Meat is an important food item for human nutrition( Reference Pereira and Vicente 1 ); however, excessive meat intake, especially red and processed meat, has been linked to chronic diseases( Reference Daniel, Cross and Koebnick 2 ) such as CVD( Reference Micha, Wallace and Mozaffarian 3 ) and cancer( 4 ). According to a recent systematic review and a meta-analysis, involving more than one million people, the intake of 50 g/d of processed meat increases the risk of CVD by 42 %, and of diabetes by 19 %( Reference Micha, Wallace and Mozaffarian 3 ). Another meta-analysis( Reference Aune, Chan and Vieira 5 ) has reported that the intake of 110 g/d of red meat is associated with a 27 % increased risk of colorectal cancer, and the intake of 50 g/d of processed meat is associated with a 29 % increased risk of colorectal cancer. Other studies have reported an association between red and processed meat intake and risk of cancer of the stomach( Reference Zhu, Yang and Zhang 6 ), oesophagus( Reference Choi, Song and Song 7 , Reference Salehi, Moradi-Lakeh and Salehi 8 ), lungs( Reference Yang, Wong and Vogtmann 9 )and other organs.
There are some reasons for meat to be linked to the risk of chronic diseases, such as heterocyclic amines (HCA) that are potentially carcinogenic substances, formed during the cooking process of meat (baking, frying and barbequing)( Reference Micha, Wallace and Mozaffarian 3 , Reference Jägerstad and Skog 10 ), besides high saturated fat content, high cholesterol content, Na and nitrites that are added to processed meat.
During HCA metabolism, reactive species are formed, especially by cytochrome P450, which can cause oxidation of lipids, proteins and nucleic acids, resulting in oxidative stress, cell damage and loss of biological function, thus increasing the risk of CVD and cancer( 4 , Reference Santarelli, Pierre and Corpet 11 – Reference Hu, La Vecchia and Morrison 13 ).
The levels of HCA formed are highly dependent on the type of meat cooked, the method, the temperature and the duration of cooking( Reference Knize, Dolbeare and Carroll 14 , Reference Skog, Steineck and Augustsson 15 ), ranging from 1 to 80 ng/g of meat for PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine), the most abundant HCA in the human diet, followed by MeIQx (2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline) with usual amounts up to 6 ng/g of meat and DiMeIQx (2-amino-3,4,8-dimethylimidazo[4,5-f]quinoxaline) with amounts up to 1 ng/g of meat( Reference Rohrmann, Zoller and Hermann 16 ).
Despite the proven genotoxicity in various organisms, such as bacteria, Drosophila, cells in vitro and experimental rodents in vivo, the data are inconsistent in humans because it is difficult to identify the specific cell damage caused by HCA metabolism, and to estimate HCA intake( Reference Jägerstad and Skog 10 , Reference Gunter, Probst-Hensch and Cortessis 17 – Reference Ollberding, Wilkens and Henderson 19 ).
The aim of the present study was to investigate the association between HCA intake and oxidative stress, and the association between meat intake and oxidative stress.
Experimental methods
Study population and data collection
Data were obtained from a representative, complex, multistage probability-based cross-sectional study titled the Health Survey for Sao Paulo (ISA-Capital) conducted in Sao Paulo, Brazil, in 2008 and 2009. This survey collected information on health, food intake and lifestyle factors of the population of Sao Paulo city, an important financial and commercial centre in Latin America, and the most populous city in Brazil with approximately eleven million inhabitants in 2008.
A two-stage cluster sampling was used: census tracts and household. In the first stage, the census tracts were drawn using the probability of the number of households. In the second stage, the households were drawn using an inverse probability of the number of households. The drawing was systematic, and six study domains were defined by age groups and sex: females and males aged 13–19 years old (adolescents), 20–59 years old (adults) and 60 years old or over (elderly). It was estimated that a minimum sample size of 300 in each of the six domains was needed based on a prevalence of 0·5 with a standard error of 0·07 at a 5 % significance level and a design effect of 1·5. A total of 2691 individuals were selected to answer questions about life conditions and sociodemographic information.
After 1 year, a new contact was attempted with the same number of participants (n 2691) to new data collection (dietetic, biochemical, anthropometrical and sociodemographic information). After three tentative visits, made at different times (during weekdays and weekends), and six tentative telephone contacts, 62 % of the initial sample (1662 individuals) agreed to participate. Of those, 750 subjects (adolescents, adults and elderly) donated a blood sample. For the present study, only adults and elderly (n 561) were included.
Although the proportion of individuals who completed the study was similar by census tract and sociodemographic characteristics compared with the original sample, sampling weights were recalculated for each individual considering the sample design, non-response and post-stratification adjustment for sex and age group, in order to equalise the sociodemographic features of the sample. The School of Public Health of the University of São Paulo Ethics Committee approved the project. An informed consent form was obtained from all the participants.
Assessment of dietary intake
For the present study, dietary intake was estimated by one 24-h dietary recall (24HR). It was collected over 1 year covering all weekdays, weekends and seasons( Reference Thompson and Byers 20 ), administered by telephone using the Automated Multiple-Pass Method( Reference Dwyer, Picciano and Raiten 21 ). The telephone calls were made to the participant's home or their mobile phone. This method is structured in five steps: (1) quick list, where participants list all the foods and beverages consumed uninterruptedly; (2) forgotten list, where participants are asked about commonly forgotten foods consumed, such as candies, coffees and sodas; (3) time and location of food and beverage intake; (4) detailing cycle, where the way of preparation and amounts consumed are described; (5) final review, that verifies whether a certain food consumed during the day was not previously recorded( Reference Dwyer, Picciano and Raiten 21 , Reference Raper, Perloff and Ingwersen 22 ).
The household measures reported in 24HR were converted into g and ml according to standard Brazilian references, which measured many foods using a precision balance( Reference Pinheiro, Lacerda and Benzecry 23 , Reference Fisberg and Villar 24 ). Recipes were broken down into ingredients in order to estimate the amount of meat in each preparation.
Data from the 24HR were entered into the Nutrition Data System for Research – NDSR (version 5.0, 2007, Nutrition Coordinating Center at the University of Minnesota, Minneapolis, MN, USA)( 25 ), and were converted into energy and nutrients. The American database for the nutrition facts (energy, protein, carbohydrate and lipid) from the NDSR and the Brazilian nutrition facts database were compared. Only the foods from the NDSR that were similar (between 0·8 until 1·2 times) to Brazilian nutrition facts in terms of energy and macronutrients were considered.
Additionally, a detailed FFQ, based on the FFQ developed and validated by Cantwell et al. ( Reference Cantwell, Mittl and Curtin 26 ), was administered to each participant, containing information regarding the frequency of consumption of each food item, meat cooking preference (pan-fried, grilled, boiled, baked and microwaved) and level of meat doneness preference (medium, well done, and very well done for poultry and pork, and rare, medium, well done, and very well done for beef).
The amount of meat intake was estimated by the 24HR. The preferences of cooking methods and degrees of cooking, collected in the FFQ, were inputted in the 24HR to estimate the intake of HCA. These data were linked to the Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease (CHARRED) database to estimate the values of three HCA (PhIP, MeIQx and DiMeIQx).
Biochemical marker
Blood samples were obtained by venepuncture after a 12-h overnight fast by a nursing assistant. They were kept in a polystyrene box with ice packs, and transported to the laboratory for immediate centrifugation for 15 min at room temperature. After centrifugation, plasma samples were aliquoted and frozen at − 80°C until analysis.
The concentration of malondialdehyde (MDA) in the plasma was determined after derivatisation with thiobarbituric acid and quantification by HPLC/diode array, as described by Bastos et al. ( Reference Bastos, Loureiro and Oliveira 27 )and Hong et al. ( Reference Hong, Yeh and Chang 28 ). Internal tests revealed within-assay CV around 5 %, and between-assay CV around 16 %. The participants were divided into two groups based on the median of MDA concentration. The variable MDA was normally distributed, as determined by the Kolmogorov–Smirnov test.
Anthropometric measures
A trained research nurse measured body weight and height, using a standardised protocol. BMI was calculated as weight (in kg) divided by the square of height (in m). Nutritional status was determined based on BMI, using the cut-off points proposed by the WHO( 29 ) for adults and by Lipschitz( Reference Lipschitz 30 ) for the elderly.
Statistical analyses
Multivariate logistic regression models were estimated to verify the associations between the dependent variable (MDA concentration) and the following independent variables: meat intake (continuous variable); HCA intake (continuous variable); PhIP intake (continuous variable); MeqIQx intake (continuous variable); DiMeqIQx intake (continuous variable). The first model was a crude model (without co-variables); the second model was adjusted for age (continuous variable) and sex (female and male); the third model was further adjusted for energy intake (continuous variable), smoking (yes, no), BMI (healthy, overweight), skin colour (white, non-white), fruit and vegetable intake (continuous variable) and physical activity (active or not). OR and 95 % CI were estimated.
In the stratified analysis, mean and 95 % CI of MDA concentration were estimated according to the cooking methods and doneness levels for each kind of meat studied.
Differences between means were analysed using the Wald test, which calculates point estimates using F-statistics and considers the weights from complex samples. For categorical variables, the χ2 test was performed to compare the distribution of demographic characteristics. All analyses were conducted using the appropriate sample weights to account for the complex survey design. For all analyses, STATA® statistical software package version 10 (Stata Corporation) was used, and P< 0·05 was considered statistically significant.
Results
The sample comprised 39 % men and 61 % women; 52 % adults and 48 % elderly. The means and standard error of food intake and sociodemographic information are presented in Table 1. Participants were divided into two groups based on the median of MDA concentration.
Table 1 Descriptive characteristics of participants in the Health Survey for Sao Paulo (ISA-Capital) study (Mean values with their standard errors)

MDA, malondialdehyde; HCA, heterocyclic amines; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; DiMeIQx, 2-amino-3,4,8-dimethylimidazo[4,5-f]quinoxaline.
* χ2 for categorical variables; Wald-test for continuous variables.
The median MDA concentration was 0·71 μmol/l (25th percentile 0·49 μmol/l; 75th percentile 0·92 μmol/l), and it was not different between sex, smoking, skin colour and physical activity. Participants, who presented a MDA concentration above the median, consumed more HCA and PhIP than the below median participants. The same was not observed with DiMeIQx, MeIQx and meat intake (Table 1).
PhIP was the most consumed HCA, and beef was the most consumed meat (Table 1). ‘Well done’ was the doneness most cited among all varieties of meat, and pan-fried was the cooking method most mentioned. Beef contributed more to HCA amounts, followed by poultry and then pork (Table 2).
Table 2 Cooking methods, doneness level preferences and heterocyclic amine (HCA) intake in the Health Survey for Sao Paulo (ISA-Capital) study

MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; DiMeIQx, 2-amino-3,4,8-dimethylimidazo[4,5-f]quinoxaline.
* No one reported meat intake in the specific cooking method.
Participants who consumed grilled beef very well done presented more MDA concentration than other participants (Table 3).
Table 3 Malondialdehyde (MDA) concentration level by cooking methods and doneness levels for each meat intake in the Health Survey for Sao Paulo (ISA-Capital) study (Mean values and 95 % confidence intervals)

* Less than two participants reported meat intake in the specific cooking method and doneness level.
Table 4 shows the multivariate-adjusted OR for MDA >0·71 μmol/l by total meat intake and types of HCA intake. Positive and significant relationships between total HCA intake and MDA, as PhIP intake and MDA (P< 0·05), were observed. They remained significant after adjustment for smoking, sex, age, BMI, skin colour, energy, fruit and vegetable intake, and physical activity. However, MeIQx, DiMeIQx and meat intake were not significantly associated with MDA concentration.
Table 4 Association between malondialdehyde concentration levels (above and below 0·71 μmol/l) and kinds of heterocyclic amines (HCA) and meat intake in the Health Survey for Sao Paulo (ISA-Capital) study (Odds ratios and 95 % confidence intervals)
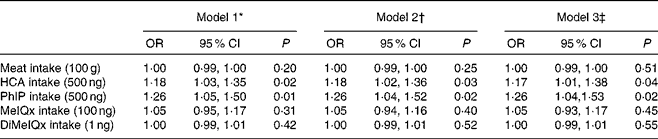
PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; DiMeIQx, 2-amino-3,4,8-dimethylimidazo[4,5-f]quinoxaline.
* Model 1: Crude model.
† Model 2: Adjusted for age and sex.
‡ Model 3: Adjusted for age, sex, smoking, BMI, energy intake, fruit and vegetable intake, skin colour and physical activity.
Discussion
The present study reported positive relationships between MDA concentration and HCA and PhIP intake in a population-based study. It showed that MDA concentration in the plasma can be increased by an intake of high total HCA and PhIP from meat, even after adjustment for sex, age, skin colour, physical activity, and intake of fruit, vegetable and energy, which can increase the risk of CVD and cancer.
Many studies( 31 – Reference Li, Li and Xie 33 ) have shown that HCA, especially PhIP, can produce reactive species and induce lipid peroxidation in vitro. A recent study has shown the relationship between PhIP intake and increased oxidative stress assessed by lipid peroxidation and protein oxidation in rats( Reference Li, Tian and Li 34 ). Other studies have reported that oxidative stress increases the risk of chronic diseases( Reference Stocker and Keaney 35 , Reference Dalle-Donne, Rossi and Colombo 36 ).
A hypothesis to explain that relationship between HCA and MDA is the biotransformation of HCA, which is a complex process to facilitate the excretion of these molecules. However, during the metabolism, unstable products (reactive species) can be formed( Reference Maeda, Sawa and Yubisui 32 , Reference Felton and Malfatti 37 , Reference Turesky and Le Marchand 38 ). These reactive species have important biological functions, such as gene activation and participation in defence mechanisms; nevertheless, when overproduced, they may favour the oxidation of lipids, proteins and nucleic acids, resulting in oxidative stress, cell damage and loss of biological function( Reference Barbosa, Costa and Alfenas 39 ), increasing the risk of chronic diseases( Reference Kryston, Georgiev and Pissis 40 , Reference Vassalle, Bianchi and Bianchi 41 ). MDA is the most used biomarker of lipid peroxidation, and its levels have been positively related to cancer and CVD( Reference Kryston, Georgiev and Pissis 40 , Reference Vasconcelos, Goulart and Moura 42 , Reference Perse 43 ).
The amount of HCA intake is variable worldwide. HCA intake was lower in the EPIC (European Prospective Investigation into Cancer and Nutrition) study( Reference Rohrmann, Zoller and Hermann 16 ) (69 ng/d) and in Sweden( Reference Augustsson, Skog and Jagerstad 44 ) (160 ng/d) than in Brazil. In the USA, more than 25 000 people consumed a mean of 455 ng/d( Reference Bogen and Keating 45 ), similar to that found in the present study.
Brazilians, in particular, are widely exposed to these compounds because overcooked meat is a very popular food item( Reference de Carvalho, César and Fisberg 46 ). In Sao Paulo, meat intake is excessive; more than 70 % of people consume above the national and international meat recommendations( Reference de Carvalho, César and Fisberg 46 , Reference Carvalho, Cesar and Fisberg 47 ). However, meat intake was not significantly associated with oxidative stress in the present study.
We reported that participants who consumed grilled beef very well done had a higher MDA concentration than other participants, evidencing that intake of very well done grilled beef may have contributed to increase oxidative stress when compared with other doneness levels. This suggests that intake of beef prepared as above could increase the exposure to a potentially harmful component of diet, supporting previous findings relating the intake of red meat cooked at high temperature to colon cancer( 48 , Reference Sinha, Peters and Cross 49 ).
An important consideration is that there are many sources of oxidative stress, such as smoking, radiation exposure, medication use, mitochondrial respiratory chain function, food intake and physical activity, in addition to HCA intake. For this reason, we adjusted regression models for other variables that could also contribute to increased oxidative stress.
HCA and PhIP intakes were reported to induce damage to other biological molecules, such as nucleic acids and proteins, leading to genetic damage and abnormal cell proliferation that may result in cancer( Reference Li, Tian and Li 34 , Reference Schraufstätter, Hyslop and Jackson 50 , Reference Lin, Kaderlik and Turesky 51 ). Further studies exploring HCA intake and other early damage biomarkers, such as DNA adducts, are needed.
The present study showed that high intake of HCA and PhIP may contribute to increase oxidative stress independently of lifestyle factors, increasing the risk of chronic diseases, such as cancer and CVD.
Acknowledgements
The authors thank the São Paulo Research Foundation (FAPESP – procedural 2009/15831-0, 2012/10965-0) and the National Council of Technological and Scientific Development (CNPq – procedural 481176/2008-0) for their financial support.
The scholarships were offered by São Paulo Research Foundation (FAPESP – procedural 2011/16225-6; 2012/10965-0; 2014/04607-0).
The authors declare that there is no conflict of interest.
The authors' contributions are as follows: A. M. C. carried out the study, biochemical analysis, data analyses, and drafted and wrote the manuscript; A. M. M. participated in the data analyses and drafted and wrote the manuscript; F. A. S. participated in the biochemical analysis and wrote the manuscript; A. P. M. L. participated in the biochemical analysis and drafted the manuscript; R. M. F. conceived the study, and participated in its design, coordination and drafted the manuscript; D. M. M. conceived the study, and participated in its design, coordination and helped to draft the manuscript.







