Psychiatrists, especially those working in liaison or old age psychiatry, frequently encounter people who have had a stroke and mood change, especially depression, is a well-recognised sequel. Estimates of frequency varying from 25 to 36% in two previous meta-analyses, Reference Ayerbe, Ayis, Wolfe and Rudd1,Reference Hackett, Yapa, Parag and Anderson2 depending on the setting, population studied, definition of depression and timing of assessments. A weakness in the current literature is the relatively short-term nature of follow-up and we therefore have a limited understanding of the longer-term risk of depression after stroke and its predictors, yet most psychiatric assessments after stroke occur years rather than months later. Ayerbe et al found only eight studies that had longer-term follow-up beyond 1 year post-stroke, Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe3 which included two population-based cohorts that had followed participants up to 5 years Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe3,Reference Sharpe, Hawton, Seagroatt, Bamford, House and Molyneux4 but no hospital-based cohorts that had such a long period of follow-up, and there are no reports of follow-up beyond 5 years. There is also a paucity of well-designed studies large enough to examine predictors of depression after stroke, and only four of these studies have followed up participants for longer than 1 year. Reference Hackett and Anderson5 Major predictors of post-stroke depression include disability, pre-stroke depression and cognitive impairment, and thus people with these factors may benefit from proactive monitoring of mood in the first year of post-stroke care. Reference Hackett and Anderson6 Since acute stroke units have resulted in improved survival after stroke, many older people survive far beyond this phase, so there is an additional issue about whether longer-term follow-up, and monitoring of both mood and cognition, would be beneficial in preventing progression of cognitive decline, deterioration in mood and functional ability. In designing randomised controlled trials to identify appropriate strategies for the long-term prevention of such deterioration, there is also a need to understand its underlying aetiology. The vascular hypothesis postulates that some depression in older people is a vascular brain disease. If this is true, then stroke patients will be at increased risk of depression after stroke, but those at greatest risk will be those with a greater burden of vascular disease, reflected in an increased number of vascular risk factors including ischaemic heart disease, peripheral vascular disease, diabetes mellitus, smoking and atrial fibrillation. Reference Valkanova and Ebmeier7 We have previously shown that a greater burden of vascular factors is associated with a greater likelihood of clinical expression of a dementia syndrome in the long term after stroke in older people. Reference Allan, Rowan, Firbank, Thomas, Parry and Polvikoski8 Whereas depression predicted the incidence of dementia in univariate analyses, it was the number of cardiovascular risk factors that was retained in multivariate analyses, along with cognitive impairment. It is therefore likely that mood disorders and cognitive decline after stroke share a common relationship with both burden of vascular risk factors and underlying neuropathology. The demonstration of any association between mood changes and vascular risk factors or vascular pathology would be important, since depression is a recognised risk factor for dementia (both Alzheimer's disease and vascular dementia), but the nature of the link remains unclear.
In this study, we examine depression in the COGFAST cohort, which is a secondary-care-based longitudinal study of older people (aged over 75) who have had a stroke, with follow-up data of up to 10 years and a brain tissue donation programme. Reference Allan, Rowan, Firbank, Thomas, Parry and Polvikoski8 The cohort provides a rare opportunity to look at predictors of mood disorders in the very long term after stroke, and also to examine their associations with neuropathology. We hypothesised that, in accordance with the vascular hypothesis of depression, an increased burden of vascular risk factors would predict depressive symptoms in the long term. We further hypothesised that those with the greatest degree of cognitive decline would account for the majority of incident depression, and that such individuals would have a greater burden of vascular neuropathology at post-mortem.
Method
Study design and participants
Older stroke patients (n = 706) ⩾75 years were screened consecutively from hospital-based stroke registers in Tyneside and Wearside in the North East of England (Fig. 1). Potential participants were evaluated at least 3 months after stroke (on the basis of the design of Desmond et al Reference Desmond, Moroney, Paik, Sano, Mohr and Aboumatar9 and to enable resolution of acute post-stroke delirium), with a standardised battery comprised of medical history, Mini-Mental State Examination (MMSE) Reference Folstein, Folstein and McHugh10 score, assessment of neurological deficits, a blood screen and a review of the computed tomography (CT) brain scan undertaken at the time of the stroke. Stroke was defined according to the World Health Organization definition. 11 We excluded those individuals with stroke (a) who were younger than 75 years, (b) with significant physical illness and disabilities that precluded neuropsychological evaluation (such as visual impairment, aphasia, hemiparesis affecting the hand used for writing), (c) who had an MMSE score less than 24 at the 3-month assessment, (d) had a diagnosis of dementia according to DSM-III-R criteria 12 or (e) declined to take part. Following general practitioner approval and discussion of the study, 355 of the original 706 patients were eligible for the post-stroke survivor study (Fig. 1). The characteristics of the full cohort have been described previously. Reference Ballard, Rowan, Stephens, Kalaria and Kenny13,Reference Ballard, Stephens, Kenny, Kalaria, Tovee and O'Brien14 In addition to this full cohort, a subgroup of 35 of the stroke survivors who already had dementia at baseline were recruited into a study of falls in dementia that was ongoing within the same department. Reference Allan, Ballard, Rowan and Kenny15 These patients received the same follow-up protocol as the main cohort (described below).
Ethical approval was obtained from the Joint Ethics Committee of Newcastle and North Tyneside Health Authority, the University of Newcastle upon Tyne and the University of Northumbria at Newcastle, and participants gave written informed consent in accordance with the declaration of Helsinki.
Baseline and follow-up assessments
All participants completed a standardised baseline medical and neuropsychological assessment at 3 months post-stroke. Medical histories taken from the participants were supported by review of hospital charts. Data on putative predictors of depression included pre-stroke factors, stroke characteristics and factors related to outcome at 3 months. Pre-stroke factors were age, gender, current treatment for depression, diagnosis of previous stroke, hypertension (a documented history of blood pressure greater than 140/90 mmHg or treatment of hypertension), atrial fibrillation, ischaemic heart disease, peripheral vascular disease, hypercholesterolaemia, diabetes (documented or treated), history of smoking prior to stroke and apolipoprotein genotype determined as described previously. Reference Ballard, Morris, Rao, O'Brien, Barber and Stephens16
Strokes were classified using the Oxford Community Stroke project (OCSP) classification. Reference Bamford, Sandercock, Dennis, Burn and Warlow17 Stroke outcome factors were activities of daily living (assessed with the Bristol scale), Reference Bucks, Ashworth, Wilcock and Siegfried18 cognition (assessed with the Cambridge Examination for Mental Disorders of the Elderly, section B (Cambridge Cognitive Examination, CAMCOG), Reference Roth, Tym, Mountjoy, Huppert, Hendrie and Verma19 which is a standardised paper and pencil test (maximum score, 107) for global cognitive performance), presence of mild cognitive impairment (based on Petersen criteria: Reference Petersen, Smith, Waring, Ivnik, Kokmen and Tangelos20 those with cognitive scores >1.5 standard deviations below an age-matched control group were defined as having amnestic mild cognitive impairment (CAMCOG memory subscore ⩽13) and/or non-amnestic mild cognitive impairment (CAMCOG executive subscore ⩽17)), and finally severity of baseline depressive symptomatology. Severity of baseline depressive symptoms was quantified using the self-rated 15-item Geriatric Depression Scale (GDS). Reference Almeida and Almeida21

Fig. 1 Flow chart of participant follow-up.
Where totals show ‘still in study’, this refers to individuals who have so far completed less than 10 years of follow-up but who have not yet died or withdrawn from the study. MMSE, Mini-Mental State Examination; VAD, vascular dementia.
Prevalent depression was defined by three methods: major depression according to the DSM-IV criteria 22 assessed by a clinical research nurse or assistant psychologist, significant depressive symptoms defined as a score of ⩾5 on the GDS (GDS depression) or depressive disorder defined as a score of ⩾10 on the observer-rated Cornell scale (Cornell depression). Reference Alexopoulos, Abrams, Young and Shamoian23 In keeping with other studies in older people, and to facilitate cross-study comparison, we utilised GDS score as our primary depression outcome for analysis. Reference Teodorczuk, Firbank, Pantoni, Poggesi, Erkinjuntti and Wallin24,Reference Teodorczuk, O'Brien, Firbank, Pantoni, Poggesi and Erkinjuntti25 As GDS scores are skewed, for analysis we converted scores to quartiles, based on GDS scores at baseline in the whole group.
Neuropsychological assessment was repeated annually, as in the baseline assessment, during a follow-up period of up to 10 years. Incident dementia according to DSM-IV criteria was assessed according to methods we have described previously. Incident depression was defined as described for prevalent depression.
Neuropathological examination and dementia diagnoses
Brains were retrieved from a total of 56 stroke survivors who came to autopsy. Six of these had dementia at baseline (Fig. 1) but were included in the assessment for comparative purposes. In the majority of cases bronchopneumonia was recorded as the cause of death.
Macroscopic and microscopic pathology was assessed with standardised protocols as described in Kalaria et al Reference Kalaria, Kenny, Ballard, Perry, Ince and Polvikoski26 and Ihara et al. Reference Ihara, Polvikoski, Hall, Slade, Perry and Oakley27 Briefly: macroscopic infarcts were detected by visual inspection while dissecting the brain, and their presence was subsequently confirmed by microscopy. Haemotoxylin and eosin was used as the standard stain for general neuropathological assessment of the structure of the brain, and for confirmation/detection of the infarcts. In this study, any infarct less than 5 mm in diameter was defined as a microinfarct. Gallyas and Bielschowsky's silver impregnation and tau immunohistochemistry were applied to assess neuritic plaques and neurofibrillary tangles for the ‘CERAD’ (Consortium to Establish a Registry for Alzheimer's Disease) plaque score Reference Mirra, Heyman, McKeel, Sumi, Crain and Brownlee28 and ‘Braak and Braak’ neurofibrillary tangle staging. Reference Braak, Alafuzoff, Arzberger, Kretzschmar and Del Tredici29 Additional staining included α-synuclein, ubiquitin and TDP-43 immunohistochemistry.
A clinical diagnosis of whether the dementia syndrome was present was made independently of neuropathological data prior to monthly clinicopathological consensus meetings where clinicians met with the pathologists to designate a final diagnosis for autopsied individuals. In all cases, additional pathologies such as that in Alzheimer's disease and dementia with Lewy bodies were noted. The pathological diagnosis of vascular dementia was then assigned if there was clinical evidence of dementia (DSM-IV) and the presence of multiple or cystic infarcts involving cortical and subcortical structures, border-zone infarcts, lacunae, microinfarcts and small vessel disease in the general absence of a high burden of neurofibrillary pathology (i.e. Braak staging <IV) Reference Kalaria, Kenny, Ballard, Perry, Ince and Polvikoski26 or obvious other primary neurodegenerative disease, and consistent with the clinical diagnostic criteria proposed by others. Reference Chui, Victoroff, Margolin, Jagust, Shankle and Katzman30-Reference Roman, Tatemichi, Erkinjuntti, Cummings, Masdeu and Garcia33 An individual's condition was designated as mixed when there was pathological evidence of cerebrovascular disease with Alzheimer's-disease-type pathology, Lewy bodies or tauopathy (frontotemporal lobar degeneration or progressive supranuclear palsy). These individuals with mixed cerebrovascular disease and Alzheimer's disease had a Braak stage of V or VI and moderate to severe CERAD scores.
Statistical analysis
Univariate analysis was used to examine correlations between putative predictors and severity of depressive symptoms (rated using the GDS) at 1 year, 4 years and 8 years. These time intervals were chosen in order to ascertain whether associations between predictors and depressive symptoms changed over time, using data-sets from early, intermediate and long-term follow-up periods. Year 8 was selected as years 9 and 10 contained insufficient data for these analyses. As depressive symptoms are on an ordinal scale, we used Spearman's rank order correlation coefficient. The presence of dementia at the respective follow-up period was used as an additional independent variable.
For multivariate analyses we used multiple linear regression to examine which predictors were independently associated with severity of depressive symptomatology at each follow-up period (years 1, 4 and 8). Age and gender were entered into all models. Other putative predictors that were significant in univariate analyses at first follow-up were entered stepwise into three multivariate models (P = 0.05 to enter, P = 0.1 to remove). Neuropathological findings were compared between those participants who had at least one GDS rating ⩾5 and those whose scores were always <5.
Results
Participants
Figure 1 shows the numbers of participants screened and recruited, and assessments of mood each year, together with deaths and withdrawals. The 355 participants recruited to the main study had a mean age of 80 years (s.d. = 4.1) and 52% were male. Hospital notes revealed that 19 (5.4%) patients had sustained a total anterior circulation stroke, 145 (41%) a partial anterior circulation stroke, 116 (33%) a lacunar stroke, 49 (14%) a posterior circulation stroke and 26 (7%) an unclassifiable stroke. A summary of baseline characteristics of the participants is shown in Table 1.
Prevalence and incidence of depression
Both baseline prevalence and incidence of depression varied according to method of assessment (Table 2), with the self-rated GDS giving the highest rates of depressive symptoms (31.7% baseline prevalence), the observer-rated Cornell scale giving an intermediate rate (9.7%) and the DSM criteria for major depression giving the lowest (1.2%). Figure 2 shows at each year of follow-up the proportion with either major depression according to DSM-IV diagnosis or depressive symptoms above cut-offs for, GDS score ⩾5 and a Cornell score ⩾10, with the 95% confidence intervals for the estimated percentages.
Table 1 Baseline characteristics of post-stroke survivors

| Characteristic | Participants |
|---|---|
| Pre-stroke factors | |
| Age, years: mean (s.d.) | 80 (4.10) |
| Male, n (%) | 184 (51.8) |
| Antidepressant treatment at time of stroke, n (%) | 35 (9.9) |
| Previous stroke, n (%) | 104 (29.3) |
| Ischaemic heart disease, n (%) | 113 (31.8) |
| Hypertension, n (%) | 193 (54.4) |
| Atrial fibrillation, n (%) | 55 (15.5) |
| Diabetes mellitus, n (%) | 29 (8.2) |
| Hypercholesterolaemia, n (%) | 45 (12.7) |
| Peripheral vascular disease, n (%) | 25 (7.0) |
| Ever smoked, n (%) | 207 (58.3) |
| Number of cardiovascular risk factors, median (IQR) | 2 (1-3) |
| APOE ε4 allele (one copy), n (%) | 64/273 (23.4) |
| Oxford Community Stroke project stroke classification, n (%) | |
| Lacunar stroke | 116 (32.7) |
| Partial anterior circulation stroke | 145 (40.8) |
| Total anterior circulation stroke | 19 (5.4) |
| Posterior circulation stroke | 49 (13.8) |
| Other | 26 (7.3) |
| Stroke outcome factors at 3 months | |
| CAMCOG total score, mean (s.d.) | 85.1 (8.96) |
| Total CAMCOG score <80 (CIND), n (%) | 90 (25.4) |
| CAMCOG total memory score ⩽17 (MCI), n (%) | 50 (14.1) |
| CAMCOG total executive function score ⩽13, n (%) | 168 (47.3) |
| Geriatric Depression Score, median (IQR) | 3 (1-5) |
| Bristol activities of daily living score | 4 (1-8) |
IQR, interquartile range; APOE, apolipoprotein E; CAMCOG, Cambridge Cognitive Examination; CIND, cognitive impairment no dementia; MCI, mild cognitive impairment.
Table 2 Number of participants identified with depression by each method of assessment each year
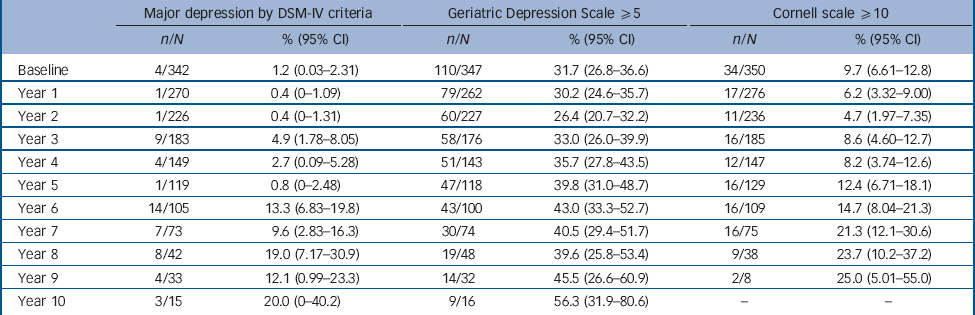
| Major depression by DSM-IV criteria | Geriatric Depression Scale ⩾5 | Cornell scale ⩾10 | ||||
|---|---|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | n/N | % (95% CI) | |
| Baseline | 4/342 | 1.2 (0.03-2.31) | 110/347 | 31.7 (26.8-36.6) | 34/350 | 9.7 (6.61-12.8) |
| Year 1 | 1/270 | 0.4 (0-1.09) | 79/262 | 30.2 (24.6-35.7) | 17/276 | 6.2 (3.32-9.00) |
| Year 2 | 1/226 | 0.4 (0-1.31) | 60/227 | 26.4 (20.7-32.2) | 11/236 | 4.7 (1.97-7.35) |
| Year 3 | 9/183 | 4.9 (1.78-8.05) | 58/176 | 33.0 (26.0-39.9) | 16/185 | 8.6 (4.60-12.7) |
| Year 4 | 4/149 | 2.7 (0.09-5.28) | 51/143 | 35.7 (27.8-43.5) | 12/147 | 8.2 (3.74-12.6) |
| Year 5 | 1/119 | 0.8 (0-2.48) | 47/118 | 39.8 (31.0-48.7) | 16/129 | 12.4 (6.71-18.1) |
| Year 6 | 14/105 | 13.3 (6.83-19.8) | 43/100 | 43.0 (33.3-52.7) | 16/109 | 14.7 (8.04-21.3) |
| Year 7 | 7/73 | 9.6 (2.83-16.3) | 30/74 | 40.5 (29.4-51.7) | 16/75 | 21.3 (12.1-30.6) |
| Year 8 | 8/42 | 19.0 (7.17-30.9) | 19/48 | 39.6 (25.8-53.4) | 9/38 | 23.7 (10.2-37.2) |
| Year 9 | 4/33 | 12.1 (0.99-23.3) | 14/32 | 45.5 (26.6-60.9) | 2/8 | 25.0 (5.01-55.0) |
| Year 10 | 3/15 | 20.0 (0-40.2) | 9/16 | 56.3 (31.9-80.6) | - | - |
n/N, number meeting criteria/total number assessed at this follow-up.
The total duration of follow-up to establish depression using DSM criteria was 1245 person years (mean 3.64 years). During this time 52 episodes of major depression according to DSM-IV criteria were detected, giving an incidence of 4.18 per 100 person years. The total duration of follow-up to establish depressive symptoms using the GDS scale was 1110 person years (mean 3.20 years). During this time 410 episodes of depressive symptoms ⩾5 were detected, giving an incidence of 36.9 per 100 person years. The total duration of follow-up to establish observer-rated depression using the Cornell scale was 1203 person years (mean 3.44 years). During this time 71 episodes according to the Cornell scale ⩾10 were detected, giving an incidence of 5.90 per 100 person years.
Univariate predictors of depression
Significant univariate predictors of depressive symptoms are shown in Table 3. At first follow-up (15 months post-stroke) significant predictors included baseline GDS score (at 3 months post-stroke), lower total CAMCOG score, previous stroke, the presence of dementia at first follow-up, Bristol activities of daily living score, the presence of cognitive impairment no dementia (CIND) and number of cardiovascular risk factors. At fourth follow-up (4 years 3 months after stroke) significant predictors included baseline GDS score, lower total CAMCOG score, Bristol activities of daily living score and the presence of CIND. At eighth follow-up (8 years 3 months after stroke) only the baseline GDS score was a significant predictor of depressive symptoms.
Multivariate predictors of depression
Table 4 shows the model summaries. Baseline GDS quartile was retained as a predictor in all three models. At year 1, number of cardiovascular risk factors was also retained. At year 4, Bristol activities of daily living score and previous stroke were retained as predictors. No additional predictors were retained at year 8, but numbers in the model were smaller (n = 32).
Neuropathological findings
The relative burdens of vascular and neurodegenerative disease were as expected in this group of older stroke survivors (Table 5). A large number of smaller vascular lesions distributed in the cortical and subcortical grey and white matter was observed. There was no clear evidence that the number or distribution of the infarctions were different in those with at least one depressive episode. We found only a few cases with a high burden of neurodegenerative pathology diagnostic for Alzheimer's disease or other neurodegenerative disorder. Neither Braak staging nor neuritic amyloid load was different in individuals who had experienced at least one depressive episode compared with those who had not (Table 5).

Fig. 2 Proportion of participants (a) meeting criteria for major depression by DSM-IV criteria, (b) Geriatric Depression Scale (GDS) ⩾5 and (c) Cornell score ⩾10, with error bars to show 95% confidence intervals for each proportion.
Y, year.
Table 3 Univariate predictors of depression rated using the Geriatric Depression Scale at 1, 4 and 8 yearsFootnote a

| Year 1 | Year 4 | Year 8 | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Pre-stroke factors | ||||||
| Age | –0.012 | 0.847 | 0.013 | 0.873 | 0.115 | 0.532 |
| Male gender | –0.109 | 0.078 | –0.264 | 0.001 | –0.053 | 0.772 |
| Antidepressant treatment at time of stroke | 0.088 | 0.163 | 0.127 | 0.135 | –0.103 | 0.576 |
| Previous stroke | 0.250 | <0.001 | 0.110 | 0.195 | –0.167 | 0.360 |
| Ischaemic heart disease | 0.007 | 0.910 | 0.093 | 0.276 | 0.193 | 0.289 |
| Hypertension | 0.094 | 0.133 | 0.224 | 0.008 | 0.082 | 0.656 |
| Atrial fibrillation | 0.034 | 0.586 | –0.008 | 0.923 | –0.174 | 0.342 |
| Diabetes mellitus | 0.043 | 0.499 | 0.098 | 0.250 | –0.103 | 0.576 |
| Hypercholesterolaemia | 0.050 | 0.429 | 0.015 | 0.860 | –0.237 | 0.191 |
| Peripheral vascular disease | 0.013 | 0.833 | 0.024 | 0.776 | –0.103 | 0.576 |
| Ever smoked | 0.028 | 0.666 | 0.003 | 0.972 | 0.192 | 0.294 |
| Number of cardiovascular risk factors | 0.169 | 0.007 | 0.175 | 0.040 | –0.027 | 0.884 |
| APOE ε4 allele (one copy) | 0.052 | 0.434 | 0.095 | 0.282 | –0.122 | 0.505 |
| Stroke outcome factors at 3 months | ||||||
| CAMCOG total score | –0.268 | <0.001 | –0.260 | 0.002 | 0.308 | 0.087 |
| Total CAMCOG score <80 (CIND) | 0.178 | 0.004 | 0.243 | 0.003 | 0.301 | 0.095 |
| CAMCOG total memory score ⩽17(MCI) | 0.107 | 0.083 | 0.182 | 0.029 | 0.027 | 0.882 |
| CAMCOG total executive function score ⩽13 | 0.105 | 0.090 | 0.190 | 0.023 | 0.293 | 0.103 |
| Geriatric Depression Score: median | 0.529 | <0.001 | 0.474 | <0.001 | 0.647 | <0.001 |
| Bristol Activities of daily living score | 0.229 | <0.001 | 0.244 | 0.003 | 0.266 | 0.141 |
| Follow-up factor | ||||||
| Dementia at time of this assessment | 0.244 | <0.001 | 0.181 | 0.031 | 0.211 | 0.688 |
APOE, apolipoprotein E; CAMCOG, Cambridge Cognitive Examination; CIND, cognitive impairment no dementia; MCI, mild cognitive impairment.
a. Results in bold are significant.
Discussion
Main findings and findings from other studies
The longer-term psychiatric sequelae of stroke have received little attention, and even for depression, which is well recognised to be common and important post-stroke. In this paper we have described the prevalence and incidence of major depression and depressive symptomatology, and identified predictors of depressive symptoms during up to 10 years of follow-up in a hospital-based cohort of survivors of first or recurrent stroke, using self-rated and observer-rated instruments as well as the researcher-rated DSM-IV criteria. We found that depression prevalence remains high and possibly increases up to 10 years. In those patients without dementia at baseline assessment 3 months post-stroke, the highest prevalence (31.7%) and incidence (36.9 per 100 person years) of depressive symptoms was found using a self-rated scale (the GDS). These estimates are in accordance with the pooled estimates of post-stroke depression reported by Hackett et al, who found a prevalence of 34% (95% CI 20-39) at 1-6 months post-stroke and 34% (95% CI 29-36) during studies following patients for more than 6 months. Reference Hackett, Yapa, Parag and Anderson2 Our study is unique in the duration of its follow-up, and confirms that the frequency of depressive symptoms following a stroke remains very high even up to 10 years post-stroke. When rated by GDS, the frequency appeared to increase only slightly over time, but observer and researcher ratings did show increasing frequencies over time. As in previous studies the frequency of depression was much lower when established using DSM-IV criteria for major depression rather than the GDS, but did appear to rise over time, ranging from 0.4 to 4.9% in the first 5 years of the study to 9.6 to 20% in the latter 5 years. This is consistent with results from the OCSP, which found a frequency of depression by DSM-III criteria to be 5% in the first 12 months, rising to 18% in years 3-5. The OCSP also found much higher frequencies of depression using a self-rated scale (Beck Depression Inventory). Astrom et al Reference Astrom, Adolfsson and Asplund34 found an initial decrease in prevalence of depression at 1 year, compared with 3 months, but the prevalence rose again over a follow-up period of 3 years. In keeping with our results, other studies with longer-term follow-up found similar prevalence of depression; 25.8% at 5 years, Reference Chausson, Olindo, Cabre, Saint-Vil and Smadja35 36% at 4.9 years. Reference Wilkinson, Wolfe, Warburton, Rudd, Howard and Ross-Russell36
Table 4 Multivariate models of predictors of depressive symptoms

| Year 1 | Year 4 | Year 8 | |
|---|---|---|---|
| Model summary | |||
| n | 249 | 138 | 32 |
| R2 | 0.305 | 0.319 | 0.416 |
| Adjusted R2 | 0.293 | 0.293 | 0.354 |
| Significant F change | 0.001 | 0.043 | 0.000 |
| Coefficients, B (95% CI for B) | |||
| Age | –0.011 (–0.042 to 0.020) | –0.020 (–0.069 to 0.029) | 0.052 (–0.061 to 0.166) |
| Gender | –0.097 (–0.353 to 0.158) | –0.313 (–0.644 to 0.019) | 0.258 (–0.487 to 1.003) |
| Baseline Geriatric Depression Scale quartile | 0.506 (0.399 to 0.613) | 0.418 (0.276 to 0.561) | 0.735 (0.389 to 1.081) |
| Number of cardiovascular risk factors | 0.176 (0.072 to 0.280) | ||
| Baseline Bristol activities of daily living score | 0.052 (0.017 to 0.087) | ||
| Previous stroke | 0.210 (0.006 to 0.414) |
Table 5 Neuropathological findings according to whether any episode of depression (Geriatric Depression Scale (GDS) ⩾5) was recorded during follow-up

| Neuropathological variable | Participants without any episodes of GDS ⩾5 | Participants with at least 1 episode of GDS ⩾5 | P |
|---|---|---|---|
| n | 16 | 35 | |
| Brain weight, g: mean (s.d.) | 1279 (143) | 1243 (134) | 0.360 |
| CERAD score, median (IQR) | 1 (1-2) | 1 (0-2) | 0.099 |
| Braak score, median (IQR) | 3 (2-4) | 3 (2-3) | 0.193 |
| CAA severity score, Reference Kalaria, Kenny, Ballard, Perry, Ince and Polvikoski26 median (IQR) | 1 (0-1) | 1 (0-1.5) | 0.881 |
| Number of infarcts, median (IQR) | |||
| All areas | 5 (2-6.5) | 3.5 (2-6) | 0.423 |
| Microscopic infarcts in all areas | 3.5 (2-6) | 2.0 (1-5) | 0.329 |
| Cortex | 1.5 (1-3.5) | 1 (0-3) | 0.533 |
| White matter | 0 (0-1) | 0 (0-1) | 0.708 |
| Basal ganglia and thalamus | 1 (0-3) | 1 (0-2) | 0.318 |
| Brainstem and cerebellum | 0 (0-0.5) | 0 (0-1) | 0.154 |
CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CAA, cerebral amyloid angiopathy; IQR, interquartile range.
Previous studies have not used depression scales with additional information provided by the carer, although the OCSP did include an informant interview in determining depression by DSM criteria. We found that the Cornell scale gave intermediate frequencies of depression between self-rated scales and DSM criteria. This scale is particularly useful in identifying depression in patients with cognitive impairment who may be less able to communicate their symptoms, and at most time points we were able to obtain data on depressive symptoms in more patients than if we had used the self-rated scale alone.
The most consistent predictor of depression at all time points in both univariate and multivariate analyses was the baseline GDS score and this has also been a consistent finding in previous studies. This emphasises the importance of follow-up for those with significant depressive symptoms soon after stroke. Consistent with our previous finding that depressive symptoms were associated with incident dementia, cognitive impairment was also a significant univariate predictor of depression up to 4 years post-stroke, as was incident dementia at year 1. This may be as a result of depressive symptoms being a common feature of early dementia, but also because of shared pathology and the performance effects of depression on cognitive tasks. Verdelho et al Reference Verdelho, Henon, Lebert, Pasquier and Leys37 and Appelros & Viitanen Reference Appelros and Viitanen38 have both also shown that dementia is more frequent in those with depression after a stroke. In the UK, the National Institute for Health and Care Excellence recommended that in the management of acute stroke there should be screening for depression and cognitive impairment 6 weeks post-stroke. 39 Guidelines on rehabilitation after stroke have also recommended that patients are screened again at 6 months and annually thereafter, 39 but it is not clear how this monitoring should be carried out. We would agree that patients with early depressive symptoms, cognitive impairment or both are monitored for incident and/or recurrent depression in the long term. Given the numbers of patients to be followed up and the long-term nature of the monitoring, it seems essential that robust methods of monitoring are developed within primary care.
Prevalent depressive symptoms appeared to be strongly associated with vascular burden in the early phase after stroke, but this effect appears to weaken over prolonged follow-up. As one might expect a dose-response effect, with an increasing dose of vascular burden over time, this is perhaps surprising. However, there are alternative explanations; we know from our previous work that those with higher baseline vascular burden may have a higher mortality suggesting that the effect may have disappeared as a result of attrition of those at highest risk. Also, we did not reassess vascular risk factor burden at each follow-up and over time there may have been convergence of risk burden in surviving participants. Finally, it is possible that the vascular hypothesis is not sufficient to account for delayed episodes of post-stroke depression, and other factors such as previous experience of depression and long-term experiences of physical disability lead to negative cognitions that perpetuate depression and outweigh the effect of vascular burden. Previous long-term studies have also consistently shown the importance of dependency in activities of daily living in predicting post-stroke depression. Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe3,Reference Sharpe, Hawton, Seagroatt, Bamford, House and Molyneux4,Reference Astrom, Adolfsson and Asplund34
In keeping with these observations regarding the effect of the vascular hypothesis over time, we were not able to show an increased burden of infarction or vascular disease at post-mortem in those participants who experienced at least one episode of depression. This may be because of the distance of post-mortem examinations from many of the depressive episodes.
Limitations
We deem there are a few limitations to our study. We did not have complete data on whether participants had depression prior to the stroke. However, we did include the use of antidepressants at the time of the stroke as a pre-stroke factor. As individual episodes of depression post-stroke were not followed clinically, it is possible that some episodes may have lasted more than the 12 months between follow-up assessments and will have been counted as individual episodes, thus overestimating the incidence. However, this is counterbalanced by the possibility that some episodes of depression will have emerged and resolved between annual assessments.
As a result of the availability of resources our determination of depression using DSM-IV criteria was carried out by research nurses and psychology assistants who may not have been as experienced in detecting a depressive episode as a medically qualified clinician, and we suspect that our prevalence and incidence figures may therefore be underestimated with DSM criteria. As a result of the low-event rate there were insufficient events to enable us to examine predictors of DSM-IV major depression. This is unfortunate as we have not therefore been able to address the important issue of whether clinically determined major depression has the same relationship with these predictors. If, as is usually assumed, major depression is the more severe subgroup, we would expect the predictors would not have been significantly different from those predicting self-rated depressive symptoms. If, however, major depression represents a different type of depression from GDS-rated depressive symptoms, then it may have different predictors.
Our cohort consists of patients who were aged at least 75 at enrolment and so the significant predictors we found may not be generalisable to a younger population. In particular, the psychosocial factors leading to depression would differ in a younger cohort, where factors such as loss of working-life roles might contribute to the onset of depression. Reference Ayerbe, Ayis, Rudd, Heuschmann and Wolfe3
Implications
In conclusion, this study has shown that the prevalence and incidence of depression and depressive symptoms in older stroke survivors are high. Significant predictors of depressive symptoms include depression at 3 months, cognitive impairment, impaired activities of daily living and in the early period, vascular risk factor burden. Post-mortem examination did not indicate an increased vascular burden in those with at least one episode of significant depressive symptoms. We recommend that long-term monitoring of mood is undertaken in those at high risk of depression, and although control of risk factor burden may be important, particularly for the maintenance of cognition, long-term psychosocial and functional support are likely to be key components of the management of these patients.
Funding
Our work is supported by grants from the UK Medical Research Council (MRC, G0500247), Newcastle Centre for Brain Ageing and Vitality (Biotechnology and Biological Sciences Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council and MRC, Lifelong Health and Wellbeing) and Alzheimer's Research UK (ARUK). Tissue for this study was collected by the Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK MRC (G0400074), by the Newcastle NIHR Biomedical Research Centre in Ageing and Age Related Diseases award to the Newcastle upon Tyne Hospitals NHS Foundation Trust, and by a grant from the Alzheimer's Society and ARUK as part of the Brains for Dementia Research Project.
Acknowledgements
We are very grateful to the patients, families and clinical house staff for their cooperation in the investigation of this study. We thank Michelle Widdrington, Carein Todd, Jean Scott, Deborah Lett and Anne Nicholson for assistance in managing and screening the cohort. We are indebted to Professors Clive Ballard and Rose Anne Kenny for guiding this longitudinal study in its early phases.










eLetters
No eLetters have been published for this article.