Bone is a dynamic tissue comprised of cellular, organic and inorganic components with a complex internal structure. Disruption of the balance between bone formation and resorption due to excessive production of osteoclasts or inadequate presence of osteoblasts leads to bone loss, and hence osteoporosis( Reference Manolagas and Jilka 1 ). The level of osteoporosis is increasing worldwide, with more than 200 million people living with osteoporosis in 2010( Reference Cooper, Campion and Melton 2 ). Despite underestimations in reports of osteoporosis prevalence( Reference Gill, Martine and Laslett 3 ), available epidemiological evidence has shown that the magnitude has increased in Australia. In 2012, 3·3 % (5·3 % men and 1·2 % women) of the general Australian population self-reported that they had osteoporosis, which was double the estimate of 1·6 % from the year 2000. This figure was higher among those aged 50 years and above (15 and 3 % in women and men, respectively)( 4 ).
Determinants of osteoporosis are multifaceted and interlinked. Genetic, lifestyle, nutritional, medical disorders, medication use and metabolic (biological) risks are identified as major contributors for osteoporosis( Reference Kanis 5 ). Evidence has demonstrated the importance of specific food items, nutrients and non-nutritive substances in maintaining bone mineral density (BMD) and preventing osteoporosis and osteoporotic fractures( Reference Sahni, Mangano and McLean 6 ). For instance, high consumption of soya( Reference Wei, Liu and Chen 7 ) and dairy products( Reference Caroli, Poli and Ricotta 8 ) has been found to be important in the prevention of osteoporosis. Nutrients and non-nutritive substances, such as Ca( Reference Skowronska-Jozwiak, Jaworski and Grzywa 9 ), PUFA( Reference Mangano, Sahni and Kerstetter 10 ) and isoflavones( Reference Messina 11 ), were also found to have an important role in the prevention of osteoporosis.
Recent epidemiological studies have focused on the effect of the overall nature of food consumption habits on disease outcomes, instead of specific foods or nutrients( Reference Newby and Tucker 12 ). This is because an outcome (disease) usually occurs as a result of natural interactions or patterns of nutrients and other components of diets rather than intake of single foods or nutrients. In line with this, new dietary analysis methods have been introduced. These methods are either ‘a posteriori’ analyses (data driven techniques), such as factor, principal component and cluster analysis, or ‘a priori’ analyses, which include dietary indices or dietary scores. Most recently, reduced-rank regression, which combines the above two, has also been used( Reference Michels and Schulze 13 ).
Studies have reported different patterns of diet that are associated with BMD( Reference Lim, Lee and Tserendejid 14 , Reference Langsetmo, Poliquin and Hanley 15 ). However, findings are inconsistent. For example, a study in South Korea among postmenopausal women showed a direct association between dairy-rich dietary patterns and BMD( Reference Lim, Lee and Tserendejid 14 ). However, another study found no such association among Canadian women( Reference Langsetmo, Poliquin and Hanley 15 ). The effect of dietary patterns on BMD can also vary by communities as the food that is available in one location may not be found in others. Hence, tailored dietary patterns that are useful for optimal bone mass should be developed for specific groups/populations.
In Australia, a few studies have explored the association between dietary patterns and BMD, and the available studies are generally conducted among children and young adults( Reference McNaughton, Wattanapenpaiboon and Wark 16 , Reference van den Hooven, Ambrosini and Huang 17 ). The present study, therefore, aims to assess the association between dietary patterns and low BMD among adults aged 50 years and above in Australia.
Methods
Study design and population
The North West Adelaide Health Study (NWAHS) data were used for this analysis. The NWAHS recruited participants from the northern and western suburbs of Adelaide, South Australia. The region represents a third of the South Australian population and half of the metropolitan area of the city of Adelaide, and was established with the purpose of providing valid and reliable data on chronic diseases and their risk factors. It is a community-based cohort study that incorporates clinical, public health, social and biochemical data. Three stages of data collection have been conducted: 1999–2003, 2004–2006 and 2008–2010. Data were collected a using self-completed questionnaire, computer-assisted telephone interview and clinical assessments( Reference Grant, Taylor and Ruffin 18 ).
Details on the objectives and methods of the NWAHS are published elsewhere( Reference Grant, Taylor and Ruffin 18 ); however, in brief, the study participants were adults aged 18 years and above when first recruited. Random sampling was initially undertaken at the household level. All households that were not connected to a landline telephone were excluded from the sampling frame (using Electronic White Pages). At the time of recruitment (1999), 97·9 % of households in South Australia were connected with a landline telephone( Reference Grande and Taylor 19 ). Randomly selected households were screened for individuals aged 18 years and above. All these individuals were then invited to participate in the study. Those who could not communicate in English were excluded. At the initial stage, 4056 males and females participated. This study used BMD data collected from those aged 50 years and over as part of Stage 2 (2004–2006, n 1588), and dietary data was collected as part of Stage 3 (2008–2010, n 2500). In total, 1182 adults (545 males, 45·9 %) aged 50 years and above provided data related to BMD and nutrition.
Dietary assessment and food groups
Dietary intake was assessed using the Dietary Questionnaire for Epidemiological Studies (DQESV2) of Cancer Council of Victoria. The DQESV2 was self-completed and designed to assess intake over the preceding 12 months. Portion size was assessed using four questions and by calculating a single portion size factor, which helps in estimating a median-sized serving of food an individual eats( Reference Giles and Ireland 20 ). The completed forms were sent to Cancer Council Victoria for analyses of total daily intakes of food items and nutrients using the Australian NUTTAB95 (Australian Government Publishing Service, Canberra) food composition database. The amount of food items consumed per day was calculated in grams for each study participant. Food items were categorised into thirty-nine food groups( Reference Schoenaker, Dobson and Soedamah-Muthu 21 ). Data on vitamin D and Ca supplementation were also collected.
Assessment of other covariates
Stage 2 covariates
Sex, age and family history of osteoporosis were determined. Annual household income was categorised as follows: up to $20 000, $20 001–$40 000, $40 001–$60 000 and more than $60 000. Marital status was determined and categorised into married or living together with partner (in union), separated/divorced, widowed and never married. Alcohol intake risk was assessed using the frequency and number of standard drinks( 22 ). Smoking status was classified into non-smokers, ex-smokers and current smokers. Height and weight of the study participants were obtained to calculate the BMI. BMI was further classified on the basis of the World Health Organization standard( 23 ). Identification of participants with diabetes was by either doctor-diagnosed self-report of diabetes or laboratory diagnosis using blood samples collected during the clinic visit, with diabetes defined as fasting plasma glucose ≥7·0 mmol/l.
Assessment of leisure-time physical activity levels (PAL) was performed using the Australian National Health Survey questions( 24 ). This was assessed considering the number of times a person exercised in the last 2 weeks and the total amount of time spent walking for exercise and performing moderate and vigorous exercise. Job-related PAL was also assessed from data related to occupation, which were obtained in Stage 1, by two occupational physicians based on the type of professions the study participants had. Both PAL were classified as sedentary or low and medium or high. In this particular analysis, home duties were considered as sedentary or low PAL. Detailed methods of both PAL are published elsewhere( Reference D’Onise, Shanahan and Gill 25 ). Total number of medications prescribed over the past 6 months (including for hypertension, high cholesterol, mental health problems, osteoporosis and asthma), menopausal status and sunlight exposure were also assessed at this stage. Data on medication use were obtained from pharmaceutical benefits scheme. Sunlight exposure was assessed using questions including average duration of direct sunlight exposure during winter and summer and timing (week day and weekend).
Stage 3 covariates
Health literacy was assessed using the Newest Vital Sign test tool( Reference Weiss, Mays and Martz 26 ). For thirty-one cases with missing values, we used data collected with the short Test of Functional Health Literacy in Adults tool( Reference Baker, Williams and Parker 27 ). Health literacy was classified as limited or adequate.
Assessment of bone mineral density
BMD of the whole body was measured using Prodigy and DPX+ dual energy X-ray absorptiometry (DXA) (GE Lunar) as part of the clinic visit at stage two. DXA was calibrated, and measurements were verified to check correct operation at the beginning of each scan day. Details of the DXA measurement procedures can be found elsewhere( Reference Appleton, Seaborn and Visvanathan 28 ). Participants were categorised into two groups using T-scores of BMD. Those who had T-scores of less than −1 were considered as osteopenic (between −1 and −2·5) or osteoporotic (less than or equal to −2·5)( 29 ) and were classified as having low BMD.
Dietary and statistical analysis
To evaluate dietary misreporting, the Goldberg method was used. In this method, the ratio of actual energy intake (EI):BMR and PAL were considered( Reference Goldberg, Black and Jebb 30 ). To take account of variations in methods, the 95 % CI of PAL was calculated. Both leisure-time and job-related PAL were considered in the calculation. Next, the ratio of EI:BMR was compared against the 95 % CI of PAL. On the basis of the recommendation by Black et al., the following values for PAL were determined: sedentary=1·4, light=1·6, medium=1·8 and strenuous=2. Individuals were classified as plausible if the ratio was in the CI range. However, if the ratio was below or above the 95 % CI, it was classified as under-reported or over-reported, respectively( Reference Black 31 ).
To represent population-level dietary patterns, factor scores and dietary patterns were calculated and constructed among 2453 (forty-seven cases with considerable (>30) missing values were excluded) study participants who provided dietary information. Data reduction technique using factor analysis with principal component analysis was used to identify dietary patterns out of the thirty-nine food groups; two dietary patterns were determined on the basis of the scree plot, eigenvalues (>1) and interpretability. To attain optimal structure and increase the interpretability of factors, varimax rotation was applied. Factor scores for each of the participants and factors were calculated as the sum of the products of factor loading coefficients, which was standardised by daily intake of each food item. Tertiles were constructed for each factor. Factor loadings are the correlation coefficients between factors (identified dietary patterns) and food groups with loadings of each food group graphically presented. Sample adequacy was checked using the Kaiser–Mayer–Olkin test.
Descriptive analysis of socio-demographic and lifestyle characteristics and chronic conditions was performed across the tertiles of the factors. Mean values and standard deviations (continuous and normally distributed variables), medians and interquartile ranges (continuous and non-normally distributed variables) and proportions were calculated (categorical variables). χ 2 Tests, Kruskal–Wallis tests and ANOVA were used to identify significant differences across different levels of dietary intake (factor scores).
To assess the association between intake of different levels of dietary patterns and low BMD, Poisson regression models were used( Reference Barros and Hirakata 32 ). For both dietary patterns, we developed four regression models in addition to the unadjusted model. The first model was adjusted for sex and age. Model two was additionally adjusted for socio-economic and lifestyle factors (smoking status, alcohol intake, marital status, income, health literacy and job-related PAL). In addition to the variables in the second model, chronic conditions (diabetes mellitus, family history of osteoporosis and BMI) were adjusted in the third model. To assess whether the association between dietary patterns and outcomes was confounded by total EI, we additionally adjusted for EI in the fourth model.
Subgroup analyses were performed to assess the association of dietary patterns with low BMD in various subgroups of the study participants. In the final models, multiplicative terms for each dietary pattern and each of the variables were used to assess the interaction in predicting low BMD. Missing data were identified across all variables. Except for leisure-time PAL, which had the highest number of missing values, others were imputed using data from the other stage of the study or were otherwise reported as ‘missing’. We did not impute leisure-time PAL in the analysis because the approaches used to assess the PAL at different stages were not the same, and it had a high number of missing values (n 128). A sensitivity analysis was undertaken by including and excluding the missing values and variables, including season of birth and DXA measurement, leisure-time PAL, vitamin D and Ca supplementation, menopausal status, medication use and sunlight exposure, in the final models. All analyses were conducted using STATA/SE version 14.1 (Stata, StataCorp LP).
Results
A total of 1182 (45·9 %, males) study participants provided dietary and BMD data and were included in the analysis. However, the total number of study participants in the multivariable analysis was 1066. Therefore, 119 (9·8 %) cases had at least one missing value among the other covariates. Variables such as leisure time PAL (128, 10·8 %) and health literacy (34, 2·9 %) had the highest proportion of missing values (Table 1). Missing values of variables including smoking status (five cases), alcohol intake risk (thirty-nine cases), diabetes (five cases), family history of osteoporosis (four cases) and marital status (four cases) were identified and imputed using data from the third stage.
Table 1 Participant characteristics across tertiles of dietary patterns in adults aged 50 years and above, South Australia (Frequency or numbers and percentages; mean values and standard deviations)

IQR, interquartile range; BMD, bone mineral density.
* χ 2 Test.
† Kruskal–Wallis.
‡ ANOVA.
Socio-demographic characteristics
The median age of the participants at the second stage of assessment was 62 years (interquartile range 56·0, 69·0). Almost half (47·7 %) of the study participants reported a household income between $20 001 and $60 000. More than two-thirds (779, 65·9 %) of the study participants were married or living with a partner (Table 1).
Dietary patterns and characteristics of study participants
Assessment of dietary misreporting showed that only 7 (0·6 %) participants had under-reporting( Reference Cooper, Campion and Melton 2 ) or over-reporting( Reference Kanis 5 ) of EI. We identified two dietary patterns. These patterns explained a total of 17·0 % variance in total food intake (10·3 and 6·7 %, respectively). Fig. 1 shows the factor loadings for each pattern. Pattern 1 (‘prudent pattern’) was characterised by high intake of fruits, vegetables, sugar, nut-based milk, fish, legumes and high-fibre bread. In contrast, pattern 2 (‘Western pattern’) was high in processed and red meat, snacks, takeaway foods, jam, vegemite (a brewers’ yeast extract commonly used as a spread in Australia), beer, soft drinks, white bread, poultry, potato with fat, high-fat dairy and eggs. Cross-loading (factor loading>0·30 in each pattern) was found for sugar, tea and water. Food groups with their constituents are provided in the online Supplementary Table S1.

Fig. 1 Factor loadings for two food patterns among adults aged 50 years and above, South Australia (n 2453).
Socio-demographic characteristics, chronic conditions, EI and BMD across intake levels of the two dietary patterns are shown in Table 1. The overall prevalence of low BMD and osteoporosis was 15·9 (12·7 % in men and 18·6 % in women) and 2·0 %, respectively. More than half (53·1 %) of the study participants had no risk of harm from alcohol. The mean BMI was 28·2 (sd 4·7) kg/m2. The prevalence of diabetes mellitus was 11·8 %. The mean whole-body BMD was 1·20 (sd 0·12) g/cm2. Family history of osteoporosis was reported in 19·3 % of the participants, with almost a quarter (24·0 %) of study participants in the third tertile (T3) of the prudent pattern having a family history of osteoporosis compared with 13·7 % in the first tertile (T1). More than two-thirds (68·8 %) of participants in T3 of the prudent pattern had adequate health literacy.
There were significant differences in dietary pattern intake by sex, smoking status, alcohol intake risk and job-related PAL. A significant difference in energy, protein, fat, carbohydrate and fruit intakes was found across the tertiles of both dietary patterns. Vegetable intake was significantly different across tertiles of the prudent pattern but not the Western pattern. In addition, family history of osteoporosis (P<0·001) and health literacy (P<0·001) had crude, significant, positive associations with different levels of the prudent dietary intake. Total BMD (P<0·001) had crude, significant, positive associations with tertiles of the Western dietary pattern.
Dietary patterns and bone mineral density
The prevalence of low BMD was 18·5, 16·4 and 12·8 % across tertiles of the prudent dietary pattern and 17·5 %, 15·7 and 14·6 % across the tertiles of the Western pattern. In the univariate regression analysis, those in T3 of the prudent pattern had a low prevalence ratio (PR) of low BMD (PR=0·69; 95 % CI 0·48, 0·99) compared with those in T1. There was no crude significant association between Western pattern and low BMD (Table 2).
Table 2 Prevalence ratio for the association between tertiles of food patterns and low bone mineral density among adults aged 50 years and above, South Australia (n 1066)Footnote † (Prevalence ratio (PR) and 95 % confidence intervals)
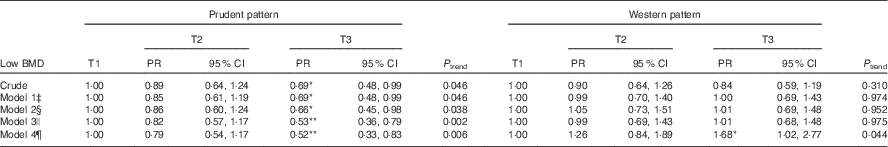
*P<0·05, **P<0·01.
† P trend was calculated by including the tertiles of the patterns as continuous variable in the models.
‡ Model 1: adjusted for sex and age.
§ Model 2: additionally adjusted for socio-economic and lifestyle factors (smoking status, alcohol intake (no risk, low risk, medium/very high risk), marital status, income, health literacy, leisure-time and job-related physical activity levels).
|| Model 3: additionally adjusted for chronic conditions (diabetes mellitus, family history of osteoporosis and BMI (continuous)).
¶ Model 4: additionally adjusted for energy intake (continuous).
Significant inverse associations between prudent pattern and low BMD were observed in multivariable regression models (Table 2). After adjustment for socio-demographic and lifestyle factors, chronic conditions and EI, participants in T3 had a significantly lower prevalence of low BMD (PR=0·52; 95 % CI 0·33, 0·83) compared with those in T1. No significant association between Western pattern and low BMD was observed after adjusting for socio-demographic, lifestyle and chronic condition covariates. However, after adjustment for EI, the study participants in T3 were 68 % more likely to have low BMD (PR=1·68; 95 % CI 1·02, 2·77) compared with those in T1.
We further conducted two sensitivity analyses: (1) by adjusting for season of birth, DXA measurement, vitamin D and Ca supplementation, total number of medications prescribed, sunlight exposure, menopausal status and leisure-time PAL in the final models; and (2) by excluding the missing values of covariates. The association between dietary patterns and low BMD remained in both sensitivity analyses (data not shown).
Interaction was examined between dietary patterns and socio-demographic and lifestyle factors. No interactions were found and these are shown in Fig. 2.

Fig. 2 Subgroup analysis of the association of third tertiles (highest intake) of prudent (left) and Western (right) dietary patterns with low BMD among adults 50 years and above, South Australia. PR, prevalence ratio (adjusted); PAL, physical activity level; sep/div/wid, separated or divorced or widowed. The first tertile (lowest intake of prudent food pattern) was the reference. Poisson’s regression was used to compute PR.
Discussion
In this study, we identified dietary patterns and the association with low BMD among adults aged 50 years and over. We identified two major dietary patterns: a ‘prudent’ (healthy) pattern characterised by fruit, vegetables, fish, medium-fat dairy products, nut-based milk, high-fibre bread and legumes and a western pattern characterised by processed and red meat, fast foods (snacks and takeaway foods), soft drinks, white bread and high-fat dairy products. A significant inverse association between prudent pattern and low BMD was observed. In contrast, a positive association between Western pattern and low BMD was found.
The finding that the prudent pattern was inversely associated with low BMD is consistent with previous studies( Reference de Jonge, Kiefte-de Jong and de Groot 33 , Reference Shin, Sung and Joung 34 ). The Rotterdam Study in the Netherlands reported that a diet with a high intake of vegetables, fruits, fish, wholegrains, legumes/beans and dairy products was positively associated with BMD( Reference de Jonge, Kiefte-de Jong and de Groot 33 ). Among Korean adults, a positive association between a food pattern characterised by high dairy products, fruits and wholegrains and BMD was found( Reference Shin, Sung and Joung 34 ). It may be that the prudent pattern prevents low BMD because of a large number of food groups within this pattern having high nutrient constituents, such as dairy products( Reference Bowes, Church and Pennington 35 ) and fish( Reference Chen Y-m and Lam 36 ), and low energy( Reference McNaughton, Wattanapenpaiboon and Wark 16 ), which play an important role in bone mass.
The prudent pattern was also characterised by a high intake of dairy products. A study among postmenopausal women found that high intake of milk and dairy products reduced the risk of osteoporosis( Reference Shin and Joung 37 ). Dairy products contain good sources of protein and Ca. Furthermore, the nutrient density of protein, Ca, Mg, K, Zn and P is higher than any other food. Vitamins, Ca and polysaccharides are also constituents of nut-based, particularly soyabean, milks( Reference Bowes, Church and Pennington 35 ). In addition, flavonoids in soyabeans, particularly isoflavones, mimic oestrogenic activity and are believed to have an effect in maintaining bone health and in preventing osteoporosis in elderly women( Reference Messina 11 ).
High consumption of fruits and vegetables were also characteristic of the prudent pattern. Fruits and vegetables are comprised of nutrients and non-nutritive substances, such as vitamin K, Mg, polyphenols and phyto-oestrogens, which are important for bone metabolism( Reference Tucker 38 ). Moreover, fruits and vegetables have an alkaline effect due to Mg andK, which buffers the acidic condition that causes bone resorption( Reference Maurer, Riesen and Muser 39 ). However, it has been proposed that the effect of fruits and vegetables on bone mass is not due to its buffering nature but rather because of the nutrients (e.g. Ca and vitamin C) they contain( Reference Macdonald 40 ). Current evidence regarding the role of fruits and vegetables in bone health is inconsistent( Reference Sahni, Mangano and McLean 6 , Reference Hamidi, Boucher and Cheung 41 ). The extent of the association between prudent pattern and BMD, due to high intake of fruits and vegetables, requires further investigation and so does the mechanism of action. Nonetheless, public health efforts should target increasing the consumption of vegetables and fruits. In South Australia, the proportion of the population consuming the recommended level of vegetables and/or fruits (consuming ≥5 vegetable servings and/or ≥2 fruit servings/d) has been consistently approximately 50 % over the past 10 years, among middle-aged and ageing people( Reference Taylor, Dal Grande and Wu 42 ).
Although there were no significant interactions between the prudent pattern and the socio-demographic and lifestyle factors in our subgroup analysis, the associations were stronger in certain groups. For instance, in the subgroup of participants who had a family history of osteoporosis, those in T3 of the prudent pattern were found to have significantly lower PR (71 % reduction in PR) of low BMD compared with those in T1. The direction of the association in those who had no family history was also similar, although the magnitude was smaller (41 % reduction in PR) and not statistically significant. We also found that the proportion of study participants who had a family history of osteoporosis and adequate health literacy significantly increased across the tertiles of the prudent pattern. Thus, the PR difference between those who had and did not have a family history could be explained by the fact that those who had a family history were aware of their susceptibility to low BMD and reduced the risk by following healthy dietary patterns.
In this study, our analysis showed a significant positive association between Western pattern and low BMD. This was observed after adjustment for all the covariates and EI, showing that the association was independent of EI, which could have arisen from differences in body size, physical activity and metabolic efficiency( Reference Willett 43 ). The association in different subgroups was also consistent with low BMD. Consistent positive associations between similar dietary patterns and low BMD have been reported in previous studies( Reference McNaughton, Wattanapenpaiboon and Wark 16 , Reference de Franca, Camargo and Lazaretti-Castro 44 ). Food items (such as soft drinks) in the Western pattern are characterised by low content of important nutrients such as Ca and high levels of energy content and P, resulting in low serum Ca, which causes bone resorption( Reference Amato, Maravilla and Montoya 45 ). In addition, evidence shows that high EI is also an important factor in the homoeostasis of nutrients (particularly macrominerals), resulting in reduced BMD( Reference Tucker, Morita and Qiao 46 ).
It is important to recognise some of the important limitations of this study: one of these is the time lapse between collection of dietary and DXA information. Although dietary data were collected between 2008 and 2010, BMD using DXA was determined between 2004 and 2006 with a 4·3-year median difference (minimum=2·8 and maximum=6·1 years). Between these years, eating behaviours of the study participants could have changed. Although habits of elderly people in relation to the choice of food groups have been found to be stable over years( Reference Jankovic, Steppel and Kampman 47 ), individuals diagnosed with chronic diseases may change their diet towards a healthy one, and this may result in an underestimation of estimates. In addition, although studies on the effect of retirement on food habits are limited( Reference Zantinge, van den Berg and Smit 48 ), the available evidence shows that more healthy food habits are likely to be developed among women while it remains similar for men( Reference Helldan, Lallukka and Rahkonen 49 ). In our study, a total of 175 (14·8 %; 44·9 % men and 55·1 % women) participants retired between the two stages of assessment, which could potentially cause underestimation of the inverse association between prudent dietary pattern and low BMD.
Although FFQ have limitations in providing valid dietary information, they are widely used to measure the usual dietary exposures and behaviours( Reference Smith, Mitchell and Reay 50 ). To evaluate the robustness of the dietary data, analysis of dietary misreporting was also conducted to identify misreporting. Furthermore, the dietary analysis we conducted was for a large population group, which can represent the consumption behaviour of the community over time( Reference Newby, Weismayer and Åkesson 51 ). Another potential limitation of this study is the number of cases with missing values of covariates and exclusion of leisure-time PAL from the analysis. However, sensitivity analyses with imputed and excluded covariates suggested that the findings remained similar.
In conclusion, to the best of our knowledge, this is the first study that assessed the association between dietary patterns and BMD among Australians aged 50 years and above. In this community-based study, we found that a dietary pattern characterised by high intakes of fruits and vegetables, medium-fat dairy products and fish was associated with higher BMD. A dietary pattern characterised by high intakes of processed and red meat, fast foods (snacks and takeaway foods), soft drinks, white bread and high-fat dairy products was inversely associated with BMD. Further longitudinal research among ageing populations is warranted.
Acknowledgements
The authors are thankful to the NWAHS participants for their participation in the study.
The NWAHS was funded by The University of Adelaide, the South Australian Department of Health and The Queen Elizabeth Hospital for which the authors are grateful.
All the authors conceived the study. Y. A. M. conducted all the analyses and wrote all drafts of the paper. Z. S. assisted with analysis and reviewed and provided comment on all drafts. T. K. G. and R. A. reviewed and commented on all drafts. All the authors read and approved the final manuscript.
The authors have no financial or personal conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516003366







