To the Editor—Hantaviruses belong to the family Bunyaviridae and mainly infect small mammals. In humans, however, they cause febrile disease, usually named hemorrhagic fever with renal syndrome (HFRS) in Asia and Europe and hantavirus cardiopulmonary syndrome (HCPS) or hantavirus pulmonary syndrome (HPS) in the Americas.Reference Kruger, Figueiredo, Song and Klempa1,Reference Chandy and Mathai2
Hantavirus transmission from rodents to humans usually occurs via inhalation of aerosolized rodent urine, saliva, and feces, but rarely by rodent bites,Reference Kruger, Figueiredo, Song and Klempa1 and person-to-person transmission has never been conclusively demonstrated. Among the genus Hantavirus, person-to-person transmission of Andes virus was documented in a physician who acquired the infection after exposure to patients infected with the Andes.Reference Padula, Edelstein, Miguel, Lopez, Rossi and Rabinovich3–Reference Martinez-Valdebenito, Calvo and Vial5 However, 2 other studies have reported no evidence of person-to-person transmission of Andes virus.Reference Chaparro, Vega and Terry6,Reference Castillo, Villagra, Sanhueza, Ferres, Mardones and Mertz7 Furthermore, there is currently no evidence of person-to-person transmission of HFRS caused by Hantaan virus. Recently, a self-limited febrile illness was noted in several healthcare workers (HCWs) who had cared for a patient with HFRS in our hospital, raising concerns about the possibility of person-to-person transmission of Hantaan virus. In this study, we evaluated whether transmission of Hantaan virus had occurred among HCWs exposed to the patient with HFRS. This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (IRB no. KBSMC2019-07-041).
We collected sera from the index patient and paired sera from most of the exposed HCWs between November 2019 and December 2019. HFRS confirmed an immunofluorescence assays (IFA) for IgG against Hantaan virus. IFA testing were performed as described in a previous study.Reference Lim, Ryou and Kim8 Blood samples were sent to the Korea Centers for Disease Control and Prevention for confirmation of HFRS infection.
The index patient was a 55-year-old man who lived in Seoul. He was admitted with high fever (39.7°C) on November 13, 2017. He denied any travel history or insect bite. Laboratory testing on admission revealed thrombocytopenia (platelet count, 117,000/m3) and white blood-cell count of 4,680/mm3. On November 16, cardiac arrest occurred after progressive tachypnea and anuria. He was transferred to the intensive care unit after cardiopulmonary resuscitation, and mechanical ventilation and renal replacement therapy was started. Despite these efforts, the patient died on November 19. On November 17, the test for Hantaan virus antibody was positive. The final diagnosis of HFRS was available on December 11, with an anti-Hantaan virus IgG of 1:2048.
In total, 6 HCWs had 1 or more symptoms corresponding to febrile illness. The characteristics of the 6 HCWs are described in Table 1. HCW 2 was a doctor who treated the index patient on a general ward. He had first had contact with the patient on November 15, and he participated in cardiopulmonary resuscitation on November 16. He complained of cough, sputum, and myalgia 3 weeks after exposure (December 7). HCW 3 was a nurse on the general ward. She participated in cardiopulmonary resuscitation on November 16 and complained of myalgia at 23 days after contact. HCW 2 showed increased anti-Hantaan virus IgG titers of 1:256 and <1:32 in sera collected at 28 days and 57 days after contact, respectively. HCW 3 exhibited increased IgG titer of 1:32 and <1:32 in sera collected at 30 days and 57 days, respectively. Upon questioning, it was apparent that HCW 2 and HCW 3 did not wear any personal protective equipment (PPE) during contact with the index case. The other HCWs showed normal IgG titers.
Table 1. Clinical, Laboratory and Serological Findings for 6 Healthcare Workers Who Had Hantaan Virus Symptoms
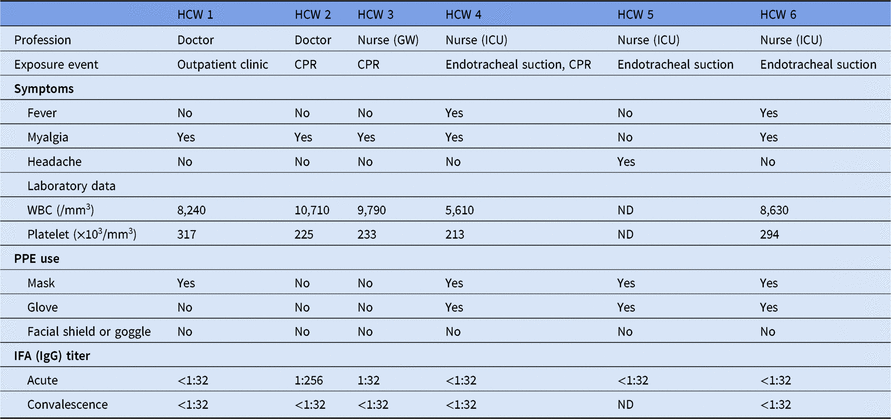
Note. HCW, healthcare worker; GW, general word; ICU, intensive care unit; CPR, cardiopulmonary resuscitation; PPE, personal protective equipment; WBC, white blood cell; ND, not determined; IFA, immunofluorescence assay.
In the present study, there was no confirmed HFRS infection among HCWs who had contact with the index patient. However, these HCWs complained of febrile illness. In fact, 2 HCWs showed elevated IgG titer against Hantaan virus. Previous studies have mentioned possible Hantavirus transmission from an index patient to HCWs.Reference Padula, Edelstein, Miguel, Lopez, Rossi and Rabinovich3,Reference Martinez-Valdebenito, Calvo and Vial5 However, studies on nosocomial transmission of hantaviruses have mainly focused on Andes virus. To date, person-to-person transmission of Hantaan virus has never been proven; therefore, there is no recommendation to isolate HFRS patients in the Korea Centers for Disease Control and Prevention. However, respiratory secretions and/or saliva may be the main route of infection in Hantaan virus, as seen in nosocomial transmission of Andes virus. Furthermore, in Andes virus nosocomial transmission, success of transmission may depend on the extent of PPE used because most HCWs had contact with the patient during the renal phase, which is considered to be the least infectious phase.Reference Padula, Edelstein, Miguel, Lopez, Rossi and Rabinovich3,Reference Martinez-Valdebenito, Calvo and Vial5 Unfortunately, the infectivity of respiratory secretions from patients with HFRS has not been studied directly. Although we found no evidence of Hantaan virus nosocomial transmission, the possibility of nosocomial transmission of HFRS cannot be completely excluded. Until additional supporting evidence is provided by studies on the transmission of HFRS, PPE should be considered.
Our study has several limitations. First, no RT-PCR data were available for the index patient therefore, Hantaan viral replication status could not be determined. Second, we did not include exposed HCWs who did not display symptoms. Third, the possibility of previous Hantaan virus infection should be considered as a potential cause of the elevated IgG titers against Hantaan virus observed in two HCWs. Finally, recall bias among the exposed workers is possible in this study.
In conclusion, we found no evidence of Hantaan virus person-to-person transmission in HCWs exposed to the index case. However, the possibility of such transmission in the future cannot be excluded. We recommend that all HCWs who care for patients with HFRS use PPE. These measures should include routine use of gloves, gowns, and goggles or facial shields to prevent direct patient contact and exposure of mucous membranes to potentially infectious droplets.
Acknowledgments
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



