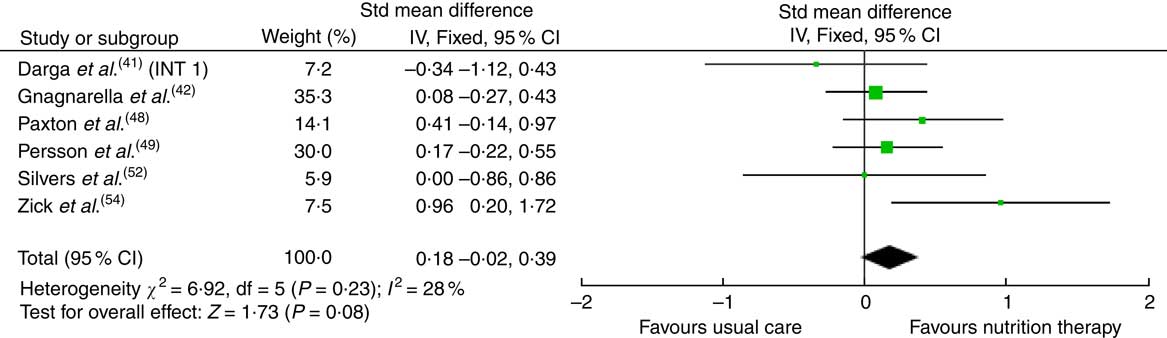
Fig. 2 Forest plot for standardised mean difference effect size in cancer-related fatigue when nutrition therapy is compared with usual care. The squares represent the pooled standardised mean difference effect size for each study, with the total pooled effect shown in the black diamond. All analyses are based on a random effects model. INT1, intervention group 1.
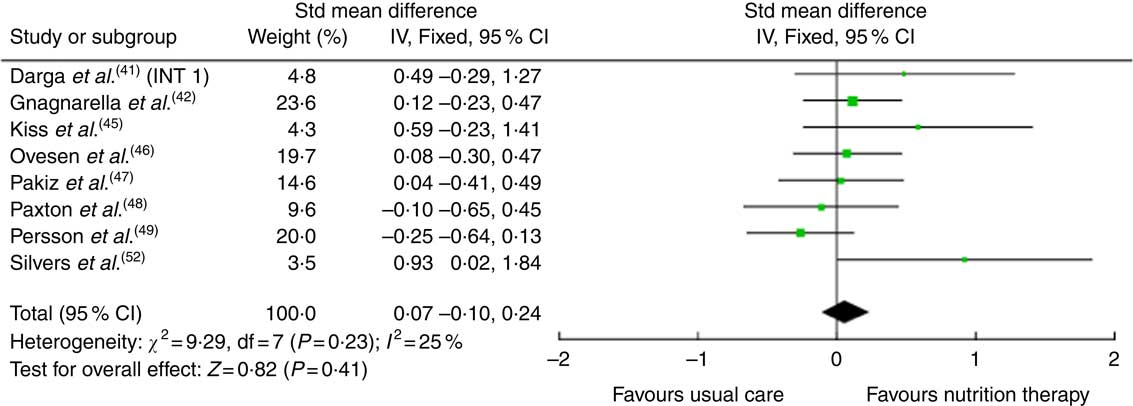
Fig. 3 Forest plot for standardised mean difference effect size in quality of life when nutrition therapy is compared with usual care. The squares represent the pooled standardised mean difference effect size for each study, with the total pooled effect shown in the black diamond. All analyses are based on a random effects model. INT1, intervention group 1.
Subgroup analysis for cancer-related fatigue
Six of eight interventions were eligible for CRF meta-analysis( Reference Darga, Magnan and Mood41 , Reference Gnagnarella, Misotti and Santoro42 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Persson, Johansson and Sjöden49 , Reference Silvers, Savva and Huggins52 , Reference Zick, Colacino and Cornellier54 ). Both Ravasco et al. ( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) studies did not report a measurement of error to the mean and was excluded in the meta-analysis. Subgroup analysis for CRF are found in online Supplementary data (File 3).
Length
There appeared to be no definitive improvement in CRF when ≤6 month (SMD 0·31 (95 % CI –0·06, 0·68) consults were compared with >6 month (SMD 0·03 (95 % CI –0·41, 0·47)). One of the three interventions showed between-group improvements in CRF compared with a control group after 12 weeks( Reference Zick, Colacino and Cornellier54 ), whilst two intervention showed within-group improvements in CRF from the interventions( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ). The one intervention of 18 weeks only showed within-group improvements from the intervention( Reference Silvers, Savva and Huggins52 ).
Duration of dietetic consults
Four of eight interventions exploring CRF reported the duration of nutrition consults in the nutrition care plan( Reference Paxton, Garcia-Prieto and Berglund48 , Reference Persson, Johansson and Sjöden49 , Reference Silvers, Savva and Huggins52 , Reference Zick, Colacino and Cornellier54 ), and as such pooled effects could not be performed. The only intervention with 15 min consults showed between-group improvements in CRF( Reference Zick, Colacino and Cornellier54 ). The only intervention prescribing 15–30 min consults showed within-group improvements in CRF( Reference Silvers, Savva and Huggins52 ).
Frequency
Weekly and fortnightly consults showed improvements in CRF (SMD 0·62 (95 % CI 0·10, 1·15)), whilst weekly (SMD 0·12 (95 % CI –0·14, 0·38)) and progressive weekly and month (SMD –0·34 (95 % CI –1·12, 0·43)) interventions showed no apparent improvements on CRF.
Mode
The mode of intervention delivery showed no apparent effect on CRF. Interventions using face-to-face with telephone consults (SMD 0·34 (95 % CI –0·17, 0·86)), or intervention that were telephone or online only (SMD 0·11 (95 % CI –0·22, 0·43)) showed no effect on CRF.
Nutrition therapy
Nutrition therapy detailing dietary patterns showed improvements in CRF (SMD 0·62 (95 % CI 0·10, 1·15)), whilst weight loss (SMD 0·01 (95 % CI –0·31, 0·33)) and interventions following dietary healthy eating guidelines (SMD 0·14 (95 % CI –0·21, 0·49)) showed no apparent effects on CRF. Of the two interventions that prescribed dietary pattern recommendations( Reference Paxton, Garcia-Prieto and Berglund48 , Reference Zick, Colacino and Cornellier54 ), one intervention reported between-group improvements in CRF when the intervention (anti-inflammatory-based diet) was compared with the control group( Reference Zick, Colacino and Cornellier54 ). Two of five interventions demonstrating between-group improvements in energy and protein intake also showed within-group improvements in CRF( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ). All three interventions showing between-group improvements in PGSGA also demonstrated within-group improvements in CRF( Reference Ravasco, Monteiro-Grillo and Marques50 – Reference Silvers, Savva and Huggins52 ).
Effects on quality of life
The effects of the interventions on quality of life are shown in Table 3. The pooled effects (SMD 0·07 (95 % CI –0·10, 0·24)) of nutrition therapy compared with usual care showed no apparent effects on quality of life. There was no heterogeneity I 2=25 %, P=0·23. Of the fourteen, two (14 %) interventions showed between-group improvements in quality of life compared with the usual care( Reference Silvers, Savva and Huggins52 ) or compared with written healthy eating information with supplements( Reference Isenring, Bauer and Capra43 , Reference Isenring, Capra and Bauer44 ). Of the fourteen, three (21 %) interventions reported within-group improvements from the nutrition intervention( Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ).
Table 3 Quality of life outcome scores
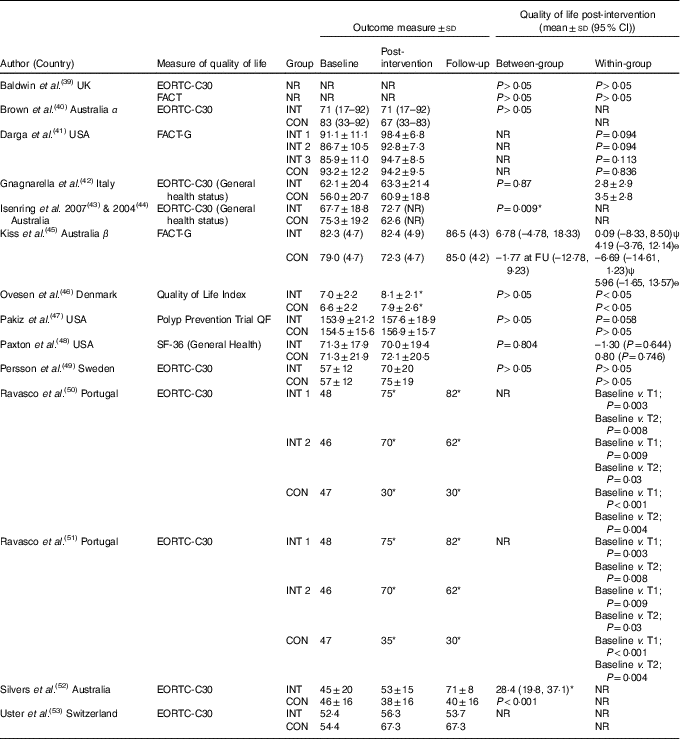
CON, control; EORTC, European Organisation for Research and Treatment of Cancer; FACT, Functional Assessment of Cancer Therapy; FU, follow-up; INT, intervention, NR, not reported; QF, quality of life factors; SF-36, Medical Outcome Study 36-Item Short-Form Health Survey; T, time point; α, data presented as median and range; β, data presented as estimated mean and standard error; ψ, measured directly after radiotherapy; ѳ, measured 4–6 weeks after radiotherapy.
* Significance <0·05.
Subgroup analysis for quality of life
Of the fourteen interventions, eight were eligible for quality-of-life meta-analysis( Reference Darga, Magnan and Mood41 , Reference Gnagnarella, Misotti and Santoro42 , Reference Kiss, Isenring and Gough45 – Reference Persson, Johansson and Sjöden49 , Reference Silvers, Savva and Huggins52 ). Studies by Baldwin et al. ( Reference Baldwin, Spiro and McGough39 ), Brown et al. ( Reference Brown, Capra and Williams40 ), Isenring et al. ( Reference Isenring, Capra and Bauer44 ), Ravasco et al. ( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) and Uster et al. ( Reference Uster, Ruefenacht and Ruehlin53 ) did not report a measurement of error and were excluded in the meta-analysis. Subgroup analysis for CRF are found in online Supplementary data (File 4).
Length
There appeared to be no definitive improvement in quality of life when ≤6 month (SMD 0·17 (95 % CI –0·09, 0·42)) consults were compared with >6 month (SMD –0·02 (95 % CI –0·37, 0·34)). One of four 12-week intervention showed between-group improvements in quality of life when compared with a control group( Reference Isenring, Bauer and Capra43 , Reference Uster, Ruefenacht and Ruehlin53 ), whilst two of four interventions showed within-group improvements in quality of life from the intervention( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ).
Duration of dietetic consults
Of the fourteen interventions, six reported the duration of the nutrition consults( Reference Brown, Capra and Williams40 , Reference Pakiz, Flatt and Mills47 – Reference Persson, Johansson and Sjöden49 , Reference Silvers, Savva and Huggins52 ), and as such pooled effects could not be performed. One of two 15–30 min interventions showed between-group improvements in quality of life from the intervention( Reference Silvers, Savva and Huggins52 ).
Frequency
Weekly (SMD 0·41 (95 % CI –0·35, 1·17)), weekly and fortnightly (SMD 0·10 (95 % CI –0·20, 0·39)) or progressive daily and weekly and monthly interventions (SMD 0·15 (95 % CI –0·24, 0·54)) did not show improvements in quality of life.
Mode
Telephone or online (SMD 0·09 (95 % CI –0·15, 0·33)), face-to-face with telephone (SMD 0·26 (95 % CI –0·86, 1·41)) or face-to-face interventions (SMD 0·20 (95 % CI –0·22, 0·62)) did not show improvements in quality of life. Zero of the three interventions using face-to-face nutrition therapy showed between-group improvements, however all three interventions showed within-group improvements from the intervention( Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ).
Nutrition therapy
Weight loss (SMD 0·18 (95 % CI –0·14, 0·50)), nutrition therapy to meet individual requirements (SMD 0·20, (95 % CI –0·22, 0·62)), guidelines and health eating (SMD 0·10 (95 % CI –0·42, 0·61)) and dietary patterns (SMD –0·10 (95 % CI –0·42, 0·61)) did not show improvements in quality of life. Five of seven interventions showed between-group improvements in energy and protein intake( Reference Isenring, Bauer and Capra43 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 ); one study showed between-group improvements in quality of life( Reference Isenring, Bauer and Capra43 ), whilst three studies showed within-group improvements in quality of life from the intervention( Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ). Five of six studies showed between-group improvements in the PGSGA( Reference Brown, Capra and Williams40 , Reference Isenring, Bauer and Capra43 , Reference Ravasco, Monteiro-Grillo and Marques50 – Reference Silvers, Savva and Huggins52 ); two interventions also showed between-group improvements in quality of life( Reference Isenring, Bauer and Capra43 , Reference Silvers, Savva and Huggins52 ) and two intervention also showed within-group improvements in quality of life( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ).
Discussion
This is the first systematic review and meta-analysis to analyse the key characteristics of the nutrition care plan, apart from adjunctive lifestyle interventions (i.e. with psychology, exercise or meditation), on CRF and quality of life in cancer. Results from this systematic review and meta-analysis demonstrate no definitive effect of nutrition therapy on CRF (SMD 0·18 (95 % CI –0·02, 0·39)) or quality of life (SMD 0·07 (95 % CI –0·10, 0·24)) in people with cancer and cancer survivors. Preliminary evidence indicates dietary pattern interventions aiming to improve fruits (2 serves/d) and vegetables (serves/d)( Reference Paxton, Garcia-Prieto and Berglund48 ), an anti-inflammatory dietary pattern high in fruits (2 serves/d), vegetables (5 serves/d), nuts and seeds (2 serves/d), and fish (1 serve/d)( Reference Zick, Colacino and Cornellier54 ) may improve CRF (SMD 0·62 (95 % CI 0·10, 1·15)). Interventions improving nutritional status through the PGSGA showed improvements in quality of life and should be a future research priority. Differences in methodological design, nutrition therapies and reporting of data in the dietetic literature impede conclusive findings regarding the efficacy of nutrition therapy on CRF and quality of life. There was a low number of participants in studies assessing CRF and only one intervention was designed with CRF as the primary outcome measure. In addition, the limited reporting of adherence or dietary outcomes in the literature limits practical recommendations specific to cancer types. In turn there is insufficient evidence to determine the optimal nutrition care plan (consult frequency, duration, mode and length) or nutrition therapy (energy, protein or dietary pattern) for outcomes of CRF or quality of life.
Cancer-related fatigue and key nutrition care parameters
There is a clear lack of dietetic interventions that effectively improve CRF, with only one of eight interventions (13 %) reporting a between-group improvement compared with usual care( Reference Zick, Colacino and Cornellier54 ). The lack of specificity of nutrition therapy studies targeting improvements in CRF is highlighted by the fact that only one of eight interventions that explored the efficacy of nutrition therapy included CRF as the primary outcome( Reference Zick, Colacino and Cornellier54 ), and there was a small sample size among the included interventions (range 30( Reference Zick, Colacino and Cornellier54 )–125( Reference Gnagnarella, Misotti and Santoro42 )). The one intervention by Zick et al. ( Reference Zick, Colacino and Cornellier54 ) with CRF as a primary outcome demonstrated between-group improvements compared with the usual care monitoring group, and combined with other interventions that aimed to increase dietary patterns of fruits and vegetables( Reference Paxton, Garcia-Prieto and Berglund48 ), collectively offer promising results for future interventions focusing on CRF in cancer survivors (SMD 0·62 (95 % CI 0·10, 1·15). Whilst the number of studies investigating dietary pattern nutrition therapies are limited, these results suggest future research is needed in larger RCT, to elucidate these findings and improve the nutrition literature in CRF.
CRF outcomes from Zick et al. ( Reference Zick, Colacino and Cornellier54 ) intervention indicated changes in dietary pattern composition (high in fruits, vegetables, fish and nuts and seeds) may be more important than focusing on total energy and/or protein prescriptions( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) for generating benefits in CRF. This is demonstrated by Zick et al. ( Reference Zick, Colacino and Cornellier54 ) intervention group showing between-group improvements (P<0·05) in dietary patterns of fruits, vegetables, fish and nuts and seeds compared with the control group after 12 weeks, whilst energy intake was similar across both groups. High adherence to the prescribed dietary patterns in the intervention by Zick et al. ( Reference Zick, Colacino and Cornellier54 ) may also explain why similar improvements in CRF were not seen in the study by Paxton et al. ( Reference Paxton, Garcia-Prieto and Berglund48 ), where there was no between-group changes in fruit (P=0·889) or vegetable (P=0·271) intake. Whilst these two dietary pattern interventions had similar fruit (2 serves/d) and vegetable (5 serves/d) prescriptions for people with cancers of the breast( Reference Zick, Colacino and Cornellier54 ) and ovaries( Reference Paxton, Garcia-Prieto and Berglund48 ), Zick et al. ( Reference Zick, Colacino and Cornellier54 ) also prescribed foods with high anti-inflammatory properties such as fish (1 serve/d) and nuts and seeds (1 serve/d). The difference seen between study inclusion criteria may also, at least in part, explain the differences seen in CRF between breast( Reference Zick, Colacino and Cornellier54 ) and ovaries cancer( Reference Paxton, Garcia-Prieto and Berglund48 ). Further, Zick et al. ( Reference Zick, Colacino and Cornellier54 ) only included CRF symptomatic breast cancer survivors, whilst Paxton et al. ( Reference Paxton, Garcia-Prieto and Berglund48 ) reported CRF as a tertiary measure with no CRF-specific study inclusion criteria.
Insights from Bower et al. ( Reference Bower, Ganz and Tao18 , Reference Bower and Lamkin20 ) suggest the associated CRF-physiological pathways appear to be alterations in inflammatory biomarker and anthropometrical parameters (body mass and composition) from the cancer stage, type or treatment. Whilst Zick et al. ( Reference Zick, Colacino and Cornellier54 ) demonstrated significant between-group improvements (P<0·001) in CRF, the absence of measurements of inflammatory biomarkers or anthropometrical parameters limits exploration of the physiological effects of dietetic intervention on CRF. Furthermore, zero studies included in this systematic review measured CRF-associated inflammatory markers (e.g. IL-6 or -8 or TNF-α), highlighting a clear need for further research in this area. Zero interventions measured change in body composition, which also limits our understanding of the effectiveness of nutrition therapy for counteracting the physical deconditioning associated with CRF. Conversely, the results from Zick et al. ( Reference Zick, Colacino and Cornellier54 ) may only be applicable to overweight breast cancer survivors, and in turn further investigations are needed to determine whether a similar dietary pattern is effective in reducing CRF in well-nourished cancer survivors or in patients undergoing treatment. Elucidating the efficacy of dietetic interventions on these CRF pathways offers novel and clinically relevant outcomes for dietetic healthcare management of CRF.
Nutrition therapy and quality of life
The current European Society of Clinical Nutrition and Metabolism (ESPEN) guidelines for cancer survivors recommend nutrition therapy to be included in the clinical management of oncology patients, with the aim to improve nutritional intake (i.e. energy, protein), maintain skeletal muscle mass, reduce treatment-related side effects, and in turn improve quality of life( Reference Arends, Bachmann and Baracos32 ). The current research in dietetic oncology predominantly focuses on improving the nutrition-related response to treatment (i.e. reducing malnutrition, nausea and early satiety); whilst this is of high clinical importance, this systematic review suggests the majority (86 %) of dietetic interventions measuring quality of life failed to find improvements relative to usual care or a comparison/usual care group, and collectively the pooled effects of nutrition therapy interventions showed no apparent effects on quality of life (SMD 0·07 (95 % CI –0·10, 0·24)). Body mass loss during cancer treatment is associated with decrements in quality of life( Reference Dewys, Begg and Lavin58 ), and in a clinical context, maintaining body mass is identified in the ESPEN guidelines as one of many clear nutrition-related goals for preventing malnutrition( Reference Arends, Bachmann and Baracos32 ). The majority (60 %) of interventions from this systematic review failed to improve body mass compared with a comparison or usual care, which may in part explain the minimal changes also seen in quality of life from the published literature. Total energy and protein intake is central to mitigating body mass and composition wasting in cancer patient; however, nutrition interventions showing between-group improvements in energy and protein compared with a usual care or comparison group( Reference Isenring, Bauer and Capra43 , Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 , Reference Uster, Ruefenacht and Ruehlin53 ), did not necessarily improve body mass from the intervention( Reference Isenring, Bauer and Capra43 ). Furthermore, nutrition therapy appears to maintain body mass during cancer treatment consistent with the ESPEN guidelines; however, maintenance of body mass may not be a surrogate marker for improving quality of life.
Cancer patients have elevated energy and protein requirements from the metabolic derangements from both the tumour and cancer treatment( Reference Fearon, Strasser and Anker59 , Reference Muscaritoli, Anker and Argilés60 ); thus, it is of high clinical importance to increase both energy and protein to address these metabolic demands and avoid potential treatment-related side effects associated with reductions in quality of life. Results from this systematic review suggest nutrition therapy is successful in improving energy and protein intake in cancer patients, with four out of the five interventions showing between-group improvements in protein and energy intake and also showing between-( Reference Isenring, Bauer and Capra43 ) or within-( Reference Ovesen, Allingstrup and Hannibal46 , Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) group improvements in quality of life. Protein intake is of high clinical importance during cancer treatment to avoid protein catabolism from muscle mass and cancer-related malnutrition( Reference Arends, Bachmann and Baracos32 ); both of which are associated with reduced quality of life( Reference Lis, Gupta and Lammersfeld61 ). Whilst energy prescription was similar between all four interventions, protein prescription in the study by Isenring et al. ( Reference Isenring, Bauer and Capra43 ) (1·2–1·5 g/kg per d) was higher compared with the other interventions of 0·8–1·0 g/kg per d( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ), and 1·0–1·2 g/kg per d( Reference Ovesen, Allingstrup and Hannibal46 ), and in part may explain the between-group improvements seen in quality of life from this intervention. Furthermore, clinically significant improvements in fat-free mass were observed in the intervention by Isenring et al. ( Reference Isenring, Bauer and Capra43 ); this may warrant future inclusion of physical functioning parameters into nutrition therapy investigations with cancer patients. Further exploration is necessary to determine the optimal protein requirements for mitigating muscle deconditioning during cancer treatment and the associated impact on quality of life.
Validated nutrition assessments, such as the PGSGA( Reference Ottery57 ) or the recommendations to assess nutrition status by Kubrak & Jensen( Reference Kubrak and Jensen62 ), determine changes in nutrition status by assessing anthropometrical parameters (body mass, subcutaneous fat and muscle wasting), symptoms impacting eating, functional status and the metabolic stress of the disease. Such tools may, in turn, be useful for isolating nutritional factors associated with improvements in the quality of life of cancer patients. When using a nutrition assessment tool, clinicians should start with a valid/reliable tool, then ensure the tool addresses cancer-specific nutritional concerns. The PGSGA is recommended in the ESPEN guidelines to be utilised by all dietitians in oncology to inform the nutrition care plan( Reference Arends, Bachmann and Baracos32 ). However, to date, systematic exploration of the PGSGA and quality of life in cancer patients has yet to be investigated. Successful interventions showing between-group improvements in the PGSGA (66 %) also showed between-( Reference Isenring, Capra and Bauer44 , Reference Silvers, Savva and Huggins52 ) and within-group( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) improvements in quality of life, further highlighting the importance of the PGSGA to inform the nutrition care plan. Minimising patient eating difficulties from cancer treatment is thought to be one strategy to improve quality of life during cancer treatment( Reference Capra, Bauer and Davidson63 ), and in turn, all interventions reducing nutrition-related difficulties (i.e. nausea, vomiting, swallowing) were associated with improvements in quality of life( Reference Ravasco, Monteiro-Grillo and Marques50 – Reference Silvers, Savva and Huggins52 ). In a clinical context, nutrition assessment tools can offer flexibility for different cancer patients, treatments and side effects to direct a patient-centred nutrition care process to improve quality of life. However, considering the majority (60 %) of studies in this systematic review failed to use a nutrition assessment tool, it is imperative future interventions consider the ESPEN guidelines and utilise the PGSGA, or another nutrition assessment tool, to identify patients at nutritional risk and inform the nutrition intervention.
Key parameters of the nutrition care plan were well reported in the studies included in this systematic review. Three of four 12-week interventions showed between-( Reference Isenring, Bauer and Capra43 ) or within-group( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) improvements in quality of life, whilst only two of seven interventions longer than 12 weeks showed between-group( Reference Silvers, Savva and Huggins52 ) or within-group( Reference Ovesen, Allingstrup and Hannibal46 ) improvements. All three interventions utilising face-to-face consults showed within-group improvements in quality of life( Reference Ovesen, Allingstrup and Hannibal46 , Reference Persson, Johansson and Sjöden49 , Reference Ravasco, Monteiro-Grillo and Marques50 ), whilst no between- or within-group differences were found with telephone or Internet interventions. There appeared to be minimal influence of the frequency of nutrition therapy delivery, with three of six interventions using weekly consults reporting between-( Reference Silvers, Savva and Huggins52 ) or within-group( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) improvements in quality of life. In contrast, the duration of nutrition consults was scarcely reported and impeded comparative analysis.
Limitations
Several methodological issues were identified among the included studies which confound the ability to explore relationships between various study variables and CRF or quality of life. The heterogeneity seen among the interventions in length of time, the duration of nutrition therapy consults, frequency and mode of consults (i.e. group-based, face-to-face), nutrition therapies (i.e. dietary requirements of energy, protein, dietary pattern interventions, weight loss interventions), compounded by the low number of articles included in this review, limits recommendations for clinical practice from this systematic review and meta-analysis. CRF was the primary outcome in only one intervention, and the low number of participants in each study suggests further investigations are needed to describe the potential effect of nutrition therapy on CRF. Furthermore, eight different cancers were included across fifteen interventions, which further limits any cancer-specific nutrition therapy recommendations for CRF and quality of life.
Nutrition guidelines for cancer survivors recommend dietary induced weight loss to achieve a healthy BMI for improved quality of life( Reference Rock, Doyle and Demark-Wahnefried33 ), however the literature to date offers minimal recommendations for clinical practice to achieve this per cancer type. Whilst some cancer (i.e. breast and prostate) treatments may induce weight gain, only two interventions( Reference Darga, Magnan and Mood41 , Reference Gnagnarella, Misotti and Santoro42 ) explored weight loss nutrition therapy on CRF and quality of life across breast and mixed cancer types. Interventions aiming to improve total energy and protein intake to avoid body mass loss were well described, however the reporting of dietary pattern and food group analysis was scarce across the literature, limiting the identification of relationships between dietary outcome characteristics with CRF and quality of life. The lack of reporting of dietary adherence was also a caveat in the literature, with only four of fifteen interventions reported adherence to the nutrition intervention( Reference Baldwin, Spiro and McGough39 , Reference Kiss, Isenring and Gough45 , Reference Paxton, Garcia-Prieto and Berglund48 , Reference Zick, Colacino and Cornellier54 ). Future nutrition investigations should include nutrition adherence measures (i.e. 24 h diet recalls or dietary pattern (serves/d)) to evaluate the feasibility and efficacy of prescriptive nutrition therapy on outcomes of CRF and quality of life. Approximately half (53 %) of the included studies did not blind data collectors, which may have confounded the accuracy of diet histories.
Limitations were also seen in the reporting of data for meta-analysis. The number of articles included for CRF and quality-of-life analysis was also fewer than ten studies, and as such this study could not perform funnel plots asymmetry of publication bias( Reference Sterne, Sutton and Ioannidis64 ). Both Ravasco et al. ( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) studies showed within-group improvements in CRF and within-group improvements in CRF; however, neither study reported measures of error in CRF and thus could not be included in the meta-analysis. Similarly for quality of life, Isenring et al. ( Reference Isenring, Capra and Bauer44 ) and Ravasco et al. ( Reference Ravasco, Monteiro-Grillo and Marques50 , Reference Ravasco, Monteiro-Grillo and Vidal51 ) showed between- and within-group improvements in quality of life but were not eligible for inclusion in the meta-analysis because neither study reported measures of error.
Future recommendations
Interventions specifically targeting oncology patients experiencing high levels of CRF will further strengthen the body of literature in dietetic oncology by limiting the ceiling effect of the CRF questionnaires. To establish comparative literature, future investigations require the inclusion of consistent and valid measures of CRF and quality of life. Results from this systematic review and meta-analysis revealed a large variety of CRF and quality-of-life measures are being used, and as such harmony is needed in these assessment measures. Future interventions should consider only using reliable and validated measures of CRF and quality of life; and from these questionnaires it is essential to select the tool that is best suited for the cancer population and the specific research question. The FACT-fatigue( Reference Yellen, Cella and Webster65 ) offers high internal consistency( Reference Cella, Eton and Lai66 ) and should be considered for future investigations in nutrition therapy on CRF. The FACT and EORTC quality-of-life tools are cancer specific, which should be utilised over generic quality-of-life measures.
Future interventions should investigate the potential CRF benefits of a high anti-inflammatory dietary pattern in different cancer types and treatments to determine the application of the dietary pattern across multiple oncological populations. Measuring diet changes through the Dietary Inflammatory Index( Reference Shivappa, Steck and Hurley67 ) may provide further insight to the efficacy of dietary therapy in CRF. Isolating and identifying the effects of nutrition therapy on biomarkers (i.e. inflammatory markers, body composition change) potentially associated with CRF will inform targeted dietetic literature and applied practice. Considering CRF as a treatment-related side effect, future interventions should also consider using the nutrition assessment tools, such as the PGSGA, and nutrition screening recommendations by Kubrak & Jensen( Reference Kubrak and Jensen62 ) to assess nutritional risk in CRF symptomatic patients.
Future investigation of all key parameters of the intervention is necessary (consult frequency, length, duration and mode) to inform the structure of the nutrition therapy. Future interventions should consider the importance of the timing of nutrition therapy (i.e. pre-, during- or post-treatment) to counteract treatment-related side effects and potentially improve quality of life. Quality (biologically available) and timing of protein, similar to that recommended in the dietetic and cancer cachexia literature( Reference Gullett, Mazurak and Hebbar68 , Reference Aoyagi, Terracina and Raza69 ), may offer novel insight to maintaining lean muscle, considering the anabolic demand from cancer-induced muscle wasting and potentially improving quality of life. In turn, future investigations are necessary to determine the optimal hypermetabolic energy and protein requirements on body composition and quality of life in cancer patients.
Conclusion
Systematic exploration of the available literature revealed dietetic intervention has no definitive benefit on CRF or quality of life in people with cancer and cancer survivors. Preliminary evidence indicates dietary pattern interventions high in fruit, vegetables, fish, nuts and seeds may improve CRF. This systematic review suggests assessing nutrition status through the PGSGA, to inform the nutrition therapy positively influences quality of life in cancer patients. Methodological caveats in the dietetic literature reveal heterogeneous reporting of nutrition therapy (i.e. dietary pattern, energy and protein requirements), length of interventions, duration, frequency and mode of consults, which limit the ability to ascertain whether dietetic interventions can improve CRF and/or quality of life. Furthermore, the low number of participants seen in the CRF literature, along with only one intervention with CRF as a primary outcome, suggests there is currently limited evidence exploring the potential effect of nutrition therapy on CRF. Harmony in CRF and quality-of-life tools are needed, with consistent and clear detail in the nutrition interventions, and adherence to dietary recommendations, to identify the optimal nutrition therapy for improving CRF and quality of life in cancer patients and survivors.
Acknowledgements
No external funding was received to support this work.
B. J. B., T. L. S. and O. R. L. W. were each responsible for the conception and design of the study. B. J. B. was responsible for the literature search, and B. J. B. together with O. R. L. W. screened for final inclusion of the articles. B. J. B. was responsible for data synthesis. B. J. B. wrote and edited the manuscript, with both T. L. S. and O. R. L. W. revising and editing the manuscript.
The authors have no relevant interests to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451800363X









