The NICE guidance on nutritional support in adults states: ‘If there is concern about the adequacy of micronutrient intake, a complete oral multivitamin and mineral supplement providing the reference nutrient intake for all vitamins & trace elements should be considered by a healthcare professional with the relevant skills & training in nutrition support who are able to determine the adequacy of a patient's dietary intake’(1). Implementation of this recommendation could carry a cost implication if it results in the increased use of vitamin and mineral supplements. The primary aim of the present audit was to assess the financial impact of implementing this guidance.
In a 4-week prospective audit the adequacy of micronutrient intake was assessed in all inpatients referred for dietetic intervention at Barts and the London NHS Trust (BLT). Inpatients are referred if they are at ‘high risk’ for malnutrition (based on the Trust Nutrition Screening Tool (NST)), require artificial nutritional support or via a blanket referral system used in certain directorates, i.e. Cancer Services and Infection and Immunity. It was beyond the capabilities of the present audit to accurately assess micronutrient intake as this aspect requires precise intake charts over a period of several days. Thus, an estimate of micronutrient intake was carried out by the dietitian using food intake charts, diet and drug history and volume of enteral feed delivered. For patients whose intake was considered inadequate, the number of vitamin and mineral pills required was recorded until intake was satisfactory or the patient was discharged.
During the 4-week audit 205 patients were assessed. The Table shows the number of patients who were considered to have an inadequate intake and the number of vitamin and mineral pills required.
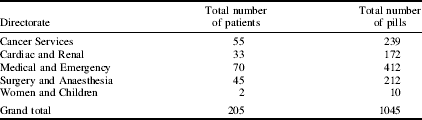
The projected cost for providing a micronutrient supplement to all patients identified in the audit was approximately £12, based on BLT issue price at the time of the audit, which may vary as cost is subject to contract prices. As the projected cost to the Trust was minimal and it has been shown that micronutrient supplementation reduces the length of hospital stay(Reference Vlaming, Biehler and Hennessey2), it has been recommended that a multivitamin and mineral supplement be prescribed to all patients identified by the NST at ‘high risk’ for malnutrition.
Guidance on patient assessment and the recommendation of vitamin and mineral supplementation needs to be developed. This guidance must identify exclusions, such as patients receiving chemotherapy, as certain vitamins decrease the efficacy of chemotherapy(Reference Werneke, Earl and Sevdel3), patients having regular dialysis (who are routinely supplemented with water-soluble vitamins), patients with cystic fibrosis (who are routinely supplemented with fat-soluble vitamins) and patients receiving full requirements from enteral or parenteral nutrition.
Whilst patients at ‘moderate risk’ for malnutrition were not included in this audit, it is likely that they might have inadequate intake and would benefit from micronutrient supplementation. A further audit is needed to assess this issue.


