Menopause is the final step of the ovarian aging process, and its timing is an important determinant of specific diseases in women(Reference Broekmans, Soules and Fauser1,Reference Brand, Van Der Schouw and Onland-Moret2) . Later menopause is protectively associated with cardiovascular disease(Reference Rosano, Vitale and Marazzi3) and osteoporosis(Reference van Der Voort, van Der Weijer and Barentsen4); however, it is associated with an increased risk of breast(Reference Nagata, Hu and Shimizu5), endometrial(Reference Dossus, Allen and Kaaks6) and ovarian cancer(Reference Tsilidis, Allen and Key7). The changes that entail ovarian aging, such as loss of ovarian function and the subsequent decline in endogenous estrogens, can exert different effects on the risk of these diseases(Reference Rosano, Vitale and Marazzi3,Reference van der Graaf, de Kleijn and van der Schouw8,Reference Dunneram, Greenwood and Cade9) .
The ovary may have negative impacts from accumulated galactose and galactose metabolites that are produced when lactose is dissolved by lactase in the small intestine, although galactose is crucial as a source of energy and a structural element in complex molecules(Reference Liu, Hale and Hughes10,Reference Coelho, Berry and Rubio-Gozalbo11) . Galactose-1-phosphate uridyl transferase is one of the enzymes responsible to metabolise galactose and relatively abound in the ovary(Reference Liu, Hale and Hughes10). Women who lack galactose-1-phosphate uridyl transferase (known as classic galactosemia) or who have reduced galactose-1-phosphate uridyl transferase activity tend to prematurely develop ovarian failure and menopause(Reference Cramer, Harlow and Barbieri12,Reference Kaufman, Kogut and Donnell13) . Irrespective of the transferase activity, high galactose intake could promote menopause(Reference Cooper, Hulka and Baird14). However, evidence on the impacts of galactose and lactose intakes on the onset of natural menopause among community-dwelling women is limited.
To date, only two epidemiologic studies have investigated the associations between galactose intake and the onset of natural menopause(Reference Rostami Dovom, Moslehi and Mirmiran15,Reference Carwile, Willett and Michels16) . Despite the presumed ovotoxicity effects of galactose, high intake of lactose, the main dietary source of galactose, was associated with a later onset of natural menopause in the Nurses’ Health Study(Reference Carwile, Willett and Michels16). By contrast, a cross-sectional study in Iran indicated that galactose and lactose intakes were associated with an elevated odds of early menopause (natural menopause occurring before the age of 45 years), although the estimates were not significant(Reference Rostami Dovom, Moslehi and Mirmiran15). Caution is warranted that only women who experienced natural menopause were included in the study and the OR showed the extent of discrepancy in the distribution of women with early menopause and non-early menopause. In the present study, we used the data from a 10-year follow-up study conducted in a Japanese community to examine the associations of galactose and lactose intakes with the onset of natural menopause. Classic galactosemia rarely occurs in Japan (approximately 1 case per 0·9 million population), and age at natural menopause varies by country or geographical region, for example, the mean age of 51·2 years in Australia, 50·1 year in Japan, 49·1 year in the USA, 47·4 years in the Middle East and 47·2 years in Latin America(Reference Dunneram, Greenwood and Cade9,Reference Schoenaker, Jackson and Rowlands17) . This study targeting Japanese community-dwelling women will aid in providing further insights into the associations.
Materials and methods
Study participants
Study participants for the present study were subjects of a population-based prospective cohort study initiated in September 1992, which targeted all residents aged 35 years or older in Takayama city, Gifu, Japan (the Takayama study). In total, 31 552 residents (85·3 %) completed a self-administered questionnaire, which included questions related to the participants’ demographic characteristics, diet, lifestyle, reproductive health and medical histories. On July 1, 2002, a follow-up survey was conducted on participants aged < 70 years at baseline (12 471 men and 14 075 women). Details of the baseline and follow-up surveys were described previously(Reference Shimizu18). For the present study, as shown in Fig. 1, 5401 premenopausal women were eligible after excluding those who were postmenopausal (n 6699) and had missing data on menstrual status (n 113) at baseline, and those who died, were physically unable to participate or had moved to another place between the dates of the baseline and follow-up surveys (n 1862). Among the eligible population, 3379 women responded to a self-administered questionnaire in the follow-up survey, which included questions about lifestyle, reproductive health and allergy and other medical histories (response rate: 62·5 %). We excluded women who previously diagnosed with cancer (n 25), used hormone therapy (n 32) and had missing data on body mass index (BMI) (n 51) at baseline. In the follow-up survey, those who were found to be already postmenopausal at baseline based on their responses to menstrual status (n 42) were excluded. Those who had missing data on menstrual status (n 94) and the duration of hormone therapy if they started after the baseline survey (n 20) were also excluded, leaving 3115 participants for analysis (35–56 years of age). The present study was approved by the Ethics Committee of Gifu University Graduate School of Medicine.
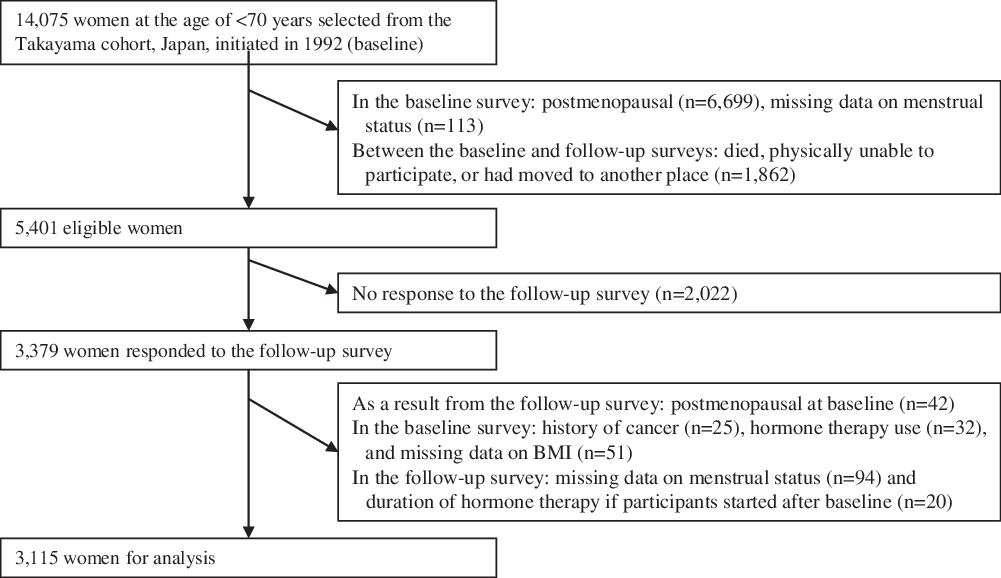
Fig. 1. Flow chart for selection of study participants from the baseline to follow-up surveys.
Natural menopause
The end point was the onset of natural menopause, which was defined as the absence of menstruation for 12 months or more. Data on self-reported menopausal status and age at menopause were obtained from the follow-up survey. Women were censored at the age when their menstrual period stopped due to surgery (n 129) and radiation therapy or chemotherapy (n 64). Since the timing of menopause for women on hormone therapy may not be accurate(Reference Ettinger, Golditch and Friedman19), those who reported to use hormone therapy in the follow-up survey were also censored at the starting age of hormone therapy (n 31).
Lactose and galactose intakes
Dietary intake was assessed at baseline using a validated 169-item semi-quantitative FFQ. The participants reported the frequency and amount of each food and beverage item they consumed during the past year. Component foods in dishes were determined in advance; a total of 520 foods were covered by the FFQ. Nutrient intake was estimated based on the frequency and portion size using the fifth revised and enlarged edition of the Japanese Standard Tables of Food Composition (20). Details of the FFQ and the methods used for calculating nutrient intake were described previously(Reference Shimizu, Ohwaki and Kurisu21). We estimated the intake of each type of carbohydrate including galactose and lactose using an available carbohydrate table, that is, a supplement to the Standard Tables of Food Composition, 2015 by the Japan Science and Technology Agency(22). We examined the validity of the intakes of total energy, lactose and galactose estimated from the FFQ by comparing with the intakes from the twelve 1-d diet records obtained at 1-month intervals over 1 year in a subsample of participants. The Spearman’s correlation coefficients were 0·51 (total energy), 0·71 (lactose) and 0·46 (galactose) in women.
Potential confounding factors
The following variables measured at baseline were considered as potential a priori confounders: i.e., age (continuous); age at menarche (≤ 12, 13–14, 15–16, or ≥ 17 years); age at first birth (≤ 25 or > 25 years); parity (0, 1 or 2, or > 2 children); oral contraceptive (OC) use (no or currently/ever); dietary intakes per day (total energy and total fat: continuous); BMI (quartile); height (quartile); physical activity (continuous); smoking status (never, former or current); marital status (married or not married [single, divorced/separated or widowed]) and years of education (≤ 11, 12–14, or ≥ 15 years). To reduce the possibility of multicollinearity(Reference Carwile, Willett and Michels16), we combined the categories of age at first birth and parity into a single category: nulliparous, ≤ 25 years and 1 or 2 children, > 25 years and 1 or 2 children, ≤ 25 years and > 2 children or > 25 years and > 2 children. Physical activity was estimated based on the average hours per week spent performing various activities during the previous year. The time spent at a specific intensity level of activity was multiplied by its corresponding energy expenditure requirement, and all the intensity levels were summed to yield a score (metabolic equivalent [MET]-hour/week). Details of the method and its validity have been described elsewhere(Reference Suzuki, Kawakami and Shimizu23).
Statistical analysis
For each participant, person-years of follow-up was calculated from the time of the baseline survey (September 1992) to the onset of menopause, the end of follow-up (July 2002) or the time when a censoring event occurred, whichever came first. To evaluate the impact of non-response to the follow-up survey and exclusion because of missing data on menstrual status and hormone therapy, we first compared the baseline characteristics between respondents (eligible population) and participants for analysis. Then, we divided the participants into quartiles according to lactose and galactose intakes, respectively.
In the Cox proportional hazards models, we first estimated the hazard ratios and 95 % CI, after adjusting for age and total energy intake, for the associations of lactose and galactose intakes with the onset of natural menopause, using the first quartile category as the reference, respectively. Next, we additionally adjusted for other potential confounders: total fat intake, age at menarche, age at first birth and parity, OC use, BMI, height, physical activity level, smoking status, marital status and years of education. The dietary intakes (lactose, galactose and total fat) were adjusted for total energy intake using the residual method of energy adjustment(Reference Willet and Willett24); the median value of each category of lactose and galactose intakes were entered into the models to analyse the linear trends in the associations.
In the sensitivity analyses, to reduce the potential impacts of genetic galactose-1-phosphate uridyl transferase deficiency and lactose intolerance on the onset of natural menopause(Reference Cramer, Harlow and Barbieri12), we first estimated the fully adjusted hazard ratios and 95 % CI after excluding women who did not consume milk. Second, to consider the potential impacts on misclassification of menopausal status(Reference Carwile, Willett and Michels16), we repeated the analyses excluding women with a history of OC use at baseline and those who used hormone therapy during follow-up. Third, to reduce the possibility of residual confounding from dietary factors other than total energy and dietary fat, we additionally adjusted for overall diet quality in the fully adjusted models. Adherence to the food guide provided by the Japanese Government (i.e. the Japanese Food Guide Spinning Top; continuous) was utilised as an indicator of overall diet quality(Reference Oba, Nagata and Nakamura25). Finally, women who perceived perimenopausal signs could be conscious about their health and consume more milk and dairy products(Reference Nagata, Wada and Nakamura26). We, therefore, repeated the analyses excluding women who experienced menopause within the first 2 years of follow-up. The proportional hazards assumption was examined using Schoenfeld residuals and visual inspection of log-log plots, with no violations detected. We conducted a complete case analysis and defined statistical significance as a two-sided P value of less than 0·05. Stata se statistical software (version 16·1; StataCorp) was used for all analyses.
Results
The baseline characteristics of the eligible population and participants for analysis are shown in Supplemental Table 1. The participants for analysis were more likely to start menarche at an early age and were less likely to be current smokers and educated compared with the eligible population. No difference was observed in the lactose and galactose intakes between the eligible population and participants for analysis.
Table 1 shows the baseline characteristics of participants for analysis according to the categories of lactose and galactose intakes. The participants consumed far more lactose than galactose. Women with low intakes of lactose and galactose consumed more total energy and less total fat and were more likely to use OC and smoke currently. Moreover, those with low intake of lactose were more likely to be married and were less likely to be nulliparous and educated.
Table 1. Characteristics of study participants according to lactose and galactose intakes, Takayama study, Japan, 1992–2002
(Number and percentages; mean values and standard deviations)
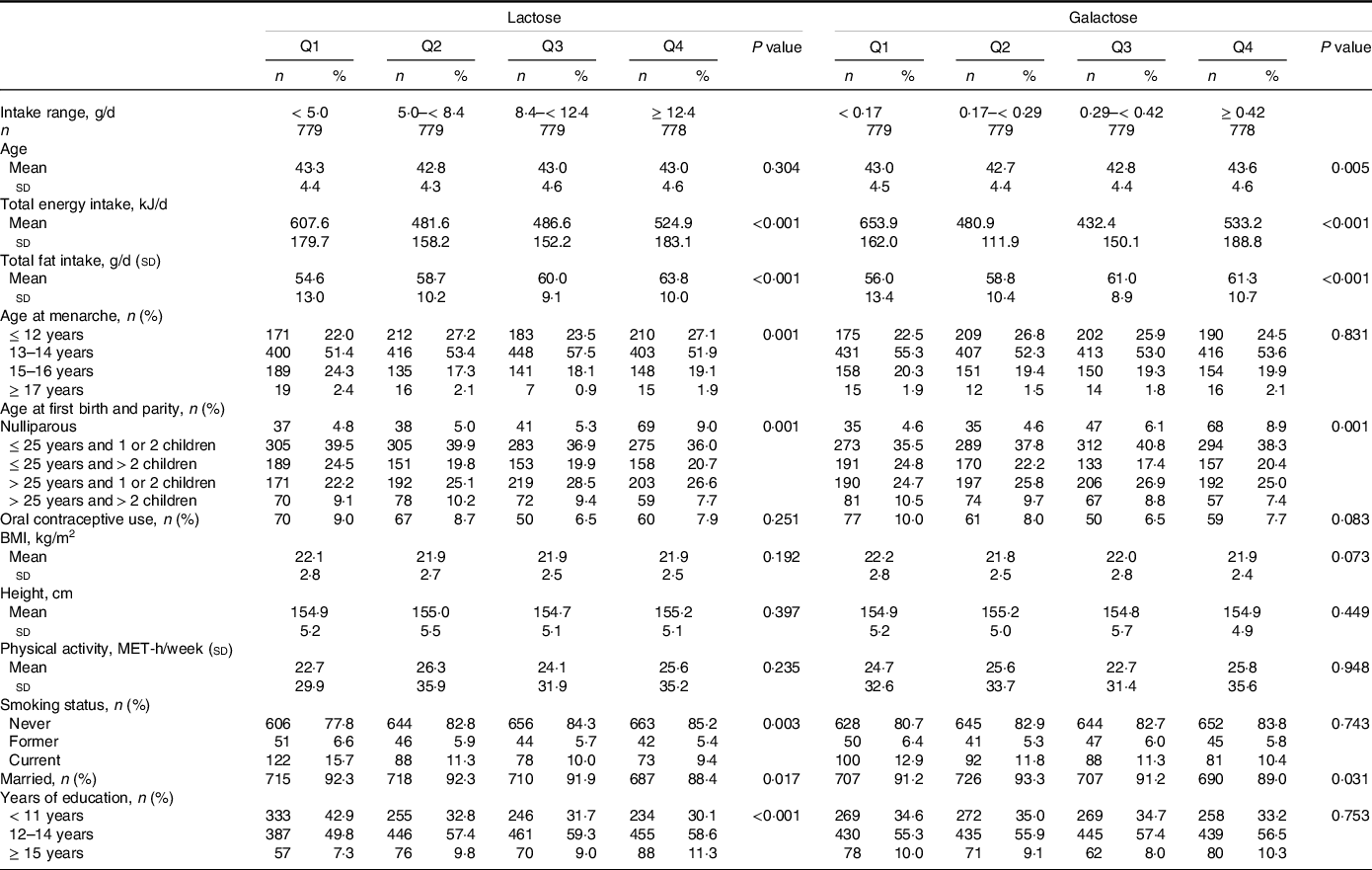
IQR, interquartile range; n, number; Q, quartile.
Values are means ± SD, or frequencies (percentages).
P values were based on linear regression analyses for continuous variables and chi-square tests for categorical variables.
Table 2 shows the associations of lactose and galactose intakes with natural menopause. During the 10-year follow-up (21 122 total person-years), 1790 women (57·5 %) had natural menopause. High intakes of lactose and galactose were associated with a later onset of natural menopause after adjusting for age and total energy intake. The associations remained significant even after adjusting for all potential confounding factors. Compared with the first quartile category, the fully adjusted hazard ratios (95 % CI) for the onset of natural menopause were 0·96 (0·83, 1·10), 0·87 (0·75, 1·01) and 0·80 (0·69, 0·92) from the second to forth quartile categories of lactose intake, respectively (P-trend = 0·001).
Table 2. HR and 95 % CI for the onset of natural menopause according to lactose and galactose intakes, Takayama study, Japan, 1992–2002
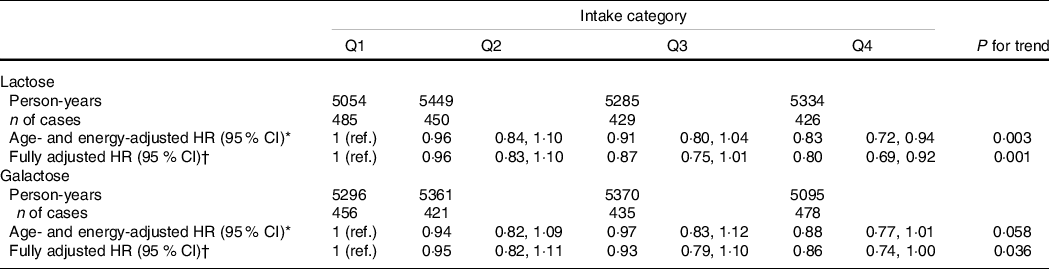
HR, hazard ratio; n, number; Q, quartile.
HR < 1 implies later menopause and HR > 1 implies earlier menopause.
* Age and total energy intake were adjusted for.
† Age, total energy intake, total fat intake, age at menarche, age at first birth and parity, oral contraceptive use, BMI, height, physical activity, smoking status, marital status and years of education were adjusted for.
In the sensitivity analyses (Table 3), excluding women who did not consume milk, with a history of OC use, or who used hormone therapy during follow-up did not substantially change the present findings. Additionally adjusting for overall diet quality did not substantially change the results. Furthermore, the results after excluding cases within the first 2 years of follow-up remained consistent with those from the main analyses, although the linear trend in the association of galactose intake turned out to be non-significant.
Table 3. Sensitivity analyses of HR and 95 % CI for the onset of natural menopause according to lactose and galactose intakes, Takayama study, Japan, 1992–2002
(Hazard ratio and 95 % CI)

HR, hazard ratio; n, number; and Q, quartile.
HR < 1 implies later menopause and HR > 1 implies earlier menopause.
In all models, age, total energy intake, total fat intake, age at menarche, age at first birth and parity, oral contraceptive use, BMI, height, physical activity, smoking status, marital status and years of education were adjusted for.
Discussion
We examined the associations of dietary lactose and galactose intakes with the onset of natural menopause using the data of premenopausal women who participated in a prospective cohort study in a Japanese community. High intakes of lactose and galactose were associated with a later onset of natural menopause, after adjusting for age, total energy intake and other potential confounding factors. The sensitivity analyses did not substantially change the findings.
Lactose and galactose intakes at usual levels may not be deleterious to the ovarian aging process. Lactose intake was not associated with infertility due to ovulatory dysfunction in the Nurses’ Health Study II(Reference Chavarro, Rich-Edwards and Rosner27), although lactose intake slightly improved the fecundability in two preconception cohort studies in the USA and Canada(Reference Wise, Wesselink and Mikkelsen28). In addition, high intakes of lactose and galactose may decrease the risk of decline in anti-Mullerian hormone level, a marker of ovarian reserve(Reference Moslehi, Mirmiran and Azizi29). In rats, high galactose diets inhibited the development of ovarian follicles(Reference Meyer, Doyle and Grifo30) and long-term exposure to high lactose diets had no harmful effects on the ovarian morphology or function, although body weights and serum progesterone concentrations decreased(Reference Liu, Shi and Blas-Machado31).
Compatible with our findings (participants aged 35–56 years), a previous study found that high lactose intake was associated with a later onset of natural menopause only among women aged < 51 years at the time of questionnaire return in the Nurses’ Health Study (median age at natural menopause in the cohort)(Reference Carwile, Willett and Michels16). This can be due to the fact that menopause had already occurred to an irreversible degree among older women.
Endogenous steroid hormones and growth factors in cow’s milk, which is a major source of lactose, could have impact on the present findings. Epidemiologic studies have suggested that milk and dairy product intakes increase the plasma concentrations of estradiol and insulin-like growth factor-I(Reference Brinkman, Baglietto and Krishnan32–Reference Romo Ventura, Konigorski and Rohrmann36). In addition, high intakes of low-fat dairy and skim milk were associated with a later onset of natural menopause(Reference Carwile, Willett and Michels16) or a reduced risk of early menopause(Reference Purdue-Smithe, Whitcomb and Manson37). The concentrations of hydrophilic conjugated estrogen metabolites (e.g. estrone sulfate) are higher in low-fat dairy products and skim milk than in high-fat dairy products(Reference Farlow, Xu and Veenstra38). In rats, decreased brain insulin-like growth factor-I signaling leads to the luteinising hormone surge, which is typically observed during the reproductive senescence(Reference Todd, Merhi and Shu39). The estradiol and insulin-like growth factor-I in milk and dairy products might extend the lifespan of the ovaries.
Furthermore, gut microbiota could mediate the associations of lactose and galactose intakes with the onset of natural menopause. Specifically, the lactic acid bacteria Lactobacillus and Bifidobacterium can utilise lactose, which is eventually decomposed into lactate, short-chain fatty acids (mainly acetate, propionate and butyrate) and gases (H2, CO2 and CH4)(Reference He, Venema and Priebe40,Reference Forsgård41) . Gut microbiota may contribute to modulation of the hypothalamic–pituitary–gonadal axis including the gonadotropin-releasing hormone, gonadotropins and sex steroids as its components, and dysregulation of the hypothalamic–pituitary–gonadal axis can have a negative effect on the metabolic and reproductive health, for example, polycystic ovary syndrome(Reference Organski, Jorgensen and Cross42). Compared with control rats, polycystic ovary syndrome rats treated with Lactobacillus showed a reduction in androgen biosynthesis and an increase in granulosa layers with formation of corpora lutea in the ovarian tissues(Reference Guo, Qi and Yang43). Lactobacillus are also colonised in the uterus(Reference Mitchell, Haick and Nkwopara44). Although the detailed mechanism is unclear, lactose and galactose intakes could help maintain healthy microbiota in the intestines and uterus, resulting in positive impacts on the ovarian aging process.
The present study has several limitations. First, the follow-up rate was low, and we could no longer obtain the data on menopausal status in women who died, were physically unable to participate, or had moved to another place. The participants for analysis were more likely to start menarche at an early age and were less likely to be current smokers and educated than the eligible population, although no difference was observed in the lactose and galactose intakes. In the present study, we adjusted for age at menarche, smoking status and years of education. Moreover, it is unlikely that women with high intakes of lactose and galactose tended to participate in the study if they reached menopause at a later age. Second, menopausal status and age at menopause are self-reported, which are of concern specifically in those who reached menopause at the beginning of the 10-year follow-up. Recall of age at menopause may not be affected by lactose and galactose intakes, but information about menopausal status and its timing should be collected repeatedly during the follow-up period, for example, biennially in the Nurses’ Health Study(Reference Carwile, Willett and Michels16,Reference Purdue-Smithe, Whitcomb and Manson37) . Third, although women with a history of OC use at baseline were excluded in the sensitivity analyses, the possibility of residual confounding from OC use cannot be ruled out because the data on OC use was not collected in the follow-up survey. Fourth, dietary intake was assessed only at baseline and might change during the follow-up period specifically in women who perceived perimenopausal signs(Reference Nagata, Wada and Nakamura26). The sensitivity analyses excluding women who reached menopause within the first 2 years of follow-up did not substantially change the present findings. Finally, as mentioned above, we cannot rule out the possibility that the observed associations might be due to confounding by the unmeasured factors, including genes, hormones and nutrients in milk and dairy products.
Conclusion
In conclusion, high intakes of lactose and galactose were associated with a later onset of natural menopause. Despite the presumed ovotoxicity effects of galactose, lactose and galactose intakes at usual levels may not exert deleterious effects on the ovarian aging process among Japanese community-dwelling women. The timing of menopause is an important determinant of future disease risk in women. Age at natural menopause varies by country(Reference Dunneram, Greenwood and Cade9). Hence, further studies in women from different countries are needed, and the associations of dietary lactose and galactose intake with the onset of natural menopause, considering microbiota in the intestines and uterus, genes and hormones and nutrients in milk and dairy products, should be examined.
Acknowledgement
This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The sponsor was not involved in deciding the study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit this paper for publication.
M. Y., K. W. and C. N. designed the study and analytical strategy; K. W. and C. N. obtained data; M. Y., Y. N. and C. N. performed analysis and interpreted data; M. Y. drafted the initial manuscript; K. W., Y. N. and C. N. reviewed and revised the manuscript; C. N. obtained the grant and supervised the study; and all authors read and approved the final manuscript as submitted.
The authors have no conflicts of interest to disclose.







