Thiamine (C12H16N4OS), also known as vitamin B1, is a water-soluble substance. Thiamine has considerable metabolic importance due to its role as a cofactor in carbohydrate and energy metabolism in organisms( Reference Bubber, Ke and Gibson 1 ). Almost 90 % of ruminal thiamine is synthesised by microorganisms, with the rest coming from feedstuff( Reference Breves, Brandt and Hoeller 2 ). In National Research Council recommendations( 3 ), the ruminal synthesis of thiamine was estimated to be 143 mg/d for a 650-kg cow producing 35 kg of 4 % fat-corrected milk/d; this amount seems to meet the requirement of dairy cows, which was assumed to be approximately 21–47 mg/d( Reference Zintzen 4 ). Therefore, there is no dietary thiamine recommendation in the National Research Council( 3 ). However, the estimated thiamine synthesis of lactating cows in the National Research Council( 3 ) were extrapolated from steer data of Miller et al.( Reference Miller, Meiske and Goodrich 5 ) and Zinn et al. ( Reference Zinn, Owens and Stuart 6 ). To investigate the validity of the extrapolated approach in the National Research Council( 3 ), Schwab et al. ( Reference Schwab, Schwab and Shaver 7 ) measured ruminal apparent synthesis (AS) of thiamine in dairy cows (51 mg/d), and calculated National Research Council( 3 ) estimation of thiamine AS (127 mg/d) by adjusting to the measured DM intake of dairy cows, and they found that thiamine synthesis was overestimated in the National Research Council( 3 ). Furthermore, thiamine deficiencies have been found in steers when diets are high in sulphate( Reference Gould, McAllister and Savage 8 ) or when diets cause a sudden drop in ruminal pH( Reference Zinn, Owens and Stuart 6 ). Indeed, numerous studies have shown positive responses in dairy cows to thiamine supplementation, such as increased milk and milk component production( Reference Shaver and Bal 9 , Reference Kholif, Hanafy and El-Shewy 10 ) and attenuated subacute ruminal acidosis (SARA)( Reference Pan, Yang and Xue 11 – Reference McDowell 13 ). To update our knowledge of thiamine in dairy cows and to ascertain the effects of thiamine on dairy cow performance and metabolism, the current literature is reviewed herein covering thiamine synthesis in the rumen, the amount of thiamine arriving at the duodenum, the effects of thiamine supplementation on milk performance and the mode of action of thiamine on rumen fermentation, especially regarding SARA attenuation.
Biochemical functions and application of thiamine in ruminants
Thiamine is an essential nutrient for dairy cows and other mammals. By serving as a cofactor of enzymes, including transketolase, α-ketoglutarate dehydrogenase, pyruvate dehydrogenase, and branched chain α-keto acid dehydrogenase, thiamine plays a critical role in carbohydrate metabolism( Reference Subramanya, Subramanian and Said 12 ). Independent of its role as a co-enzyme, thiamine also has specific roles in neuronal communication, immune system activation, signalling and maintenance processes in cells and tissues( Reference Subramanya, Subramanian and Said 12 ). Severe thiamine deficiency in cattle leads to various clinical effects, from anorexia to polioencephalomalacia( Reference McDowell 13 ), while the clinical polioencephalomalacia is not the main point of this review and will not be discussed in detail. For rumen bacteria, thiamine is indispensable for the growth of the rumen bacteria Ruminococcus albus ( Reference Bryant and Robinson 14 ) and Ruminococcus flavefasciens ( Reference Ungerfeld, Rust and Burnett 15 ), serves as a cofactor of phosphoenolpyruvate decarboxylase in Bacteroides fragilis ( Reference Zhang, Dai and Lu 16 ) and participates in the production of acetyl-CoA via pyruvate-ferredoxin oxidoreductase in Megasphaera, Selenomonas ruminantium, Butyrivibrio fibrisolvens and Ruminococcus ( Reference Russell 17 ).
Due to the key functions of thiamine in dairy cows and rumen bacteria, a thorough understanding of thiamine status in dairy cows is crucial, and there has been more focus on supplying thiamine in ruminant diets. For example, Shaver & Bal( Reference Shaver and Bal 9 ) found that the milk and component production tended to be increased by thiamine supplementation (300 mg/d) when dairy cows fed diets high in non-fibre carbohydrate. Similarly, Kholif et al. ( Reference Kholif, Hanafy and El-Shewy 10 ) also found that feeding lactating cows a daily ration supplemented with 340 mg of thiamine increased milk yield, milk fat and protein yields. The improvement in milk performance may be related to an increase in precursors of milk components due to thiamine supplementation, since Solouma et al. ( Reference Solouma, Kholif and Hamdon 18 ) demonstrated that adding 40 mg/d thiamine to the diet of sheep significantly increased the blood concentrations of albumin, globulin and glucose. However, there are discrepancies surrounding the effects of thiamine on ruminant’s metabolism. Rowghani et al. ( Reference Rowghani, Zamiri and Ebrahimi 19 ) found that thiamine supplementation (0, 4 and 6 mg/kg DM) in lambs fed a high concentrate diet had no effect on blood glucose level. Silzell et al. ( Reference Silzell, Hellwig and Kegley 20 ) reported that supplemental thiamine did not improve zantibody response and cell-mediated immune response. The inconsistent results concerning the effect of thiamine on ruminant metabolism may be related to differences in thiamine supplementation levels, dietary nutritional composition and physiological stage.
Microbial synthesis, degradation and absorption of thiamine in the gastrointestinal tract of ruminants
The requirement for thiamine in ruminants is mainly met by microbial synthesis, with a small portion coming from feedstuffs degradation. The thiamine content is higher in cereal feeds than in forage feedstuffs (Table 1). Similarly, Tafaj et al. ( Reference Tafaj, Schollenberger and Feofilowa 25 ) reported a higher thiamine content in concentrate than in hay (3·78 v. 0·4 mg/kg DM), and different concentrate:hay ratios influenced dietary thiamine intake. However, ruminal thiamine concentration was negatively correlated with dietary thiamine intake and was assumed to be more closely related to the concentrate level than to dietary thiamine content because of the effect of concentrate on rumen conditions( Reference Tafaj, Schollenberger and Feofilowa 25 ). The actual ruminal thiamine synthesis by microbes is extremely difficult to measure as the rumen is a dynamic system in which thiamine synthesis, degradation and absorption occur simultaneously during passage along the digestive tract( Reference Miller, Meiske and Goodrich 5 , Reference Schwab, Schwab and Shaver 7 , Reference Beaudet, Gervais and Graulet 23 , Reference Kon and Porter 26 , Reference Castagnino, Kammes and Allen 27 ). Therefore, ruminal thiamine AS is calculated by subtracting daily orts-corrected thiamine intake from the amount reaching the duodenum. This calculation does not reflect actual thiamine synthesis, as it ignores ruminal degradation, microbial use, or potential absorption across the rumen wall. The thiamine AS has been measured in some studies and data are provided in Table 2. Schwab et al.( Reference Schwab, Schwab and Shaver 7 ) reported that the average ruminal thiamine AS in lactating dairy cows is 50·6 mg/d, which is similar to the value of 51·7 mg/d reported by Breves et al. ( Reference Breves, Brandt and Hoeller 2 ) and >26·0 mg/d reported by Santschi et al. ( Reference Santschi, Berthiaume and Matte 29 ). In the recent studies by Beaudet et al. ( Reference Beaudet, Gervais and Graulet 23 ), Castagnino et al. ( Reference Castagnino, Kammes and Allen 27 ) and Seck et al. ( Reference Seck, Linton and Allen 28 ), the ruminal thiamine AS was negative (from −39·8 to 0·8 mg/d), which they hypothesised was due to thiamine destruction by thiaminase enzymes or degradation by the ruminal microflora. Based on the above studies, we can conclude that dietary factors such as feed type and nutrient composition influences thiamine synthesis. Castagnino et al. ( Reference Castagnino, Kammes and Allen 27 ) reported that long cut alfafa silage reduced thiamine AS which proved that feed type affects thiamine synthesis in the rumen. In addition, Buziassy & Tribe( Reference Buziassy and Tribe 30 ) found that the thiamine concentration in rumen fluid was significantly reduced when dietary protein levels decreased. Breves et al. ( Reference Breves, Brandt and Hoeller 2 ) showed that ruminal actual thiamine synthesis and the amount of thiamine flowing to the duodenum decreased when 26 % of dietary N was deducted from the diet, and the amount of ruminal thiamine synthesis positively correlated with duodenal microbial N flow (R 0·76), indicating that dietary N levels and microbial metabolism in the rumen affect thiamine synthesis.
Table 1 Summary of thiamine concentrations of several feeds

Table 2 Duodenal flow and ruminal apparent synthesis of thiamine in dairy cows

ND, data are not detected.
* Composition of concentrate: 32·8 % barley, 24 % wheat, 14 % tapioca meal, 2·6 % maize, 23·6 % soyabean meal, 1·0 % soyabean oil, 2·0 % minerals.
† Composition of concentrate: 32·5 % barley, 6·1 % wheat, 36·8 % tapioca meal, 15·7 % maize, 5·9 % soyabean meal, 1·0 % soyabean oil, 2·0 % minerals.
‡ Composition of concentrate: 31·0 % barley, 5·8 % wheat, 35·0 % tapioca meal, 14·9 % maize, 5·5 % soyabean meal, 1·0 % soyabean oil, 2·0 % minerals, 4·8 % urea.
§ Composition of concentrate: the same as concentrateFootnote ‡ but an increased frequency of feeding.
In addition to dietary N, dietary carbohydrate sources and levels also affect ruminal thiamine synthesis. Schwab et al. ( Reference Schwab, Schwab and Shaver 7 ) discovered that increasing non-fibre carbohydrates from 30 to 40 % tended to decrease daily ruminal thiamine AS in cows fed a 35 % forage diet, and the opposite effect occurred for cows fed a 60 % forage diet. Castagnino et al. ( Reference Castagnino, Kammes and Allen 27 ) reported that thiamine AS was negatively correlated with the amounts of organic matter and ruminally digested starch. Tafaj et al. ( Reference Tafaj, Schollenberger and Feofilowa 25 ) also found that ruminal thiamine content was higher under conditions of 40 and 25 % concentrate in the diet than under conditions of 50 and 60 % concentrate, despite higher thiamine content in concentrate than in forage feedstuff. Thus, Tafaj et al. ( Reference Tafaj, Schollenberger and Feofilowa 25 ) assumed that the ruminal thiamine concentration was more closely related to the concentrate level, that is the intake of energy and digestible organic matter, than to the dietary thiamine content. The effect of dietary carbohydrates on thiamine concentration may be due to its influence on rumen fermentation and the microbial community. Castagnino et al.( Reference Castagnino, Kammes and Allen 27 ) and Seck et al. ( Reference Seck, Linton and Allen 28 ) reported a positive correlation between ruminal pH and thiamine AS. The impact of pH on thiamine status may be related to the increasing thiaminase production at low ruminal pH, since pH values below 5·8( Reference Boyd and Walton 31 ) are optimal for thiaminase-producing bacteria (C. sporogenes and a few species of Bacillus; Brent & Bartley( Reference Brent and Bartley 32 )), as a result, thiamine degradation by microbial thiaminase increased. Besides, thiamine synthesis by Bacteroidetes, Fibrobacter and Pyramidobacter decreased under low ruminal pH condition( Reference Pan, Xue and Nan 33 ), which also contribute to their positive correlation between ruminal pH and thiamine AS. However, Schwab et al. ( Reference Schwab, Schwab and Shaver 7 ) reported a negative correlation between thiamine AS and ruminal pH values, whereas Beaudet et al. ( Reference Beaudet, Gervais and Graulet 23 ) didn’t find any relationship between these two parameters. This discrepancy may be related to the different extent of pH decrease under different dietary conditions: in the study of Castagnino et al.( Reference Castagnino, Kammes and Allen 27 ), the average ruminal pH values for dairy cows fed alfalfa silage and grass silage are 6·26 and 5·84, respectively; Seck et al. ( Reference Seck, Linton and Allen 28 ) reported that pH values of dairy cows fed high forage and low forage are 6·0 and 5·86, respectively; whereas the ruminal pH values in the study by Schwab et al. ( Reference Schwab, Schwab and Shaver 7 ) were approximately above 6·0, thus there was less thiaminase available to degrade thiamine.
Ruminal thiamine exists in a free or bound form or contained in rumen microorganisms. Currently, the proportion of thiamine absorbed in the rumen and intestine is difficult to determine and is unclear. The disappearance rate was used to indicate thiamine fate in the gastrointestinal tract in the study by Santschi et al. ( Reference Santschi, Berthiaume and Matte 29 ), who found that 67·8 % of thiamine disappears before the duodenum and that there is almost no disappearance of postruminally infused thiamine before the duodenum, suggesting extensive ruminal destruction or utilisation of thiamine. Zinn et al. ( Reference Zinn, Owens and Stuart 6 ) reported the ruminal disappearance rate of thiamine is 47·7 %. The fate of thiamine that disappears from the rumen is unclear, and it is unknown whether thiamine absorption can occur in the rumen. Through permeability measurements on sheep rumen mucosa at several thiamine concentrations (0·1–12·8 μg/ml), Hoeller et al. ( Reference Hoeller, Fecke and Schaller 34 ) found that the rumen wall mucosa has a low permeability to thiamine. Similarly, Smith & Marston( Reference Smith and Marston 35 ) demonstrated that thiamine is not absorbed in appreciable amounts through the rumen wall. However, Rérat et al. ( Reference Rérat, Champigny and Jacquot 36 ) and McDowell( Reference McDowell 37 ) reported that the rumen wall is just not permeable to bound thiamine or thiamine contained in rumen microorganisms, but ruminants can absorb free thiamine from the rumen wall by an active transport mechanism. Regarding intestinal thiamine absorption, the intestinal disappearance of thiamine is 75 %( Reference Santschi, Berthiaume and Matte 29 ), and 90–96 % of thiamine that reaches the duodenum in sheep is in a microbial fraction( Reference Breves, Hoeller and Harmeyer 38 ), indicating the high absorption of microbially produced thiamine in the intestine. Free thiamine can be degraded by ruminal microbes or absorbed through the rumen wall, while the absorption of bound thiamine or thiamine in ruminal microorganisms mainly occurs in the intestine and plays an important role in supplying this nutrient to host ruminants.
Subacute ruminal acidosis induction altered thiamine status in ruminants
As mentioned above, increasing dietary non-fibre carbohydrate levels can decrease the daily AS of thiamine in the rumen( Reference Schwab, Schwab and Shaver 7 ) and may cause thiamine deficiency. SARA caused by overfeeding a high-grain diet is known to reduce ruminal pH and microbial activity( Reference Mao, Huo and Liu 39 ) and may, therefore, affect thiamine production. The relationship between thiamine status and SARA has attracted increasing attention. Recently, researchers( Reference Pan, Yang and Xue 11 , Reference Dabak and Gul 40 , Reference Karapinar, Dabak and Kizil 41 ) have reported that thiamine deficiency occurs when sheep or cattle have subacute or acute ruminal acidosis. To better understand how SARA induction interacts with thiamine status, the methods for diagnosing thiamine deficiency and the impact and possible mechanisms of SARA on ruminal and blood thiamine concentrations are reviewed in the following section and summarised in Fig. 1.
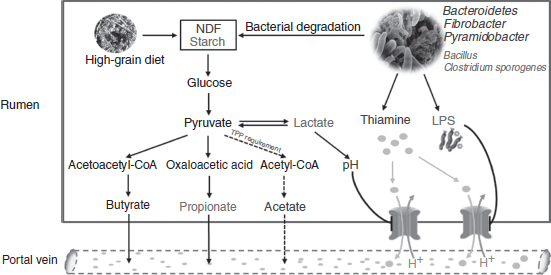
Fig. 1 The potential mechanisms by which high-grain-induced subacute ruminal acidosis alters thiamine status in the rumen and blood of dairy cows. When dairy cows are fed a high-grain diet, more starch and less neutral-detergent fibre (NDF) reach the rumen. Then, carbohydrates are decomposed to volatile fatty acids (VFA) through pyruvate by bacterial degradation. During this process, pyruvate accumulates, and more thiamine (in the form of the cofactor thiamine pyrophosphate (TPP)) is needed for the conversion of pyruvate to acetyl-CoA. As a result, thiamine concentrations decrease, and more pyruvate flows to lactate and propionate, resulting in decreased pH. In addition, the abundance of thiamine-synthesising bacteria, including Bacteroidetes, Fibrobacter and Pyramidobacter, decrease, whereas those of thiamine-degrading bacteria, such as Bacillus and C. sporogenes, increase under high-grain feeding; these changes also contribute to decreased thiamine concentrations in the rumen. Decreased ruminal thiamine content and hampered thiamine transport in response to low ruminal pH and lipopolysaccharide (LPS) accumulation act together to alter thiamine status in the blood. Red text, increase; blue text, decrease.
Thiamine deficiency diagnosis
The erythrocyte thiamine pyrophosphate (TPP) effect is a criteria for assessing thiamine status, and thiamine deficiency can be diagnosed when TPP effect is >45 %( Reference Rehm, Zerobin and Christeller 42 ). Recent literatures demonstrated that blood TPP effect increased along with rumen acidosis induction, for example, Dabak & Gul( Reference Dabak and Gul 40 ) found that the mean TPP effect was 25·5 % in normal sheep and 59·4 % in sheep with chronic ruminal acidosis; Karapinar et al. ( Reference Karapinar, Dabak and Kizil 41 ) also demonstrated that the TPP effect was significantly higher in feedlot cattle fed a high concentrate diet (47·2 %) than in control cattle (19·5 %).
Other criteria of thiamine deficiency include increased lactate and pyruvate concentrations in the blood due to the impaired co-enzyme function of thiamine in enzymatic decarboxylation reactions( Reference Pill 43 ). Dabak & Gul( Reference Dabak and Gul 40 ) found that increased l-lactate and pyruvate concentrations in sheep with chronic ruminal acidosis were probably related to thiamine deficiency. In addition, blood thiamine concentrations are suggested as an indicator of thiamine status. Hill et al. ( Reference Hill, Rammell and Forbes 44 ) proposed a reference range of 19·90–49·09 μg/l for cattle, and concentrations below 13·27 μg/l are considered indicative of deficiency. Gooneratne et al.( Reference Gooneratne, Olkowski and Klemmer 45 ) found that the average blood thiamine concentration was 49·2 (sd 14·9) μg/l in dairy steers, and Olkowski et al. ( Reference Olkowski, Christensen and Rousseaux 46 ) reported this concentration to be 24·85 (sd 10·1) μg/l in beef cattle.
Subacute ruminal acidosis alters thiamine status in ruminal fluid
Our previous study( Reference Pan, Yang and Xue 11 ) demonstrated that high-grain-induced SARA alters the thiamine status in ruminal fluid, the thiamine concentration in SARA cows (2·97 μg/l) was lower than that in control cows (7·88 μg/l). This decrease in thiamine concentrations in rumen fluid is mainly caused by an increased thiamine requirement, decreased bacterial thiamine synthesis and increased thiamine degradation in the rumen compared with a high forage diet, and these three possible reasons are explained separately below.
First, when SARA is induced by a high-grain diet, many dietary carbohydrates are fermented and converted into large amounts of pyruvate. Then, pyruvate is converted to lactate by lactate dehydrogenase( Reference Chen, Luo and Wang 47 ) or degraded to acetyl-CoA and formate by pyruvate formate-lyase( Reference Asanuma and Hino 48 ). TPP is the cofactor of pyruvate formate-lyase( Reference Knappe, Schacht and Mockel 49 ), therefore, more thiamine is required and used for the decarboxylation of pyruvate, which thus contributes to decreasing the thiamine content in the rumen. Once thiamine deficiency occurs, the conversion of pyruvate to acetyl-CoA is blocked, and the flow of pyruvate to lactate is enhanced, which will aggravate SARA in dairy cows.
Second, the thiamine requirement in ruminants is mainly met by bacterial synthesis in the rumen( Reference Miller, Meiske and Goodrich 5 ). Magnusdottir et al.( Reference Magnusdottir, Ravcheev and de Crecy-Lagard 50 ) noted that thiamine synthesis is most prevalent in Bacteroidetes and Fusobacteria among thiamine-synthesising bacteria. Silverman & Werkman( Reference Silverman and Werkman 51 ) reported that certain propionate-producing bacteria make thiamine or its intermediates, and Bacteroidetes is the main genus that produces propionate from carbohydrates by the succinate pathway( Reference Louis, Hold and Flint 52 ). Pan et al. ( Reference Pan, Xue and Nan 33 ) found that the ruminal thiamine content was positively correlated with the genera Bacteroidetes, Fibrobacter and Pyramidobacter and deduced that these genera play important roles in thiamine biosynthesis. However, the abundance of Pyramidobacter ( Reference Pan, Xue and Nan 33 ), Bacteroidetes ( Reference Mao, Zhang and Wang 53 , Reference Khafipour, Krause and Plaizier 54 ) and Fibrobacter ( Reference Fernando, Purvis and Najar 55 ) in the rumen was reduced by SARA induction. As a result, total bacterial thiamine synthesis in the rumen decreases, which may contribute to the altered thiamine status in ruminal fluid.
In addition, thiamine deficiency can occur due to increased thiamine degradation by thiaminase in the rumen( Reference Brent 56 ). Harmeyer & Kollenkirchen( Reference Harmeyer and Kollenkirchen 57 ) reported that approximately 90 % of thiamine in the rumen is present in particle-free rumen fluid as free thiamine and is readily accessible to extracellular microbial thiaminase( Reference Edwin and Jackman 58 ). A decrease in ruminal pH caused by high-grain diets was thought to increase thiaminase production( Reference Brent 56 ). C. sporogenes and a few species of Bacillus are the main culprits of ruminal thiaminase( Reference Brent and Bartley 32 ); these species have optimum pH values of 5·2 and 5·6, respectively( Reference Boyd and Walton 31 ). The growth of C. sporogenes and Bacillus increased when SARA (ruminal pH below 5·8) was induced by high-grain feeding, resulting in increased thiaminase production and consequent thiamine degradation( Reference Randhawa, Ahuja and Rathor 59 , Reference Dunlop 60 ). Enhanced thiaminase activity in acidotic ruminal fluid has been reported previously( Reference Brent 56 , Reference Randhawa, Ahuja and Rathor 59 ). The above studies indicate that thiamine degradation in the context of SARA also contributes to the altered ruminal thiamine status and that both the synthesis and degradation of thiamine should be considered when explaining the ruminal thiamine status.
Subacute ruminal acidosis alters the thiamine status in blood
Ruminal bacteria first synthesise thiamine, which is then absorbed and transported via the portal vein to the liver by active transport and simple diffusion( Reference Bräunlich and Zintzen 61 ). Pan et al. ( Reference Pan, Yang and Beckers 62 ) investigated the effect of high-grain feeding on blood thiamine status and found that the blood thiamine concentration in high-grain-fed cows (11·66 μg/l) decreased below 13·27 μg/l, indicating that high-grain feeding results in thiamine deficiency and alters the blood thiamine status in dairy cows. The blood thiamine content was positively correlated to rumen thiamine concentration( Reference Pan, Yang and Beckers 62 ), so insufficient bacterial thiamine synthesis in the context of SARA partially accounts for the low blood thiamine level( Reference Dabak and Gul 40 ). On the other hand, ruminants can absorb free thiamine from the rumen by an active transport mechanism( Reference McDowell 37 ) involving transporter-1 and -2 (THTR1 and THTR2)( Reference Zhu, Fang and Subramanian 63 ). THTR1 and THTR2 are pH sensitive( Reference Arun, Antoinette and I, David 64 ), and their expression can be decreased by lipopolysaccharide (LPS) and pro-inflammatory cytokines( Reference Zhu, Fang and Subramanian 63 ). During SARA challenge, the ruminal pH declines and LPS production increases in response to high-grain overfeeding, which down-regulates THTR2 expression and consequently represses thiamine transport, thereby contributing to low thiamine concentrations in the blood( Reference Pan, Yang and Beckers 62 ).
Taken together, SARA induction affects ruminal thiamine status by increasing the thiamine requirement, decreasing bacterial thiamine synthesis, and increasing thiamine degradation in the rumen. The decreasing ruminal thiamine concentrations and reducing thiamine absorption and transport contributed to the altered thiamine status in blood.
Thiamine supplementation helps to attenuate subacute ruminal acidosis
It is well known that SARA induced by overfeeding a high-grain diet decreases the ruminal pH, alters the rumen microbial population, and increases the concentration of LPS in rumen fluid( Reference Khafipour, Krause and Plaizier 54 , Reference Plaizier, Li and Le Sciellour 65 ). Specifically, SARA challenge leads to a reduction in the abundance of cellulolytic bacteria( Reference Fernando, Purvis and Najar 55 ), an increase in the proportion of starch-fermenting and lactic acid-producing bacteria( Reference Khafipour, Li and Plaizier 66 ), and enhanced lysis of gram-negative bacteria associated with increased ruminal LPS( Reference Khafipour, Krause and Plaizier 54 ). The alterations in ruminal LPS and pH could act synergistically to disrupt barrier function( Reference Emmanuel, Madsen and Churchill 67 ), once the epithelium has been breached, mucosa-associated lymphoid tissue cells respond by triggering local inflammation via the LPS/toll-like receptor 4 (TLR4) signalling pathway, leading to the excessive production of pro-inflammatory cytokines( Reference Kurashima, Goto and Kiyono 68 , Reference Zhang, Zhu and Mao 69 ). Moreover, an impaired gastrointestinal epithelium facilitates the translocation of LPS from the digestive tract into circulation, causing metabolic alterations and systemic inflammation in host cattle( Reference Gressley 70 ), which greatly impacts the production and health of dairy cows.
Currently, there is an increasing focus on SARA prevention. Interestingly, our recent studies have shown that dietary thiamine supplementation (180 mg/kg DM intake) may be a new strategy for SARA prevention. As illustrated in Fig. 2, thiamine supplementation attenuates SARA mainly by improving rumen fermentation( Reference Pan, Yang and Xue 11 ), balancing the ruminal bacterial community( Reference Pan, Xue and Nan 33 , Reference Wang, Pan and Wang 71 ) and exerting anti-inflammatory effects( Reference Pan, Yang and Beckers 62 ) in dairy cows. Specifically, Pan et al. ( Reference Pan, Xue and Nan 33 ) showed that thiamine administration promotes the growth of the bacterial community associated with the degradation of fibre (Ruminococcus 1, Pyramidobacter and Succinivibrio)( Reference Bainbridge, Cersosimo and Wright 72 ) and polysaccharides (Bacteroides)( Reference Flint, Bayer and Rincon 73 ), and decreases the abundance of bacteria positively related to ruminal lactate content (Succiniclasticum and Ruminococcaceae NK4A214). As a result, ruminal fermentation and lactate degradation improved, and ruminal pH increased( Reference Pan, Yang and Xue 11 ). Meanwhile, ruminal acetate content in SARA cows supplemented with thiamine increased with a higher abundance of fibre-digesting bacteria, and acetate was then transported to the mammary gland to produce more milk fat( Reference Pan, Yang and Beckers 62 ). On the other hand, during a SARA challenge, the death and lysis of gram-negative rumen bacteria, especially Bacteroidetes spp., are the main sources of free LPS in the rumen( Reference Mao, Zhang and Wang 53 ). Then, free LPS triggers the release of pro-inflammatory cytokines by activating the TLR4/NFκB signalling pathway( Reference Lin, Wang and Xue 74 ). In the study by Pan et al. ( Reference Pan, Yang and Beckers 62 ), the proportion of Bacteroides increased, with a subsequent decrease in free LPS, upon infusion of thiamine in SARA cows. The decreased LPS content and inhibited NFκB activation by exogenous thiamine act together to reduce the production of pro-inflammatory cytokines, and thereby attenuate local inflammation in the rumen epithelium. The anti-inflammatory effects of thiamine have also been demonstrated in rats( Reference Shoeb and Ramana 75 ) and humans( Reference Gonzalez-Ortiz, Martinez-Abundis and Robles-Cervantes 76 ).
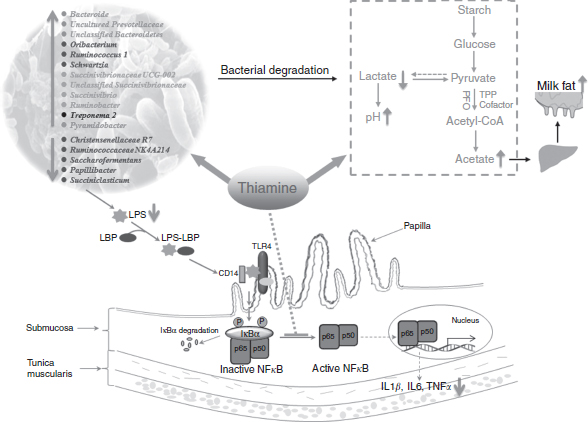
Fig. 2 The potential mechanisms by which thiamine supplementation attenuates high-grain-induced subacute ruminal acidosis in dairy cows. Thiamine supplementation can balance the bacterial community by increasing the abundance of cellulolytic bacteria, including Bacteroides, Ruminococcus 1, Pyramidobacter, Succinivibrio, and Ruminobacter. Such increases enhance fibre degradation and ruminal acetate production; then, increased concentrations of acetate are transported to the mammary gland to increase milk fat synthesis. On the other hand, thiamine supplementation suppresses the ruminal epithelium inflammatory response by decreasing ruminal lipopolysaccharide (LPS) production and repressing NFκB protein activation. TPP, thiamine pyrophosphate; LBP, LPS-binding protein.
Conclusions and future research
Notwithstanding the knowledge gaps described above, the vital function of thiamine in dairy cows is obvious, including its participation in carbohydrate metabolism in the rumen and host ruminants, regulation of rumen fermentation conditions, and stimulation of milk performance. However, several issues must be addressed to thoroughly understand the thiamine nutritional status in dairy cows. The following are some of the limitations of the current knowledge that need to be resolved:
(1) There is no conclusive dietary recommendation of thiamine in dairy cows. To establish a recommendation, the minimum requirement of thiamine must be estimated. During this process, the amount of thiamine from dietary sources that escapes degradation in the rumen and the amount of thiamine synthesised in the rumen must be ascertained. Unfortunately, knowledge of the factors controlling the amount of thiamine escaping the rumen and thus available for absorption by dairy cows is limited. Therefore, the major challenge in determining the thiamine requirement in dairy cows is predicting thiamine supply dynamically according to different dietary chemical compositions. Hence, more studies are needed to reveal how diet composition (e.g. type of forage, starch concentration and protein concentration) affects the fate of thiamine in the rumen and the amount available for cows at different physiological stages. In addition, the simultaneous determination of ruminal, duodenal, blood and milk biomarkers of thiamine would be useful to understand the fate of thiamine in dairy cows.
(2) Although Pan et al. ( Reference Pan, Xue and Nan 33 ) deduced the possible genera associated with thiamine synthesis based on the positive correlation between thiamine content and the abundance of a particular genera, whether these genera actually participate in thiamine synthesis is still inconclusive. Hence, more experiments, such as the isolation and culture of related bacteria in vitro, are necessary to verify their thiamine synthesis ability. In addition, the rumen is inhabited by a multitude of microorganisms, including bacteria, protozoa, and yeast. Ruminal bacteria on the rumen epithelium, in the liquid or solid fraction, are different because of their distinct ecological niche and metabolic function. Thiamine supplementation has a positive impact on the liquid-associated bacterial community, but whether solid-attached bacteria are affected by thiamine supplementation and whether the anti-inflammatory effect of thiamine is related to its regulation of rumen wall-adherent bacteria are unknown. Moreover, protozoa represent 50 % of the total microbial mass in the rumen and may play crucial roles in thiamine metabolism; therefore, more research on the relationship between ruminal protozoa and thiamine is necessary to understand thiamine metabolism in the rumen.
(3) Most studies on the response of dairy cows to thiamine supplementation have been confined to rumen fermentation and milk performance. In non-ruminant research, the activity of non-thiamine-dependent enzymes, such as succinate dehydrogenase, succinate thiokinase and malate dehydrogenase in the TCA cycle, has been shown to be altered by thiamine deficiency. Therefore, it is necessary to explore the systematic changes in carbohydrate metabolites under conditions of different thiamine status in dairy cows using metabolomics, which will improve the understanding of thiamine function in dairy cows and identify a more sensitive biomarker for thiamine status. In addition to its role in carbohydrate and energy metabolism, the effects of thiamine on cell regulation, immune function, and oxidative damage should be evaluated. The discovery of unknown functions and cattle responses to thiamine will help to improve cow health and productivity and enhance the nutritional value of milk and other dairy products.
Acknowledgements
The study was financially supported by the Project of National Nature Science Foundation of China (grant no. 31572435). We declare that the National Nature Science Foundation of China had no role in the design and writing of this article.
The authors’ contributions are as follows: X. P. wrote and revised the manuscript, X. N. and L. Y. contributed to the preparation of figures, L. J. and B. X. provided critical comments. All authors read and approved the final manuscript.
None of the authors had any conflicts of interest to declare.







