INTRODUCTION
The leopard (Panthera pardus) appeared in Europe as a newcomer of African origin 1.2–1.1 Myr ago, with the oldest record known from the Vallonnet Cave (Moullé Reference Moullé1992; Turner Reference Turner2009; Michel et al. Reference Michel, Shen, Woodhead, Hu, Wu, Moullé, Khatib, Cauche, Moncel, Valensi, Chou, Gallet, Echassoux, Orange and Hd2017). O’Regan (Reference O’Regan2002) highlighted that the arrival of P. pardus in Europe might have placed pressure on the jaguar (Panthera gombaszoegensis). The leopard would have focused on smaller animals, which were at the lower end of the jaguar prey size range (Hemmer Reference Hemmer2003, Reference Hemmer, Baquedano and Rubio2004). An unanswered question is why, despite its enormous environmental and hunting adaptability, the occurrence of P. pardus in the Middle Pleistocene is limited to a few sites (von Koenigswald et al. Reference von Koenigswald, Nagel and Menger2006). At the same time, the leopard was widespread in Africa and Asia (Geraads Reference Geraads2008; Geraads et al. Reference Geraads, Raynal and Sbihi-Alaoui2010). It was present in Europe until the end of the Late Pleistocene, even during the very cold and continental climatic phase after the glaciation (Spassov and Raychev Reference Spassov and Raychev1997). O’Regan and Turner (Reference O’Regan and Turner2004) described P. pardus of the Middle Pleistocene as a relatively small cat of a gracile posture and with narrow teeth. The small size of the early leopard may have been a response to reduce competition with other large carnivores (mostly the jaguar) in the areas where these two species coexisted (Nowell and Jackson Reference Nowell and Jackson1996; Hemmer Reference Hemmer2001, Reference Hemmer, Baquedano and Rubio2004; García and Virgós Reference García and Virgós2007; Marciszak and Lipecki Reference Marciszak and Lipecki2021). Only after the disappearance of the jaguar did P. pardus spread widely in Europe, increasing in size and ecologically substituting P. gombaszoegensis (García and Virgós Reference García and Virgós2007; Marciszak and Lipecki Reference Marciszak and Lipecki2021). The number of late Middle Pleistocene localities with leopard remains, younger than 300 kyr, increased considerably (Diedrich Reference Diedrich2013; Sauqué and Cuenca-Bescós Reference Sauqué and Cuenca-Bescós2013). The leopard reached the maximum extension of its geographical range during the Late Pleistocene. The spread of P. pardus was accompanied by a trend for a size increase, when the initially relatively small and gracile leopard had become larger in size and weight. The size of the largest Late Pleistocene specimens is comparable to that of the small Middle Pleistocene jaguar, and they held the ecological niche that had been occupied by P. gombaszoegensis (Hemmer Reference Hemmer, Baquedano and Rubio2004; García and Virgós Reference García and Virgós2007).
There is a small number of direct dates of P. pardus, like that of the Radochowska Cave (Diedrich Reference Diedrich2013; Sauqué and Cuenca-Bescós Reference Sauqué and Cuenca-Bescós2013; Sauqué et al. Reference Sauqué, Sanchis and Madurell Malapeira2017). Every new date is critical to obtain a thorough understanding of the history of the evolution of a species such as the leopard, which is known from numerous sites, but usually only represented by sparse material at each site. In thus study, we have dated a leopard sample from the Radochowska Cave. Apart from the new date we have obtained, in addition to the first record of the leopard from Poland (Marciszak et al. Reference Marciszak, Krajcarz, Krajcarz and Stefaniak2011a, Reference Marciszak, Socha, Nadachowski and Stefaniak2011b), the list of Polish localities with remains of this species has recently increased to six cave sites. We briefly discus some palaeoecological aspects of this species and possible reasons for its final disappearance from the territory of Europe.
SITE
Radochowska Cave (50°21'31"N, 16°49'9"E; 460–468 m above sea level), is a karst, 265-m-long cave developed in the lenticular marble at the foot of Mount Bzowiec in the Złote Mountains (eastern Sudety Mountains, southwest Poland). Radochowska Cave was formed during the Pliocene by the leaching of water-soluble marbles in a lens of white marble, partly in contact with schist (grey-yellow, friable rock). It was formed under the erosion made by water filling the entire cross-section of its passages flowing under high pressure. The intensification of erosion of the surrounding slopes at the end of the Pliocene led to deepening of the valley. This caused the channel of a nearby flowing stream to lower (Pulina Reference Pulina1996). A decrease in the karst water level led to dehydration of the area near the cave opening and the formation of hollows. Corridors have been developed along tectonic fissures, and larger halls have been formed at their intersections. In the corridors drained in this way, the alluvial deposition process has begun. The alluvium in the site consists of weathered schist that falls from the cave ceiling and walls, rubble, precipitated calcium carbonate and organic material like animal bones deposited by water from the surface. The alluvium has almost completely filled the cave chambers and passages.
The locality has been known for more than 300 years (Kahlo Reference Kahlo1757; Ostrowicz Reference Ostrowicz1881). Shortly afterwards, it became a popular destination for spa patients from Lądek-Zdrój and tourists. From 1933 to 1947, the cave had a permanent custodian and guide, the retired miner Heinrich Peregrin. He also removed a significant part of the sediment while preparing the cave for visits and opening its corridors. Systematic excavations were carried out by J. Frenzel in 1935 and by L. Zotz in 1936. Brown and grey loams represented deposits of this cave with limestone rubble (Utescher Reference Utescher and Zotz1939). L. Zotz also found quartzite products and pine wood charcoal. In one of the niches, he also found a cave bear’s skull with the mandible and three cervical vertebrae. This gave rise to assumptions about the presence of the Paleolithic people in the cave and created a theory of a “bear cult among Paleolithic people” (Zotz Reference Zotz1937a, Reference Zotz1937b, Reference Zotz1939, Reference Zotz1951). The excavations in 1983 were taken to check the results of German studies from the 1930s, and this theory is no longer supported (Bieroński et al. Reference Bieroński, Burdukiewicz and Wiszniowska1985; Wiśniewski et al. Reference Wiśniewski, Stefaniak, Wojtal, Zych, Nadachowski, Musil, Badura and Przybylski2009; Marciszak et al. Reference Marciszak, Sobczyk, Kasprzak, Gornig, Ratajczak, Wiśniewski and Stefaniak2020). Unfortunately, most of the bone remains from the pre-Second World War studies have been lost, and thus only a small part could be re-examined. These excavations have revealed two main faunal assemblages. The older, Late Pleistocene fauna is roughly dated at MIS 3, and it contains 23 species (Table 1, Table S2). The second, younger assemblage is dated at MIS 1 and includes 30 species (Table S2; Frenzel Reference Frenzel1936, Reference Frenzel1937a, Reference Frenzel1937b; Pax Reference Pax1937; Zotz Reference Zotz1937a, Reference Zotz1937b, Reference Zotz1939, Reference Zotz1951; Kowalski Reference Kowalski1954; Bieroński et al. Reference Bieroński, Burdukiewicz and Wiszniowska1985, Reference Bieroński, Socha and Stefaniak2007, Reference Bieroński, Burdukiewicz, Socha, Stefaniak, Hercman and Nadachowski2009; Pulina Reference Pulina1996; Stefaniak and Bieroński Reference Stefaniak and Bieroński2009; Wiśniewski et al. Reference Wiśniewski, Stefaniak, Wojtal, Zych, Nadachowski, Musil, Badura and Przybylski2009; Marciszak et al. Reference Marciszak, Stefaniak and Gornig2016, Reference Marciszak, Sobczyk, Kasprzak, Gornig, Ratajczak, Wiśniewski and Stefaniak2020; 2021a, Reference Marciszak, Kropczyk and Lipecki2021b, Reference Marciszak, Lipecki, Pawłowska, Jakubowski, Ratajczak-Skrzatek and Nadachowski2021c).
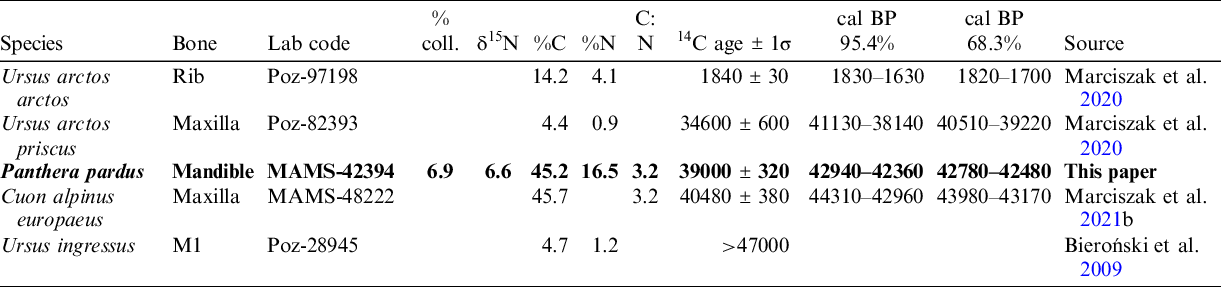
Table 1 AMS radiocarbon results dating of carnivores from Radochowska Cave. The newly obtained date of Panthera pardus is in bold. All dates were calibrated using the program IntCal20, according to Reimer et al. (Reference Reimer, Austin, Bard, Bayliss, Blackwell, Bronk Ramsey, Butzin, Cheng, Edwards, Friedrich, Grootes, Guilderson, Hajdas, Heaton, Hogg, Hughen, Kromer, Manning, Muscheler, Palmer, Pearson, van der Plicht, Reimer, Scott, Southon, Turney, Wacker, Adolphi, Büntgen, Capano, Fahrni, Fogtmann-Schulz, Friedrich, Köhler, Kudsk, Miyake, Olsen, Reining, Sakamoto, Sookdeo and Talamo2020).
SAMPLES AND METHODOLOGY
The leopard sample from the Radochowska Cave used for dating was taken from a fragment of a left mandible (JR/Pp/1), with preserved p4 alveolus and worn m1, stored in the Department of Palaeozoology, University of Wrocław (Figure 1, Table 2). The sample was pretreated at the Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology (MPI-EVA), Leipzig, Germany, using the method described by Talamo and Richards (Reference Talamo and Richards2011) and Talamo et al. (Reference Talamo, Fewlass, Maria and Jaouen2021). The outer surface of bone samples is first cleaned by a shot blaster and then 500 mg of whole bone is taken. The samples are then decalcified in 0.5 M HCl at room temperature until no CO2 effervescence is observed. Then, 0.1 M NaOH is added for 30 min to remove humics. The NaOH step is followed by a final 0.5 M HCl step for 15 min. The resulting solid is gelatinized as described by Longin (Reference Longin1971) at pH 3 in a heater block at 75°C for 20 hr. The gelatine is then filtered in an Eeze-Filter™ (Elkay Laboratory Products [UK] Ltd.) to remove small (> 80 μm) particles. The gelatine is then ultrafiltered with Sartorius “VivaspinTurbo” 30 kDa ultrafilters. Prior to use, the filter is cleaned to remove carbon-containing humectants (Talamo et al. Reference Talamo, Fewlass, Maria and Jaouen2021). The samples are lyophilized for 48 hr. The date was corrected for a residual preparation background estimated from a pretreated 14C-free bone sample, kindly provided by the Mannheim laboratory and pretreated in the same way as archaeological samples (Korlević et al. Reference Korlević, Talamo and Meyer2018). To identify the quality of the collagen, the C:N ratio was also estimated. It should be between 2.9 and 3.6, and the collagen yield should not be < 1% of the weight (van Klinken Reference van Klinken1999; Talamo et al. Reference Talamo, Fewlass, Maria and Jaouen2021). Stable isotopic analysis was performed at MPI-EVA, Leipzig (Lab Code R-EVA), using a ThermiFinnigan Flash EA with a Delta V isotope ratio mass spectrometer. The sample was sent to the Curt Engelhorn Centre for Archaeometry (CEZA) in Mannheim, Germany (lab code MAMS), where it was graphitized and dated (Kromer et al. Reference Kromer, Lindauer, Synal and Wacker2013). All new dates were calibrated by using the program IntCal20, according to Reimer et al. (Reference Reimer, Austin, Bard, Bayliss, Blackwell, Bronk Ramsey, Butzin, Cheng, Edwards, Friedrich, Grootes, Guilderson, Hajdas, Heaton, Hogg, Hughen, Kromer, Manning, Muscheler, Palmer, Pearson, van der Plicht, Reimer, Scott, Southon, Turney, Wacker, Adolphi, Büntgen, Capano, Fahrni, Fogtmann-Schulz, Friedrich, Köhler, Kudsk, Miyake, Olsen, Reining, Sakamoto, Sookdeo and Talamo2020).

Figure 1 Radiocarbon dated specimen of Panthera pardus from Radochowska Cave: 1. fragment of a left mandible (JR/Pp/1, a—buccal view, b—occlusal view); 2. left m1 belonging to the same specimen (a—buccal view, b—lingual view). Scale bar 10 mm.
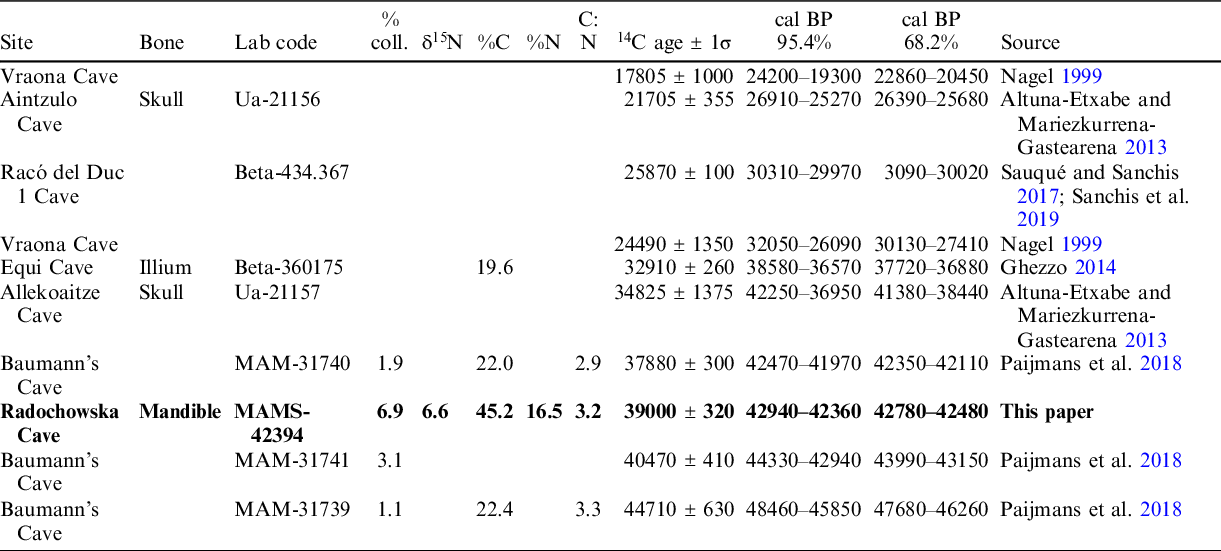
Table 2 Radiocarbon results dating of Panthera pardus from Europe. All dates were calibrated using the program IntCal20, according to Reimer et al. (Reference Reimer, Austin, Bard, Bayliss, Blackwell, Bronk Ramsey, Butzin, Cheng, Edwards, Friedrich, Grootes, Guilderson, Hajdas, Heaton, Hogg, Hughen, Kromer, Manning, Muscheler, Palmer, Pearson, van der Plicht, Reimer, Scott, Southon, Turney, Wacker, Adolphi, Büntgen, Capano, Fahrni, Fogtmann-Schulz, Friedrich, Köhler, Kudsk, Miyake, Olsen, Reining, Sakamoto, Sookdeo and Talamo2020).
RESULTS
Although the dated bone fragment was relatively small, a precise date was acquired (MAMS-42394 in Table 1). The date indicates the middle part of MIS 3 (∼43–42 kyr BP). Three more dates were obtained that also fall within the range of MIS 3. The subfossil, a brightly colored and light rib of Ursus arctos arctos, was dated to the Romanian period (∼1.8–1.7 kyr BP). These dates confirmed the presence of two different faunal assemblages, dated at MIS 3 (older) and MIS 1 (younger). Both assemblages were partially mixed, although bones dated at MIS 3 are grey, brown or black; deeply mineralized; and heavy. Subfossil bones dated at MIS 1 are white or bright grey, lightly mineralized and delicate. These findings confirm the conclusions of Bieroński et al. (Reference Bieroński, Burdukiewicz and Wiszniowska1985, Reference Bieroński, Burdukiewicz, Socha, Stefaniak, Hercman and Nadachowski2009) about the mixed character of a part of the sediments in the Radochowska Cave, partial redeposition and a significant role of outside transport in the formation of cave sediments.
DISCUSSION
Polish Records of Panthera pardus Based on the European Background
A broad query revealed the presence of the leopard at 312 European sites, which cover the timespan of the last ca. 1.1 Myr (Figure 2, Table S1). The list of Polish localities with leopard remains has recently increased to six cave sites. Among them, four are located in the Sudety Mountains (the Naciekowa, Obok Wschodniej, Radochowska, and Wschodnia Caves) and two others are located in the Kraków-Częstochowa Upland (the Biśnik and Dziadowa Skała Caves). All records are from rocky regions with rugged terrain convenient for ambushes and hiding, and all of them are located on the territory of Silesia (southern Poland). There is a small number of direct dates of P. pardus, like the date we have obtained for the Radochowska Cave sample (e.g., Diedrich Reference Diedrich2013; Sauqué and Cuenca-Bescós Reference Sauqué and Cuenca-Bescós2013).
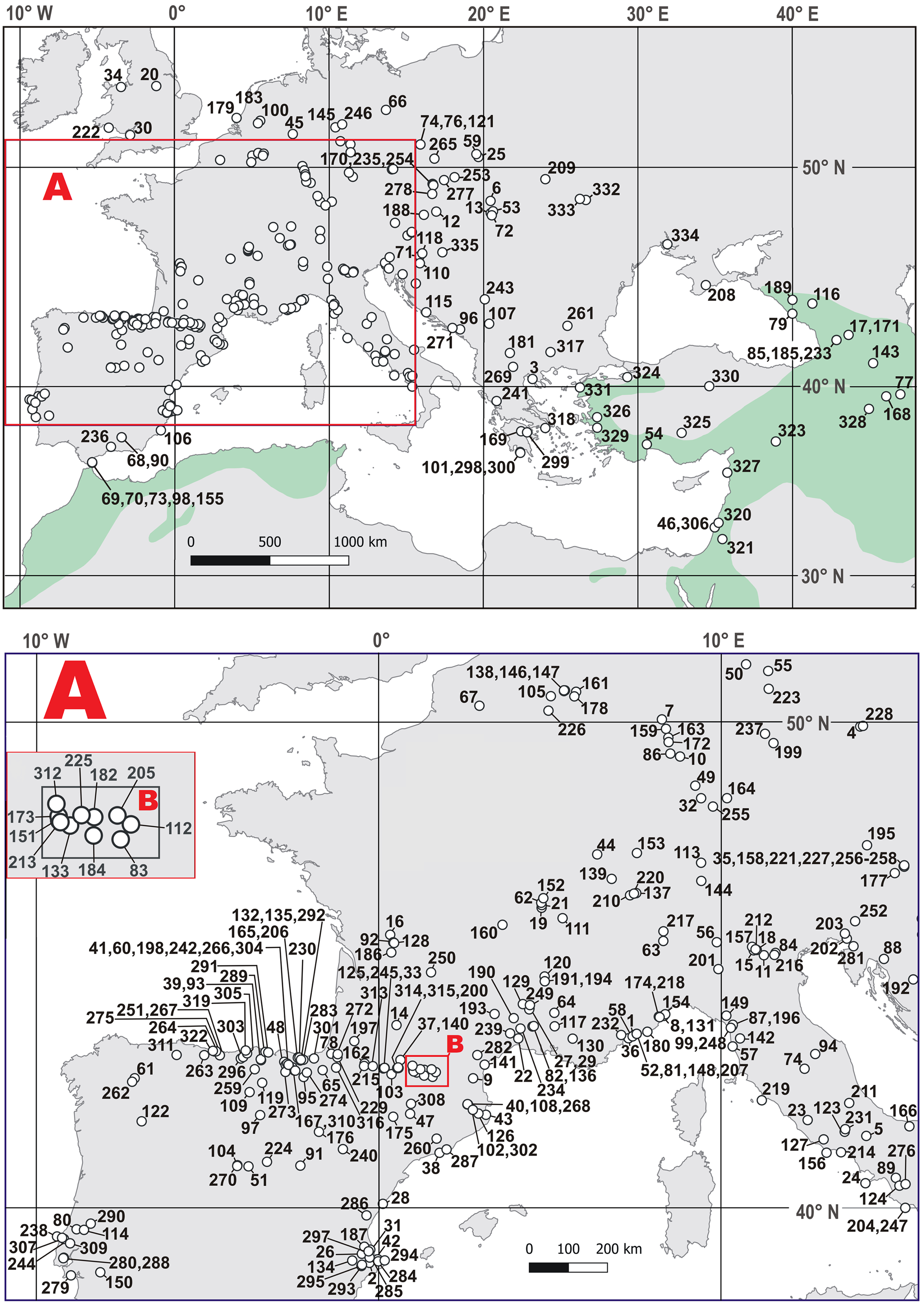
Figure 2 Distribution of the Pleistocene and the Holocene sites with Panthera pardus within Europe and southwest Asia. See Table S1 for site numbers. The greenish color indicates the areas where the leopard occurred in historical times and where the extant isolated populations currently living. (After Jacobson et al. Reference Jacobson, Gerngross, Lemeris, Schoonover, Anco, Breitenmoser-Würsten, Durant, Farhadinia, Henschel, Kamler, Laguardia, Rostro-García, Stein and Dollar2016. Please see electronic version for color figures.)
The presence of the leopard during MIS 3 at the Sudety Mountains had already been suggested (Marciszak et al. Reference Marciszak, Stefaniak and Gornig2016, Reference Marciszak, Sobczyk, Kasprzak, Gornig, Ratajczak, Wiśniewski and Stefaniak2020), but our direct dating has confirmed it. Other Polish records indicate the presence of this species since the late Middle Pleistocene, as confirmed by the study of remains from the lowermost layers 19a–d of the Biśnik Cave, dated at MIS 10/9 (Marciszak et al. Reference Marciszak, Krajcarz, Krajcarz and Stefaniak2011a, Reference Marciszak, Socha, Nadachowski and Stefaniak2011b). Between MIS 7 and MIS 3, P. pardus was probably present as a rare hunter in Poland. No younger individuals have been found, so nothing is known about the species survival or re-colonization in the Polish territory during the Late Glacial or Holocene. However, this problem with the reconstruction of the European history of P. pardus, due to the nearly complete absence of direct dates of its remains, hampers a detailed time resolution of the leopard distribution in Europe (Pacher and Rabeder Reference Pacher and Rabeder2016). The two oldest dates, 88.5 kyr (LEB-14069) and 98.4 kyr (LEB-14070), obtained directly from the remains of a leopard from the Spanish site Artazu VII and based on the amino acid racemization (AAR) method, documented the presence of the species during MIS 5b (Suárez-Bilbao et al. Reference Suárez-Bilbao, Garcia-Ibaibarriaga, Castaños, Castaños, Iriarte-Chiapusso, Arrizabalaga, Torrese, Ortize and Murelaga2016). Three other dates from Baumann’s Cave (Germany) and one from the Equi Cave (Italy) also fall into MIS 3 (Table 2). Two conventional dates from the Vraona Cave (Greece) have documented the presence of this species during the MIS 3/MIS 2 transition and MIS 2 (Nagel Reference Nagel1999). These data corroborate well with the occurrence of the Balkan refugee of the species during cool phases—for example, the Last Glacial Maximum (LGM). It also corresponds well with the idea that the leopard seems to have survived up to the LGM in southern and eastern parts of Europe. The history of the last European leopards is still incomplete. The supposed Holocene survival of P. pardus in Europe is still highly unresolved and based on quite unclear records—for example, Spassov and Raychev (Reference Spassov and Raychev1997), Bartosiewicz (Reference Bartosiewicz, Buitenhuis and Prummel2001, Reference Bartosiewicz2015), and Galik et al. (Reference Galik, Horejs and Nessel2012). It is difficult to reconstruct the last occurrence and extinction of P. pardus in Europe. The two imprecisely dated records, one Mesolithic from northern Spain (Altuna Reference Altuna1972) and the second from Greece (Nagel Reference Nagel1999), are two indicators that the species could have survived there until the Holocene (Sommer and Benecke Reference Sommer and Benecke2006; Sauqué and Cuenca-Bescós Reference Sauqué and Cuenca-Bescós2013). The leopard retreated from Europe eastward and south-eastward, but this process has been poorly documented. In western Europe, the Iberian population remained detached from the rest. Multiple post-LGM records indicate their presence and eventual survival until the Middle Holocene in the Iberian Peninsula (Vega del Sella Reference Vega del Sella1930; Altuna Reference Altuna1972; Sauqué and Cuenca-Bescós Reference Sauqué and Cuenca-Bescós2013). This area seems to have been the last refuge for the European leopard, similarly to the last European dhole Cuon alpinus europaeus Bourguignat, 1868 (Pérez Ripoll et al. Reference Pérez Ripoll, Morales Pérez, Serra, Aura Tortosa and Sarrión Montañana2010; Sanchis et al. Reference Sanchis, Real, Sauqué, Núñez-Lahuerta, Égüez, Tormo, Pérez Ripoll, Carrión Marco, Duarte and la Rasilla2019, Reference Sanchis, Gómez-Olivencia, Real, Boudadi-Maligne and Mallye2020; Marciszak et al. Reference Marciszak, Kropczyk and Lipecki2021b).
Disappearance of P. pardus in Europe: a Working Hypothesis
The Polish fossil record is highly incomplete and, for this reason, the species succession history in Poland should be treated with caution. The presence of P. pardus in Poland was restricted to highland and mountain areas of Silesia. This animal probably avoided open, extensive Bohemian, Moravian, and Silesian grasslands because of the presence of other large, fast-moving and social carnivores, like the steppe wolf or the cave hyena. Both species constituted a serious threat for a solitary cat, especially when trees or other potential shelters were absent or very rare (Eaton Reference Eaton1979).
Regarding the disappearance of the leopard in Europe, the key questions are not only when but also why. First, we must clearly state that our discussion is based on hypotheses and not proven facts. Only positive evidence is reliable in palaeontology due to the incompleteness of the fossil record. The absence of leopard remains in some regions or chronological intervals does not necessarily mean that they were absent from the surroundings. It is possible that the remains were not preserved or have not yet been identified or excavated. Sudeten cave regions favor fossil bone preservation, but open, extensive Bohemian, Moravian, and Silesian grasslands do not. Hence, only knowing the geographic or chronological distribution of the localities is not enough to draw conclusions about the distribution of past populations. Second, the deductions on population replacements over time and on interspecies relationships are difficult to support by using only the fossil record. Genetics and/or isotopic dating are crucial to conclude about ecological relationships.
The leopard is an extremely adaptive carnivore: it is now able to inhabit highly urbanized areas. Unlike, for example, the lion, the progressive warming and the associated increase in afforestation should have no negative impact on the leopard population (Nagel et al. Reference Nagel, Lindenbauer, Kavcik-Graumann and Rabeder2018). On the contrary, forested areas gave it shelter and the density of potential prey—for example, deer, which was quite sufficient. P. pardus probably found a favorable ecological niche in the Sudety Mountains during the Late Pleistocene, when there were coniferous forests with some number of deciduous trees growing mostly in valleys during warmer periods. The presence of trees is an important factor for their environment, while deep snow cover is a limiting one (Heptner and Naumov Reference Heptner and Naumov1967; Jacobson et al. Reference Jacobson, Gerngross, Lemeris, Schoonover, Anco, Breitenmoser-Würsten, Durant, Farhadinia, Henschel, Kamler, Laguardia, Rostro-García, Stein and Dollar2016). However, even if the presence of trees is one of the crucial factors for this species, the ecologically extremely flexible leopard could have been adapted even for the coolest phases of the Late Pleistocene (i.e., the LGM), which was characterized by nearly treeless and rocky habitats (Nagel Reference Nagel1999). Despite the fact that P. pardus is not a typical cave dweller, recent leopards use caves to catch their prey to avoid competitors like lions and spotted hyenas in treeless regions (Brain Reference Brain1981; de Ruiter and Berger Reference de Ruiter and Berger2000, Reference de Ruiter and Berger2001; Hayward et al. Reference Hayward, Henschel, O’Brian, Hofmeyr, Balme and Kerley2006; Diedrich Reference Diedrich2013; Yang et al. Reference Yang, Zhao, Han, Wang, Mou, Ge and Feng2018). In African cave regions, up to 33% of the carcasses are dragged by leopards into deeper cave parts to avoid conflicts with lions and hyenas. Simultaneously, only 7% of prey remains were pulled up into trees, which were located more than 500 m from those caves. In cases where trees grow near the entrances, 83% of carcasses were stored in caves and only 17% in trees (de Ruiter and Berger Reference de Ruiter and Berger2001; Diedrich Reference Diedrich2013). Leopards drag even larger carcasses very deeply into caves, especially to avoid stealing by hyenas, which cannot climb slopes within those caves (de Ruiter and Berger Reference de Ruiter and Berger2001; Diedrich Reference Diedrich2013). If possible, they usually preferred small rock shelters with narrow entrances that made them less accessible to lions. In that context, the area of the Sudety Mountains, full of such small rock shelters, can be regarded as a potential preferable habitat for the leopard.
According to Diedrich (Reference Diedrich2013: 189), this ibex/chamois/deer prey–adapted felid is extremely rare in lowlands. The remains of P. pardus have not been found in any of the open-air faunal assemblages in Poland (Wolsan Reference Wolsan1989; Marciszak et al. Reference Marciszak, Kotowski, Przybylski, Badura, Wiśniewski and Stefaniak2019). This scenario has already been discussed for Balkan sites, where the situation is similar to those in Silesia and the Sudety Mountains (Spassov and Raychev Reference Spassov and Raychev1997). This cat also preferred rocky regions with a rugged terrain that are convenient for ambushes and hiding (Heptner and Sludskij Reference Heptner and Sludskij1972; Spassov and Raychev Reference Spassov and Raychev1997; Jacobson et al. Reference Jacobson, Gerngross, Lemeris, Schoonover, Anco, Breitenmoser-Würsten, Durant, Farhadinia, Henschel, Kamler, Laguardia, Rostro-García, Stein and Dollar2016). The leopard lived in relatively low densities while the density of the wolf population is high (Heptner and Sludskij Reference Heptner and Sludskij1972; Sludskij Reference Sludskij1976; Spassov and Raychev Reference Spassov and Raychev1997). The lowlands surrounding the Sudety Mountains (Bohemian, Moravian, and Silesian) were overwhelmed by packs of robust steppe wolves, whose remains have been recorded regularly from open-air sites (Herr Reference Herr1924; Kowalski Reference Kowalski1959; Wiśniewski et al. Reference Wiśniewski, Stefaniak, Wojtal, Zych, Nadachowski, Musil, Badura and Przybylski2009; Marciszak et al. Reference Marciszak, Kotowski, Przybylski, Badura, Wiśniewski and Stefaniak2019, Reference Marciszak, Sobczyk, Kasprzak, Gornig, Ratajczak, Wiśniewski and Stefaniak2020). In addition, the presence of wolves might be a limiting factor, because as a solitary hunter, the leopard usually avoided aggressive competitions with large dogs (Heptner and Sludskij Reference Heptner and Sludskij1972; Eaton Reference Eaton1979; Spassov and Raychev Reference Spassov and Raychev1997; Miquelle et al. Reference Miquelle, Stephens, Smirnov, Goodrich, Zaumyslova, Myslenkov, Ray, Berger, Redford and Steneck2005). Given the similar size of wolves and leopards, in a one-on-one confrontation, the leopard has an advantage, but the confrontation of a solitary cat with a pack of wolves is a different story (Figure 3). In this regard, we agree with the hypothesis proposed by Spassov and Raychev (Reference Spassov and Raychev1997) that a set of different factors caused the species not to form vital populations after the LGM (GS-2.1a interval, 17.5–14.7 kyr), when intensive deglaciation processes started. Among them, there are a lack of sufficient food sources combined with a relatively small number of adequate rocky shelters, the high density of the wolf population, and intense human pressure. Isolated, relict populations such as those in the Iberian Peninsula, which survived without probable contact with the North African populations, were probably exterminated by humans at a fairly early date. Eastern re-colonization, in turn, was probably hindered or even prevented by a strip of wide, almost treeless plains that effectively cut off central and western Europe from the eastern populations of P. pardus, where the leopard has survived (Bartosiewicz Reference Bartosiewicz, Buitenhuis and Prummel2001).

Figure 3 Scenes showing conflict between the leopard Panthera pardus with one of his main competitors during the Late Pleistocene, cave wolves Canis lupus spelaeus near the Radochowska Cave. (Drawing by W. Gornig.)
CONCLUSIONS
A critical revision of all available literature sources shows the presence of P. pardus at 312 European sites, with the last documented ∼1.1 Myr. Among them, there are six Polish records (five of which are new), including four sites located in the Sudety Mountains (the Naciekowa, Obok Wschodniej, Radochowska and Wschodnia Caves), as well as two localities in the Polish Jura (the Biśnik and Dziadowa Skała Caves). All records are from rocky regions with rugged terrain convenient for ambushes and hiding, located in the territory of Silesia (southern Poland). These records document the probable presence of the species in Poland between MIS 10/9 and MIS 3. In addition, a newly obtained radiocarbon date of 43–42 kyr from the Radochowska Cave directly confirms the occurrence of P. pardus in the Sudety Mountains in the middle part of MIS 3. Together with other AMS dates from this site, we can confirm the presence of two faunal assemblages, one of which is older (MIS 3), and another that is younger (MIS 1). The post-LGM history of P. pardus in Europe has been poorly defined. Only the Iberian Peninsula, with numerous records of P. pardus dated in the range of 17–11 kyr or even later, can be regarded as a reliable documentation of the last European refugium for this cat. Eastern or southern European records are usually based on imprecisely dated records, and it is still full of ambiguities. A set of different factors probably underscored the inability of the species to establish vital populations after the LGM, including a lack of sufficient food sources combined with a relatively small number of adequate rocky shelters, a high density of the wolf population and human pressure. Isolated relict populations were probably exterminated by humans at a fairly early date. Eastern re-colonization, in turn, was probably hindered or even prevented by a strip of wide, almost treeless plains that effectively cut off central and western Europe from eastern populations of P. pardus, where the leopard has survived.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/RDC.2022.33.








