INTRODUCTION
Campylobacter infection is the leading cause of gastrointestinal illness in Australia among all the notified enteric pathogens [Reference Miller1]. The incidence of notified campylobacteriosis in Australia has steadily increased during the past decade, from 68·5/100 000 population (8074 cases) in 1993 to 116·8/100 000 in 2004, with a peak in 2001 of 124·0/100 000 (16 123 cases). However, in total there are an estimated 277 000 campylobacter infections each year in Australia and the majority of these infections are thought to be sporadic in nature [Reference Hall2].
Case-control studies used to identify risk factors for sporadic infection have been conducted in a number of developed countries worldwide including the United States [Reference Potter, Kaneene and Hall3–Reference Effler5], Canada [Reference Michaud, Menard and Arbeit6], the United Kingdom [Reference Neal and Slack7–Reference Rodrigues9], Norway [Reference Kapperud10], Sweden [Reference Studahl and Andersson11], Switzerland [Reference Schorr12], Denmark [Reference Neimann13], Finland [Reference Schonberg-Norio14] and New Zealand [Reference Eberhart-Phillips15]. These studies have demonstrated that poor handling and/or consumption of raw or undercooked chicken is the single most important risk factor for infection. Other frequently identified risk factors include the consumption of raw milk, drinking untreated water, contact with farm animals, contact with pets, especially puppies, and travel abroad. The identification of certain risk factors in some studies and not others, suggest that the ecology and primary sources of campylobacter may vary somewhat across different countries. Furthermore, the limited study size of some case-control studies may restrict their ability to detect some risk factors. Outbreaks of campylobacteriosis, although rarely reported, are predominantly associated with consumption of raw milk, poultry or untreated water [Reference Frost, Gillespie and O'Brien16–Reference Wood, MacDonald and Osterholm19].
There is still much to be learnt about the epidemiology of campylobacter infection despite the large number of case-control studies that have been conducted in recent years. In many of these studies, a large proportion of cases had unexplained risk factors. Epidemiological studies of campylobacter infection in Australia are limited and there has been only one published case-control study of risk factors for sporadic campylobacter infection and this was among children aged <3 years [Reference Tenkate and Stafford20]. This paper presents the findings from the first national study of risk factors associated with sporadic campylobacter infection in Australia.
METHODS
Study design and population
A multi-centre prospective case-control study was conducted in five of the eight states and territories in Australia with data collected over a 12-month period, beginning September 2001. These jurisdictions represented all states that have legislation that requires doctors and laboratories to notify patients infected with campylobacter. The total population of Australia at the time of this study was ∼19 million and the five states involved in this study covered ∼12 million people. Based on historical notification data, ∼12 000 campylobacter cases were expected to be notified from these five states during the study period. Each state aimed to recruit ∼200 cases for the study. Because of disparities in the number and source of notified cases between states, three states selected one private pathology laboratory that provided statewide services as their source of notified cases and two states recruited cases notified from all pathology laboratories within their jurisdiction. Systematic sampling was undertaken as cases were notified.
Cases
New cases were sought on a daily basis and were defined as a person notified from any of the five participating states with a culture-positive stool result for campylobacter infection and a recent history of acute diarrhoea, who was not part of an outbreak investigation unless identified as the index case. Cases were excluded from participating in this case-control study if (1) they did not have a telephone number available for their primary residence, (2) they were not reachable after at least six telephone attempts, (3) they were not English-speaking or they were unable to answer questions for some other reason, (4) they were unable to recall the date of onset of their diarrhea, (5) their onset of diarrhoea was ⩾10 days before the collection date of the positive specimen, (6) another member of the household had a history of diarrhoea or had been diagnosed with campylobacter infection in the previous 4 weeks, or (7) another enteric pathogen other than campylobacter was isolated or detected in their stool. Children aged <5 years were recruited for a similar study, which is not presented here. It was at the parent's or guardian's discretion as to whether a child aged between 15 and 17 years was interviewed directly. Information from a subject aged <15 years was obtained from the parent or guardian who was most familiar with their diet and behaviour. Cases were interviewed as soon as possible, preferably within 14 days of onset of illness but no later than 30 days after onset of illness.
Controls
Controls were sourced from a control bank made up of household members recruited from a National Community Gastroenteritis Survey, which was a retrospective, cross-sectional survey of the Australian community conducted over a 1-year period in 2001 [Reference Hall21]. Households were selected using random digit dialling. A total of 14 021 controls were recruited into the national control bank from the 5123 households who consented to participate.
Controls were excluded from participating in this case-control study if (1) the telephone had been disconnected subsequent to recruitment onto the database, (2) they were not reachable after at least six telephone attempts, (3) they were not English-speaking or they were unable to answer questions for some other reason, (4) they had suffered diarrhoea in the 4 weeks prior to interview, or (5) another member of the household had a history of diarrhoea or had been diagnosed with campylobacter infection, with an onset of illness within 4 weeks prior to interview.
Controls were frequency matched to cases by age bands in each state. The age bands were 5–9 years, 10–19 years, 20–29 years, 30–59 years and ⩾60 years. The ratio of controls to cases was one to one. Persons who agreed to participate and who met the specified criteria above were eligible for enrolment in the study as controls. If a person did not wish to participate or failed to meet these criteria, a subsequent person was sought from the control bank. Once a control had been selected from a household, that household was no longer eligible for future selection of controls. Controls were interviewed within 30 days of interview of a notified case.
Questionnaire
A telephone-administered structured questionnaire was used to collect information regarding exposures, in the 7 days prior to onset of illness in cases and in the 7 days prior to interview for controls. The questionnaire comprised several sections, each representing a different exposure group including: host factors; overseas travel; water consumption; dining locations outside of the home; produce consumption; meat and poultry consumption; seafood consumption; consumption of eggs and dairy products; animal and pet exposures; and demographic information. Cases were asked additional questions about the clinical course of their illness and treatment. Questions on travel, water consumption, dining locations, food consumption and animal and pet exposures were asked based on a 7-day history. Host factor information on prior antibiotic and antacid consumption and any immunosuppressive treatment were based on a 4-week history.
Sample size
Sample size estimates were generated with Epi-Info software (Epi-Info version 6.04d; CDC, Atlanta, GA) using three exposure levels among controls of 20%, 15% and 10%. To detect an odds ratio (OR) of 1·5 at the 5% significance level with 80% power then 1124 (562 cases and 562 controls), 1380 and 1914 subjects are required for each exposure level respectively.
Data analysis
Univariate analysis was conducted on all variables to generate crude odds ratios with 95% confidence intervals. Logistic regression modelling was conducted separately on each exposure group to investigate associations between potential risk factors and campylobacteriosis after controlling for location (state), sex and education. Significance was assessed using Wald's statistic. Potential collinearity between independent variables was identified with associations yielding χ2 >100, examining parameter stability and studying model convergence. In the absence of any other information among a group of collinear variables, the factor with the highest estimate of effect with illness was chosen for inclusion in the model. A multivariable logistic regression main-effects model was then developed, using sequential backward elimination of non-significant variables (based on the model deviance statistic) [Reference Dupont22]. Once the most parsimonious main-effects model was identified, two-factor interactions were introduced into the model and stepwise elimination of non-significant terms were undertaken (again based on the model deviance statistic) until the final model was ascertained. The two-factor interactions considered were based on biological plausibility or prior knowledge from the literature. SPSS software version 11.0 (SPSS Inc., Chicago, IL, USA) was used for all computations and a significance level of α=0·05 was used to define statistical significance.
Population attributable risk (PAR) proportions were calculated using adjusted odds ratios (aOR) from the final logistic regression model for each variable that was significantly associated with an increased risk of infection, excluding host factors [Reference Rockhill, Newman and Weinberg23]. For trichotomous variables, category-specific attributable fractions were calculated to estimate the proportion of total disease risk that would be prevented in the study population following elimination of that specific exposure category, assuming the exposure is causally related to infection. stata statistical software, release 7 (Stata Corp, College Station, TX, USA) was used for calculating 95% confidence intervals (CI) around the PAR estimates.
RESULTS
Descriptive analysis
Study population
During the study period, a total of 7225 campylobacter cases aged ⩾5 years were notified from the five states and of these, 1550 cases were reported from laboratories that participated in the case-control study. Of the 1550 cases, 362 cases were ineligible. Of the remaining 1188 eligible cases, there were 881 (74·2%) cases recruited and interviewed for the study. Among the eligible cases who did not enter the study, there were 85 (7·2%) cases in which the treating doctor could not be contacted or refused consent to contact the patient, 24 (2·0%) cases who refused to participate, 59 (5·0%) cases who did not have a telephone number available for their primary residence and 139 (11·7%) cases who were not reachable after at least six telephone attempts. The median interval between onset of symptoms among cases and their interview date was 20 days (25th–75th percentile range: 15–24 days). The distribution of study cases across the five age groups and for both sexes were similar to the distribution of all population cases that were notified in Australia during the study period (Table 1).
Table 1. Frequency and percentage of study cases (n=881) vs. notified cases in Australia (n=12 565) by age group and sex
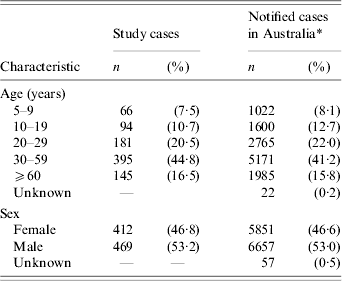
* Number of campylobacter cases notified during study period, September 2001 to August 2002.
A total of 1253 individuals were randomly selected from the bank of controls, 1127 were found to be eligible for inclusion of which 833 (73·9%) were recruited and interviewed for the study. Among the eligible controls who did not enter the study, 81 (7·2%) refused to participate, 52 (4·6%) no longer had a telephone number available for their primary residence and 161 (14·3%) were not reachable after at least six telephone attempts.
Male subjects comprised 53·2% of cases and 45·9% of controls (aOR 1·3, 95% CI 1·1–1·6). There was no significant difference between cases and controls in terms of: indigenous ethnicity; language other than English spoken in their household; ‘Health Care Card’ or ‘Pensioner Concession Card’ status; total household income; and residential rurality. However, case households were more likely to have persons with an apprenticeship or a year 11 or 12 secondary level of education compared to controls (aOR 1·5, 95% CI 1·1–2·1).
Univariate analysis of risk factors
Overseas travel
Cases were significantly more likely to have travelled outside of Australia in the 7-day exposure period compared to controls (OR 7·4, 95% CI 2·2–38·7), although only 23 cases and three controls had travelled overseas during their exposure period. These subjects were excluded from any risk factor analysis for local exposures.
Meat and poultry
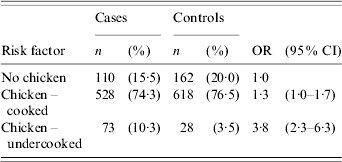
There was no significant association between consumption of beef, lamb, veal, pork, game meat or any delicatessen meats during the 7-day exposure period and illness (Table 2). Cases were significantly more likely to have eaten chicken (OR 1·6, 95% CI 1·2–2·1) and offal (OR 2·2, 95% CI 1·2–4·4) during the 7-day exposure period than controls. Among the common chicken types, consumption of chicken fillet/breast (OR 1·2), chicken kebabs (OR 1·7) and purchased barbecued chicken (OR 1·2) were all significantly associated with an increased risk of illness. Other less frequently reported chicken types including chicken nuggets, chicken schnitzel, chicken sandwiches, chicken casseroles, chicken pies, chicken rolls and chicken wraps were grouped together as a single variable and this variable was significantly associated with illness (OR 1·8, 95% CI 1·3–2·5). When chicken meat was categorized as either cooked or undercooked, there was no significant association between consumption of cooked chicken and illness. However, cases were significantly more likely to have eaten undercooked chicken than controls (OR 3·8, 95% CI 2·3–6·3) (Table 3). There was no significant association between eating barbecued chicken meat and illness, irrespective of whether the chicken meat was undercooked or cooked.
Table 2. Frequency and sample size (n/N), percentages (%) and crude odds ratios (OR) together with the 95% confidence intervals (95% CI) for the association between type of meat and poultry consumed and campylobacter illness

Table 3. Frequency (n), percentages (%) and crude odds ratios (OR) together with the 95% confidence intervals (95% CI) for the association between cooked or undercooked chicken and campylobacter illness
Produce
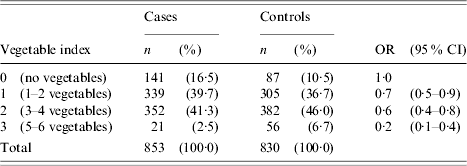
In general, cases were significantly less likely to have eaten uncooked fruit or vegetables than controls during the 7-day exposure period (Table 4). Cases were also significantly less likely to have eaten organically grown fruit and vegetables, home-grown fruit and vegetables or home-grown herbs. To further explore the relationship between consumption of raw produce and the risk of campylobacter infection, a variable (vegetable index) was created to indirectly measure the range of raw produce consumed in the 7-day exposure period. The values of this index variable represented a count of the number of different types of salad/vegetable food items eaten during the exposure period. The following six salad/vegetable food items were selected for inclusion in the vegetable index variable: lettuce/salad greens, spring onions, tomatoes, cucumber, broccoli/cauliflower and alfalfa/bean sprouts. Crude odds ratios were generated for each index value using an index value of zero as the reference category (Table 5). There was a decreasing linear trend in the risk of campylobacter infection as the vegetable index value increased (χ2 for linear trend=26·3, P<0·001). This decreasing magnitude of association between vegetable index and campylobacter illness was maintained when stratified across chicken consumption measures.
Table 4. Frequency and sample size (n/N), percentages (%) and crude odds ratios (OR) together with the 95% confidence intervals (95% CI) for the association between type of produce consumed and campylobacter illness

Table 5. Frequency (n), percentages (%) and crude odds ratios (OR) together with the 95% confidence intervals (95% CI) for the association between vegetable index and campylobacter illness
No single produce item was selected for inclusion in the multivariable analysis. Rather, variables which represented a group of produce and demonstrated a significant low odds ratio in the univariate analysis were chosen. These included the vegetable index, organic fruit and vegetables and home-grown fruit. Home-grown vegetables and home-grown herbs were not included as both variables were identified to be collinearly related to home-grown fruit.
Other food exposures
No other foods were significantly associated with an increased risk of illness. There was no significant association between illness and eating food outside of the home or eating food from specific types of food venues including fast-food chicken outlets.
Non-food exposures
There was no significant association between drinking untreated water and illness (OR 1·3, 95% CI 0·9–1·8), however, cases were significantly more likely than controls to have commercial bottled water as their primary source of drinking water (OR 1·5, 95% CI 1·0–2·3). Cases were also more likely to have dogs aged <6 months (OR 3·2, 95% CI 1·8–5·7) and chickens aged <6 months (OR 3·6, 95% CI 1·3–9·7) as domestic pets. There was no significant association between campylobacter illness and contact with any farm animals or native animals nor was there any significant association with living on a farm or property of five acres or more or visiting a farm or petting zoo during the 7-day exposure period.
Multivariable analysis of risk factors
Among the food exposures, consumption of undercooked chicken (aOR 4·7, 95% CI 2·6–8·4) and offal (aOR 2·0, 95% CI 1·0–4·0) were found to be significantly associated with illness after adjusting for all other variables in the model. Consumption of cooked chicken was also associated with illness but was of borderline statistical significance (aOR 1·4, 95% CI 1·0–1·9, P=0·06) (Table 6). Eating fresh fish, homemade foods containing raw eggs, organically grown fruit and/or vegetables and home-grown fruit were independent factors associated with a reduced risk of infection. Eating raw salads or vegetables, as measured by the vegetable index variable was also associated with a reduced risk of infection and this risk was reduced further as the number of different types of raw salad/vegetable food items consumed during the exposure period increased.
Table 6. Final multivariable logistic regression analysis with adjusted odds ratios (aOR) together with the associated 95% confidence intervals (95% CI), the derived population attributable risk percentages (PAR) % and (95% CI) showing exposures associated with an increased or decreased risk of campylobacter infection

* 95% CI unable to be calculated due to small numbers.
Among the animal exposures, ownership of domestic chickens aged <6 months old (aOR 12·4, 95% CI 2·6–59·3) and domestic dogs aged <6 months (aOR 2·1, 95% CI 1·1–4·2) were found to be independent risk factors for illness. The final independent risk factor associated with illness from the multivariable analysis was persons who reported a medical history of a chronic gastrointestinal condition (aOR 2·3, 95% CI 1·5–3·4). The association between having commercial bottled water as a primary source of drinking water and illness did not remain significant in the final multivariable model. No statistically significant first-order interactions were detected among the independent variables or with any potential effect modifiers in the final model. There was no evidence that the final model failed to fit the data (Hosmer–Lemeshow goodness-of-fit test, P=0·20).
Population attributable risk proportions
The proportion of campylobacter illness in the study population that could be prevented by eliminating the consumption of undercooked chicken was estimated to be 8·1% (95% CI 5·2–11·1) (Table 6). For cooked chicken, a further 21·2% (95% CI 0·0–36·9) of campylobacter infections in the population could be prevented through better handling of raw chicken and more thorough cooking during the preparation of cooked chicken dishes. The overall PAR associated with chicken was 29·3%. The PAR for other independent risk factors was small ranging from 1·9% to 3·0%.
DISCUSSION
Risk factors
This study identified that sporadic campylobacter infections among persons aged ⩾5 years in Australia were associated with eating undercooked chicken, eating offal and having contact with dogs or chickens aged <6 six months. There was also an increased risk associated with the consumption of cooked chicken. The risk associated with consumption of cooked chicken might be explained in that some subjects may have reported the consumption of chicken as cooked, unaware that the chicken they had consumed was in fact, undercooked. In addition, the risk associated with poor handling of raw chicken during the preparation of chicken dishes may contribute to the observed effect for cooked chicken. The association between chicken consumption and campylobacter infection has been extensively reported in the literature and this risk factor appears to be the major source of infections in Australia as well. Our study has shown that almost one-third of campylobacter infections that occur in Australia each year can be attributed to eating chicken meat.
The biological plausibility of this association is supported by prevalence studies of raw poultry meat, in particular raw chilled chicken, which often show campylobacter frequencies in excess of 50% [Reference Kramer24–Reference Zhao26]. Furthermore, contamination levels in excess of 105 organisms per carcass at retail level have been reported [Reference Hood, Pearson and Shahamat27, Reference Jorgensen28]. A relatively recent finding has been the detection of campylobacter on the outer packaging of retail raw chickens. In the United Kingdom, Jorgensen et al. found Campylobacter spp. on the outer packaging of 9/140 (6%) whole raw chickens while in New Zealand Wong et al. reported the detection of Campylobacter jejuni on the outer surfaces of 72/300 (24%) retail raw chicken packs [Reference Jorgensen28, Reference Wong29]. These studies suggest the external packaging of raw chilled meats represent a further potential vehicle of transmission for campylobacter in retail stores and consumers' homes.
Two previous case-control studies have reported an association between campylobacter illness and consumption of chicken liver [Reference Schorr12, Reference Eberhart-Phillips15]. Our study identified an association with consumption of offal, however, there were insufficient cases to determine the specific types of offal associated with illness. Twenty of the 36 cases who reported eating offal consumed liver with the predominant type being lambs' liver (10 cases). Four cases consumed chicken liver and six cases did not specify the type of liver eaten. The other major type of offal consumed by cases was kidneys (eight cases). These findings suggest that poor handling or consumption of raw or inadequately cooked offal other than chicken liver may constitute a further vehicle of infection for campylobacter illness. This is not unexpected since campylobacter frequently colonize the intestinal tract of a variety of farm animals used in food production and contamination of carcasses during the slaughtering process is not uncommon. In addition, several microbiological studies of campylobacter prevalence among retail raw meats have demonstrated high frequencies (54–83%) of contamination among different types of offal, including chicken, lamb, pig and ox liver [Reference Kramer24, Reference Cornelius, Nicol and Hudson30].
The failure to find an association between consumption of any of the red meats and campylobacter illness was not unexpected as several microbiological studies have shown the frequency of campylobacter contamination of retail red carcass meats to be low [Reference Zhao26, Reference Nielsen31, Reference Vanderlinde, Shay and Murray32]. It has been suggested that this may be a reflection of the more hygienic slaughter procedure for ruminants than poultry [Reference Stanley and Jones33]. Our study found a weak crude association between consumption of commercial bottled water and illness, nevertheless we believe this association was probably due to confounding with another factor as the association disappeared in the multivariable analysis. However, it should be noted that bottled water has been reported as a potential risk factor for campylobacter infection in at least three recent studies and probably warrants further investigation, particularly as none of the three studies included a ‘healthy community-based’ comparison group [34–Reference Evans, Ribeiro and Salmon36]. Although consumption of untreated water was not identified as a major risk factor for sporadic infection in this study, untreated water has been implicated as the cause of at least one outbreak of campylobacter infection in Australia in recent years [Reference Merritt, Miles and Bates37].
Contact with puppies and chickens have been previously reported as risk factors for campylobacter infection, including Australia, and transmission of C. jejuni from a puppy to a child has recently been verified [Reference Friedman4, Reference Studahl and Andersson11, Reference Eberhart-Phillips15, Reference Tenkate and Stafford20, Reference Wolfs38]. Although biologically plausible, the increased risk of infection associated with having a chronic gastrointestinal condition could possibly be a selection bias as controls were excluded from the study if they had experienced any diarrhoea in the previous 4 weeks.
Factors associated with a reduced risk of infection
A number of foods were independently associated with a reduced risk of infection, in particular raw fruit and vegetables. Several other campylobacter case-control studies have found similar negative associations with raw vegetables and/or fruits [Reference Rodrigues9, Reference Kapperud10, Reference Neimann13, Reference Eberhart-Phillips15]. Salad vegetables have also been reported as a risk factor for infection [Reference Evans, Ribeiro and Salmon36]. In our study, the different measures of exposure for intake of uncooked fruit and vegetables consistently showed a strong negative association with campylobacter infection. The validity of these findings was further strengthened by using a vegetable index to demonstrate a decreasing risk of infection as the number of different types of uncooked vegetables consumed increased. The interpretation of these findings is still unclear despite the consistency of findings among the different studies. Differences in dietary behaviour between cases and controls is frequently reported as a likely explanation for this effect [Reference Austin39]. In our study it was postulated that the observed effect could be due to controls being more likely to eat fruit and vegetables than cases as their frequency of chicken consumption was lower. However, subsequent analysis demonstrated that the negative association was maintained independent of chicken meat consumption.
Several biological mechanisms have been proposed that might explain a causal effect associated with fruit and vegetable consumption. For example, it has been suggested that fruit and vegetables which are known to contain high levels of antioxidants and carotenoids could boost general immunity to infection. In addition, some have been shown to inhibit bacterial growth [Reference Kapperud10, Reference Neimann13]. A large randomized, double-blind control study in Brazil found vitamin A supplementation reduces the severity of diarrhoea in young children in developing countries [Reference Barreto40]. Another postulated mechanism includes the effect of diet (e.g. certain fruit or vegetables) on the intestinal microflora which may alter host susceptibility to infection [Reference Rodrigues9, Reference Cameron41]. Recent studies have found consumption of fruit and vegetables to be associated with a lower risk of intestinal cancer [Reference Negri42, Reference Levi43]. There is accumulating evidence from these studies to suggest that some fruit and vegetables have the capacity to prevent or reduce the risk of infection with campylobacter. We are unable to provide any biologically plausible explanation for the ‘protective’ effect of eating fresh fish or homemade foods containing raw egg and we suggest that these observed effects are more likely the result of random error.
Limitations of study
The study size and case representativeness are important strengths when considering our findings. Furthermore, all four independent risk factors identified in this study had moderate to strong measures of association which strengthens their validity. However, study biases cannot be excluded as alternative explanations for these observed effects. Participation rates for cases and controls in this study were very similar at around 74%. There is no reason to suspect that the exposure distribution between participating and non-participating cases, or between participating and non-participating controls would differ greatly either. Attempts were made to minimize information bias during the design of the study, including limiting the recall period to 7 days prior to illness (cases) and interview (controls) and strict adherence to exclusion criteria. To minimize interviewer bias, structured questionnaires were developed and all interviewers were provided with thorough pre-trial training. However, interviews were not conducted blindly and interviewer bias cannot be excluded as having some affect on our results.
CONCLUSIONS
The PAR proportions from this study shows that chicken meat may account for ∼30% of campylobacter cases that occur each year in Australia whereas consumption of offal, and contact with puppies and chickens are of less importance as risk factors. These results justify the continued need for education of consumers and foodhandlers about the risks associated with the handling of raw chicken and the potential for cross-contamination. In addition, efforts to reduce the contamination of chicken carcasses with campylobacter either through improved on-farm control or interventions during processing would have a significant impact on reducing the overall burden of this infection in Australia. Given the size of this study, it is unlikely that further observational studies would further contribute to the mechanism of the ‘protective’ effect from fruit and vegetables. Specific experimental studies might better elucidate the role of fruit and vegetables in reducing the risk of infection.
APPENDIX
The OzFoodNet Working Group for this study consisted of: Russell Stafford, Martyn Kirk, Leanne Unicomb, Craig Dalton, Tony Merritt, Rosie Ashbolt, Cameron Sault, Joy Gregory, Robert Bell, Gillian Hall, Rod Givney, Jane Raupach, Barry Combs, Lillian Mwanri, Jennie Musto, Nola Tomaska, Geoff Millard, Mohinder Sarna, Geoff Hogg, Craig Williams, Janet Li, Karin Lalor, Nittita Prasopa-Plazier, Lyn Mueleners and Ian McKay.
ACKNOWLEDGEMENTS
We thank all subjects, parents and guardians who kindly participated in the study and the medical practitioners and pathology laboratories who notified the cases. We are also grateful to Dr Scott Cameron (member of the OzFoodNet Scientific Review Panel) who provided constructive comments on the manuscript. The Australian Government Department of Health and Ageing provided funding for this study under the OzFoodNet surveillance programme.
DECLARATION OF INTEREST
None.








