Brief historical and anthropological perspective on the use of hallucinogenic mushrooms
Indigenous use of psilocybin mushrooms
Psilocybin-containing mushrooms have been used in rituals by the Indigenous Peoples of Mesoamerica long before being consumed as recreational drugs or treatments in Western medicine, as evidenced by art from 3500 years ago.Reference Carod-Artal1 Sculptures of “mushroom stones” with faces carved on their stalks, and other art pieces dating back centuries depict fungi in religious and ceremonial contexts.Reference Carod-Artal1 The first written accounts suggesting psilocybin consumption were documented by 16th-century Spanish naturalists. Notably, the friar Bernardino de Sahagún studied the life and customs of the Aztecs and mentioned in his accounts teonanacatl, a Nahuatl term translated as “flesh of the gods.” Sahagún recalled the use of teonanacatl for medicinal and spiritual purposes in rituals that induced intoxication, visions, and “sensuousness”.Reference Sahagún3 Diego Durán, another Spanish friar, described the mass consumption of mind-altering mushrooms at the coronation of Aztec emperor Montezuma II.Reference Durán4 Francisco Hernández de Toledo, a naturalist and physician, also reported the ritualistic use of mushrooms that could induce “madness” and visions.Reference Piore5 Beyond these early accounts, there is little documentation of the mysterious teonanacatl, likely due to cultural suppression by the Catholic Church and colonial authorities.Reference Guzmán2, 6
Today, psilocybin is still used in ritual vigils (often called veladas) by multiple Indigenous Peoples including the Nahuas, Matlatzinca, Totanac, Mazatec, Zapotec, and Chatinos.Reference Guzmán2 The rituals may serve a wide range of spiritual and therapeutic purposes, and their objective is not necessarily to provide a cure. Sometimes they aim to unravel the cause of a problem.Reference Guzmán2, Reference Wasson7, Reference Sabina8 Understanding the original nature of these ceremonies is difficult since outside influences have inexorably reached these communities and altered their traditions. Even the earliest descriptions of veladas mentioned the presence of Christian symbolism in these ceremonies.Reference Guzmán2, Reference Wasson7 Since the early 1960s, rituals were further modified as they became tourist attractions for Westerners. Nonetheless, certain essential features of a velada can still be identified.Reference Guzmán2 The ceremony is generally held at night after a period of fasting and abstinence. It is led by a shaman or an older experienced person who usually invites the participants to their house. The mushrooms are incensed with copal resin, then counted and offered in pairs. Throughout the experience, chants, poetry, and dances are often performed by the leader of the velada. Reference Sabina8, Reference Estrada and XXI9
While psilocybin-containing mushrooms are found all around the world, it is only in current-day Mexico that their traditional use has been documented with certitude. Limited archeological data may support their ethnobotanical use in parts of Africa, Columbia, or New Guinea.Reference Guzmán2, Reference Akers, Ruiz, Piper and Ruck10–Reference Guerra-Doce13
Introduction to Westerners
Shortly before the Second World War, Austrian-born physician Blasius Paul Reko (who later adopted the name Blas Pablo) reported to Richard Evans Schultes and Robert J. Weitlaner that Mazatec Indians in Huautla de Jiménez were using mushrooms in religious ceremonies. Intrigued by this information and by the mysterious tales of early Spanish settlers, Schultes and Weitlaner traveled to the region of Oaxaca to collect fungi samples and to describe their traditional use.Reference Schultes14, Reference Schultes15 In 1938, a group composed of Weitlaner, Jean Bassett Johnson, Irmgard Weitlaner-Johnson, Bernard Bevan, and Louise Lacaud were the first known Westerners to attend and describe a mushroom ceremony.Reference Guzmán2, Reference Wasson and Wasson16
Around 1952, New York banker Robert Gordon Wasson became intrigued by the works of Schultes and initiated a series of trips to Mexico with his wife Valentina Pavlovna Wasson. Accompanied by the photographer Allan Richardson, Wasson was allowed to participate in a mushroom velada with Mazatec sabia María Sabina during the summer of 1955. To be allowed access to the velada, Wasson deceived the community by pretending he worried about the well-being of his son back home.Reference Beyer17 Shortly after, their experience was narrated in the photo essay “Seeking the Magic Mushroom” published in Life magazine, which brought widespread attention to this phenomenon.Reference Wasson7
In 1957, Wasson and his wife were accompanied on a follow-up expedition by French mycologist Roger Heim, who correctly identified several of the mushrooms as species of Psilocybe. Heim cultivated the mushrooms in France and sent a sample of dried Psilocybe mexicana to Swiss chemist Albert Hofmann.Reference Nichols18 Hofmann used paper chromatography to separate the different components of the fungi. The paper chromatogram was cut into pieces that Hofmann and several colleagues voluntarily ingested to identify the active fraction. Hofmann successfully characterized psilocybin and invented a synthesis process that led to its commercialization by Sandoz under the name Indocybin.Reference Hofmann19
Revealing the secret of psilocybin mushrooms came with devastating consequences for the Mazatec. Without María Sabina’s consent, Wasson disclosed her identity and location.Reference Wasson and Wasson16, Reference Pollan20 The popularity of his work brought a surge of Westerners to Oaxaca seeking psychedelic trips, which profoundly disrupted the local community. Sabina was blamed for the actions of intoxicated foreigners, while the Mexican police suspected her of being a drug dealer.Reference Pollan20 She was ostracized, her house burned down, and her son was murdered. Regretful, María Sabina later died in poverty.Reference Pollan20, 21 Many regret how the Mazatec traditional knowledge was commodified and profited from.Reference Fotiou22 Indigenous communities from Mexico do not profit from the lucrative commercialization of psilocybin.Reference Gregoire23, Reference Gerber, Flores, Ruiz, Ali, Ginsberg and Schenberg24
Occidental research on psilocybin
The 1960s ushered in a wave of research investigating the pharmacology and the psychological effects of psychedelics such as psilocybin, LSD, and mescaline. Prominent and charismatic figures such as Timothy Leary, Richard Alpert, and Ralph Metzner were at the vanguard of this movement. Trials were performed using psychedelic substances in an eclectic array of situations. Many investigated their power to facilitate psychodynamic-oriented therapies.Reference Cavarra, Falzone, Ramaekers, Kuypers and Mento25 The Concord Prison Experiment explored psilocybin’s effect on convicted inmates in the hopes of reducing recidivism.Reference Doblin26 In the Marsh Chapel Experiment (also known as the Good Friday Experiment), the entheogen power of psilocybin was studied on students of Harvard Divinity School. Unethical projects such as the CIA-led MK-Ultra investigated the use of psychedelics for psychological torture, brainwashing, and forcing confessions during interrogations.Reference Schell27 Many concerns were raised about the ethics and the scientific validity of research conducted during this period. While psychedelic substances were widely used recreationally, psilocybin research became tied with the counter-culture movement of the 1960s.
Mounting concerns and tensions lead to the abrupt end of this first era of psychedelic research. In 1966, the FDA demanded the shutdown of almost all psychedelic research programs. Soon after, the U.S. Congress criminalized the possession of psilocybin in a 320–2 vote. In 1970, the Controlled Substances Act was passed, which categorized psilocybin as a Schedule I substance, therefore supposing it has no medical use and high potential for abuse.28 Around that time, many other countries adopted similar approaches to criminalize activities involving psychedelics. All these factors contributed to the long hiatus in psychedelic research.
Psychedelic research began to re-emerge in the late 1990s and early 2000s.Reference Vollenweider, Vollenweider-Scherpenhuyzen, Bäbler, Vogel and Hell29 Organizations such as the Multidisciplinary Association for Psychedelic Studies (MAPS) and academic institutions such as Johns Hopkins University were among the first to reexplore the therapeutic potential of psychedelics. Contemporary research is now generally led with higher ethical standards and improved scientific methodology. Meanwhile, modern techniques in neuroscience allow for a better understanding of psilocybin’s effects.
Subjective experience propelled by hallucinogenic mushrooms or psilocybin
The ingestion of psilocybin or hallucinogenic mushrooms produces mighty modifications in perceptions, thoughts, and moods. Despite the ineffable quality of psychedelic journeys and although each experience is unique, we will do our best to summarize the complex and intertwined sensory, cognitive, and emotional manifestations that often characterize them. The importance of set and setting will be addressed in Section 4 on the delivery of psilocybin therapy (PT) and the duration of these experiences will be discussed in Section 5 with other notions of pharmacokinetics.
Sensory and perceptual alterations
Characteristic visual manifestations are soon noticed after the ingestion of psilocybin or hallucinogenic mushrooms. Wavy or rhythmic moving patterns can be observed when contemplating objects or surfaces. Colors often appear brighter and more vivid, and flashes or streaks of light can be seen. Objects or body parts can appear smaller or larger than their actual size. Closing the eyes gives rise to visions of abstract patterns, geometric shapes, or complex scenes involving people, landscapes, and objects.Reference Preller, Vollenweider, Halberstadt, Vollenweider and Nichols30 Psychedelic substances commonly produce audiovisual synesthesia, that is music inducing dynamic images, shapes, or colors.Reference Sinke, Halpern, Zedler, Neufeld, Emrich and Passie31 Synesthesia can less commonly occur between other sensory modalities. Even though the term “hallucinogen” is often used to classify substances like psilocybin, LSD, DMT, and mescaline, they are unlikely to cause hallucinations such as those classically described in psychotic disorders.Reference Preller, Vollenweider, Halberstadt, Vollenweider and Nichols30, Reference Passie and Halpern32
During the psychedelic experience, the perception of time is greatly altered. It might appear slower to some, while others will perceive its acceleration.Reference Studerus, Kometer, Hasler and Vollenweider33 Feelings of “timelessness” or experiences of “eternity” have even been described by many.Reference Barrett, Johnson and Griffiths34 Beyond these sensory alterations, the subjective experience of perception is often enhanced by a surge of curiosity and amazement. This typically translates into the adoption of a contemplative stance, through which the perception of beauty can be expanded.Reference Studerus, Kometer, Hasler and Vollenweider33
Changes in thoughts and cognition
When attention is turned inward, subjects of psychedelic experiences can fall into a state of internal absorption where arising thoughts are often felt with a sense of deep and intuitive understanding. These internal phenomena can sometimes be felt as “more real than reality”.Reference Barrett, Johnson and Griffiths34 Akin to revelations of great importance, many consider these experiences with reverence and strive to remember their details. Culturally loaded terms such as “sacred” or “mystical” are often used to describe these subjective experiences.
Thoughts and feelings of being united with something that transcends the personal self are cardinal features of many psychedelic experiences,Reference Barrett, Johnson and Griffiths34, Reference Prugger, Derdiyok, Dinkelacker, Costines and Schmidt35 and terms such as “oceanic boundlessness” have been used to describe these profound experiences of connection with a larger whole. These experiences of unity may be characterized by a blurring of the boundary between the external environment and what is commonly considered the “I” or the “self.” Some subjects may recall losing ownership of their body or surrendering their mind.Reference Preller, Vollenweider, Halberstadt, Vollenweider and Nichols30 After the notion of “self” has been stripped of its familiar quality, usual ideas, and behaviors can often be considered with a renewed curiosity. Automatic or mindless thoughts and actions can seem puzzling and remarkable. Symbolic imagery such as archetypal themes can be brought to the forefront of consciousness, and identifying with some of these symbolic patterns can nourish a deeper reinterpretation of one’s personal narrative.Reference Preller, Vollenweider, Halberstadt, Vollenweider and Nichols30 Although thinking is generally less target-oriented, subjects may seek answers to existential and ontological questions.
Changes in mood and affect
Most experiences with psilocybin are characterized by a heightened mood, although difficult or challenging psychological experiences of varying severity are not uncommon.
Many people experience joy or happiness, while some even recall feeling bliss or ecstasy. Psychedelics often produce spells of uncontrollable laughter. Overall, a sense of deep contentment often envelops the experience and its aftermath.Reference Barrett, Johnson and Griffiths34, Reference Nicholas, Henriquez and Gassman36
Unfortunately, emotional hardship can be the cost of psychedelic-altered thought patterns. Deep alterations in the sense of “self” can come with the impression of losing control, becoming insane, or even dying. These distressful impressions can be felt with anxiety and panic.Reference Barrett, Bradstreet, Leoutsakos, Johnson and Griffiths37 Overinterpreting insignificant occurrences or events by giving them a personal meaning can also result in feelings of paranoia. Some also experience vivid recollections of personal conflicts or traumatic memories.Reference Barrett, Bradstreet, Leoutsakos, Johnson and Griffiths37 Facing difficult emotions, conflicts, or memories, rather than pushing them aside is often followed by a sensation of breakthrough, which can lead to feelings of relief or closure.Reference Roseman, Haijen, Idialu-Ikato, Kaelen, Watts and Carhart-Harris38
Efficacy of psilocybin therapy: state of the current knowledge & methodological challenges in research
Clinical studies have explored the therapeutic potential of psilocybin for a wide range of conditions. Owing to the encouraging results of prior trials, there is now considerable enthusiasm and momentum in favor of interventions using psilocybin, and the literature on this subject is rapidly expanding as many new studies are underway. We will briefly summarize current knowledge on the efficacy of psilocybin therapy (PT) for the treatment of mental health problems. Safety considerations and possible adverse events from these treatments will be reported in Section 6.
It must be noted that many studies were conducted on psilocybin and other psychedelic drugs during the 1960s and 1970s. While this pioneering work was of considerable importance, it often lacked the methodological or ethical standards required in today’s research.Reference Rucker, Iliff and Nutt39 Therefore, the information we summarize will focus on clinical trials conducted since the rebirth of psychedelic research in the 2000s.
Treatment of depressive episodes
Major depressive disorder (MDD) is a burdensome condition, and current treatment options are unfortunately ineffective in helping many patients who suffer from it.Reference Lam, Kennedy and Adams40, Reference McAllister-Williams, Arango and Blier41 Therefore, there is great interest and need for new treatments against MDD. In recent years, PT has emerged as a promising intervention and many studies have evaluated its efficacy.
A thorough review of the literature allowed us to identify published results for seven controlled trials and four open-label trials evaluating PT for MDD. Trials that solely recruited patients suffering from anxiety or depressive symptoms occurring in the context of a life-threatening medical illness will be discussed in Section 3.2. The main characteristics of these studies are presented in Table 1. Most recruited participants suffered from moderate to severe MDD, which was commonly defined by a score ≥ 17 on the Hamilton Depression Rating Scale (HAMD). Some studies recruited patients with treatment-resistant depression (TRD), meaning the patients’ symptoms persisted despite prior antidepressant trials. All studies had a small sample size, the largest having only recruited 233 participants. The interventions varied significantly between clinical trials. Most offered around 10–30 mg of psilocybin on a fixed-dose regimen, while others calculated a weight-based dose. Protocols had between one to three medication administration sessions, and there was a wide variation in the type of psychological intervention offered. All studies reported short-term primary outcomes (from 1 to 6 weeks after PT), while only a few reported outcomes after a longer follow-up period (from 6 to 12 months after PT). By drafting funnel plots for psychedelic interventions in MDD or TRD and by performing Egger’s test, some authors have suggested publication bias or small study effects that could also limit our current knowledge of the efficacy of psychedelic treatments.Reference Fang, Chan, Chan, Jiao, Wang and Li42, Reference Galvão-Coelho, Marx and Gonzalez43
Table 1. Characteristics of Clinical Studies Evaluating PT for MDD or TRD
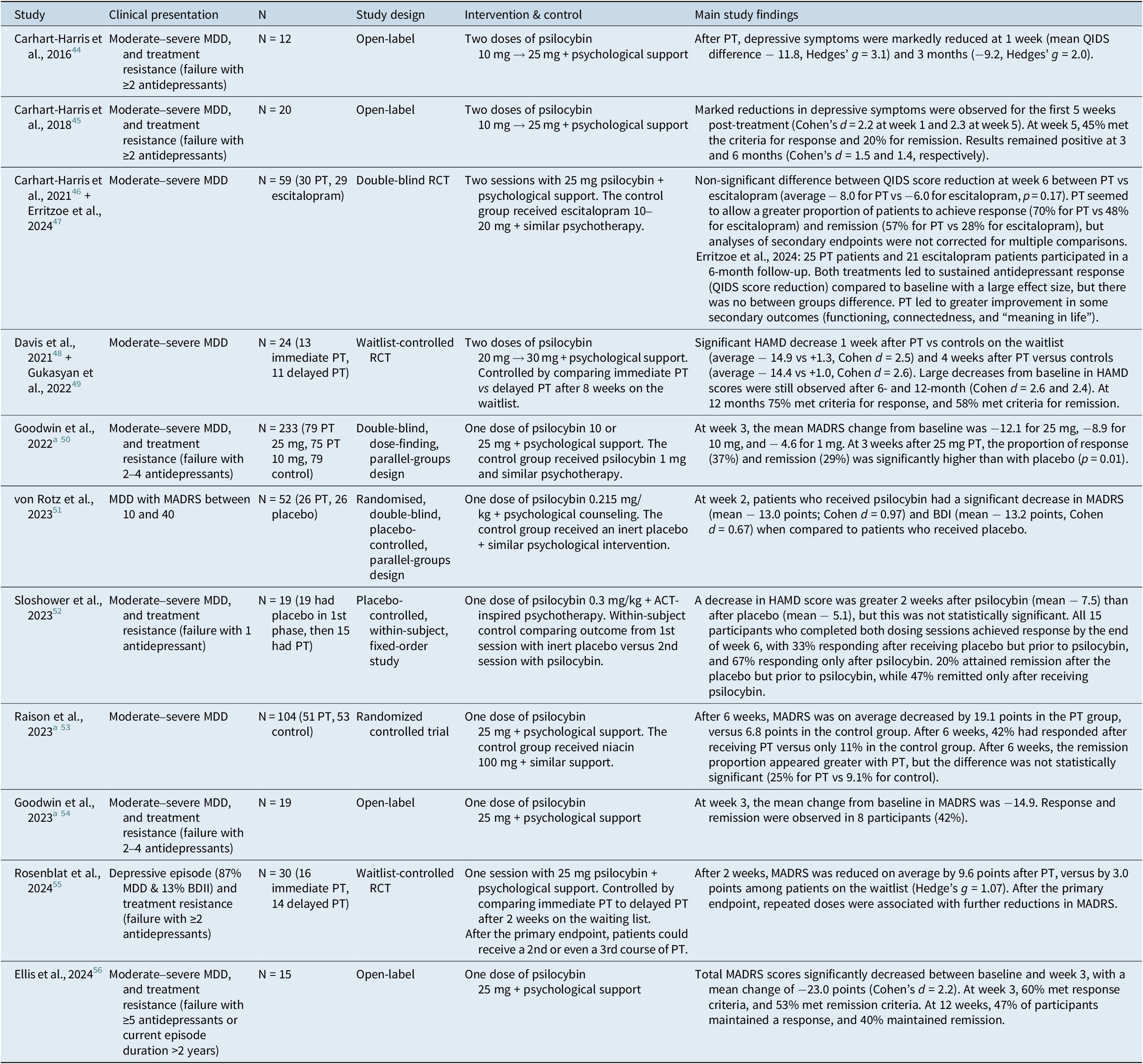
a Industry-sponsored trials. PT, psilocybin therapy; MDD, major depressive disorder; TRD, treatment-resistant depression; QIDS, Quick Inventory of Depressive Symptomatology; RCT, randomized controlled trial; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; BDI, Beck Depression Inventory.
At short-term endpoints, most studies found that PT offered a meaningful reduction in depressive symptoms (with an average MADRS reduction between 13 and 23 points or with an average HAMD decrease between 7 and 15 points). The effect sizes were generally large as illustrated by Cohen’s d commonly reported between 0.9 and 2.5. At short-term endpoints, these small studies found response rates ranging from 40% to 75%, while remission rates vary between 20% and 60%.
The long-term efficacy of PT has not yet been thoroughly described. A study by Gukasyan et al. reported 12 months of follow-up results for a cohort of 24 patients who initially suffered from moderate to severe MDD. This study showed a 75% response rate (>50% reduction in HAMD score) and a 58% remission rate (HAMD score ≤ 7) one year after a single psilocybin treatment.Reference Gukasyan, Davis and Barrett49 Soon-to-be-published data from our laboratory also suggests that response and remission rates can remain high, even 12 months after PT. In a small sample of veterans suffering from moderate to severe TRD, sustained response was observed for 50% of participants, and persistent remission for 40% at a 12-month endpoint (n = 10).
Currently, PT has limited evidence as a treatment for depression in bipolar disorders. Aaronson et al. reported the safety and efficacy of psilocybin in 15 patients with type II bipolar disorder and a current depressive episode. In this study, all participants had a reduced MADRS score at week 3, with a mean improvement of 24 points on this scale. At the same study endpoint, 80% of participants met remission criteria. The intervention was well tolerated, and no manic or hypomanic episode was observed.Reference Aaronson, Van Der Vaart and Miller57 Four participants with type II bipolar disorder were also included in a recent study.Reference Rosenblat, Meshkat and Doyle55
We reviewed clinical practice guidelines for the treatment of depression published across the United States, Canada, Europe, and Asia-Pacific. None has yet recommended PT for the treatment of MDD or TRD. Because of limited data regarding its efficacy, methodological limitations in published studies (that will be discussed further), and challenges surrounding its implementation, PT remains an investigational treatment.Reference Lam, Kennedy and Adams40, 58
Treatment of psychological distress in patients with life-threatening illness
The mainstream rebirth of psychedelics research was initially sparked by seminal works on PT to relieve anxiety and depressive symptoms in patients with advanced cancer. We were able to find 4 studies (some having led to multiple publications) comprising a total of 104 patients with psychological distress related to cancer. The main characteristics of these small studies are presented in Table 2.
Table 2. Characteristics of Clinical Studies Evaluating PT for Anxiety and Depressive Symptoms in Cancer Patients
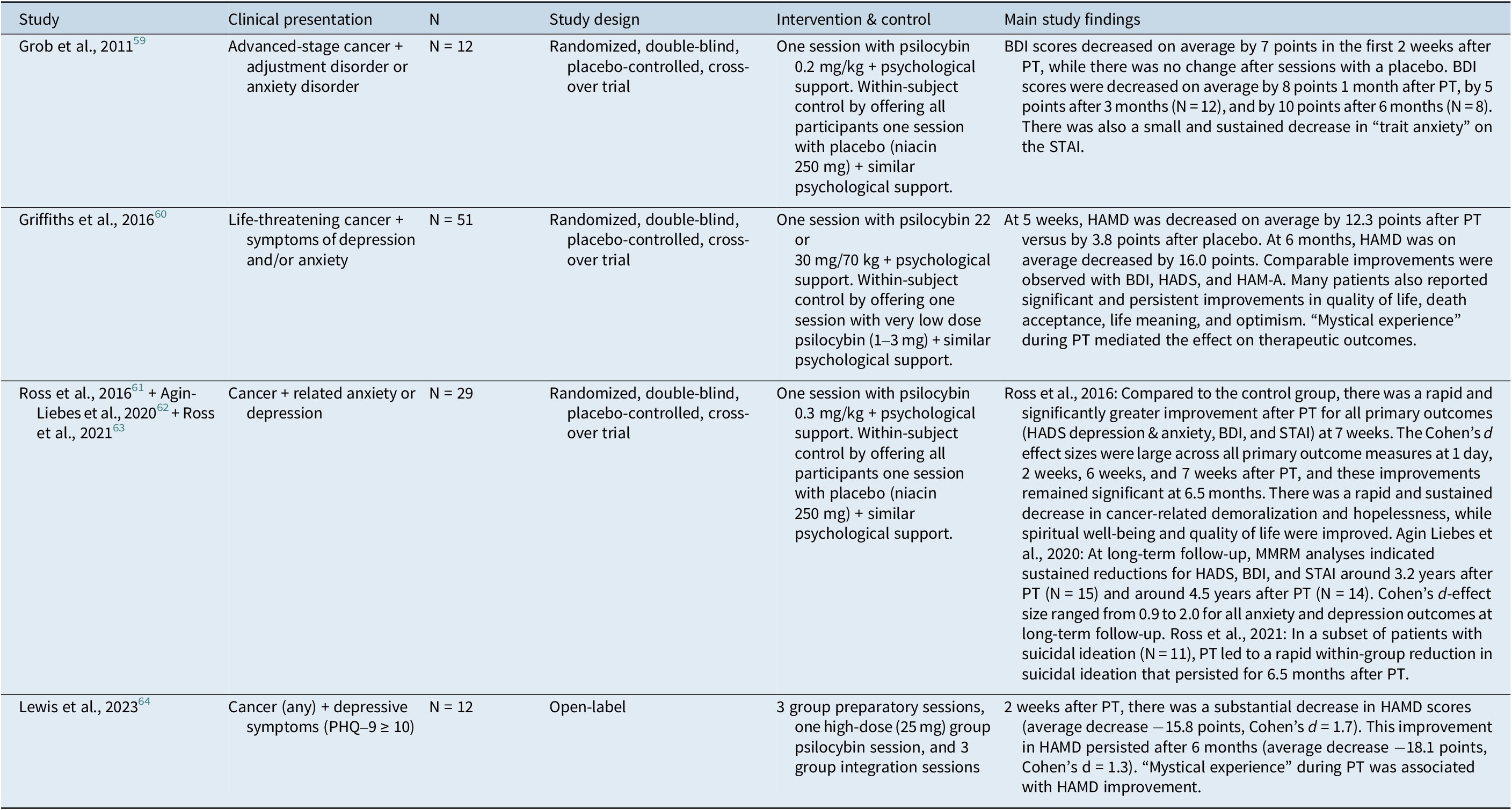
PT, psilocybin therapy; BDI, Beck Depression Inventory; STAI, State–Trait Anxiety Inventory; HAMD, Hamilton Depression Rating Scale; HADS, Hospital Anxiety and Depression Scale; HAM-A, Hamilton Anxiety Scale; MMRM, Mixed models for repeated measures; PHQ-9, Patient Health Questionnaire-9.
These studies demonstrated that PT can remarkably improve depressive symptoms, anxiety, sense of life meaning, and fear of death in some patients. Large effect sizes were generally reported for these outcomes. Remarkably, one study explored long-term follow-up until an average of 4.5 years after treatment and showed sustained response in approximately three-quarters of patients.Reference Agin-Liebes, Malone and Yalch62 Because of limited clinical data and implementation challenges, no clinical practice guideline has yet recommended PT to alleviate psychological distress among oncology patients.
Alcohol use disorder
Two recent studies (n = 105) could be found for PT against alcohol use disorder. The characteristics of these small studies are detailed in Table 3. These promising results suggest a meaningful reduction in alcohol consumption after PT, but these interventions have currently not made their way into clinical practice because of very limited data on their efficacy and innocuity.
Table 3. Characteristics of Clinical Studies Evaluating PT for Alcohol Use Disorder
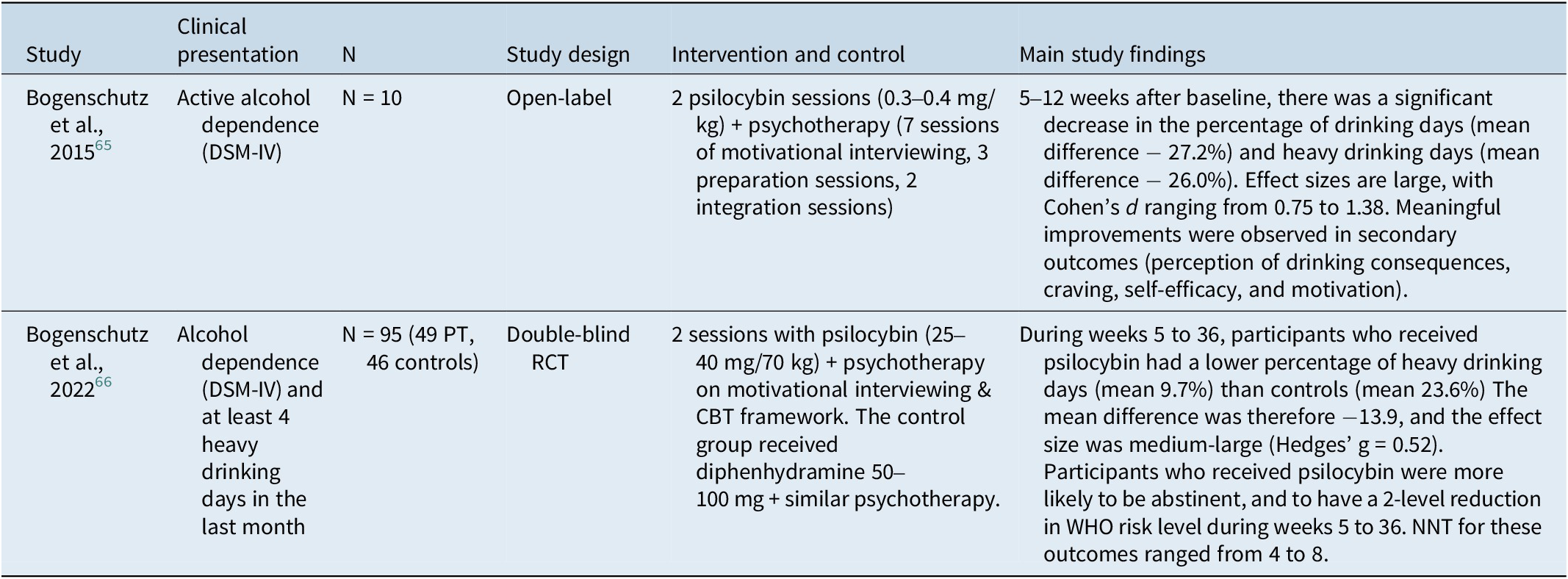
PT, psilocybin therapy; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; RCT, randomized controlled trial; CBT, Cognitive behavioral therapy; WHO, World Health Organization; NNT, Number needed to treat.
Other conditions
Results from exploratory studies suggest that PT might be versatile in treating a wide array of disorders. A small but rapidly expanding literature supports the promising potential of psilocybin for tobacco addiction (2 studies, n = 30),Reference Johnson, Garcia-Romeu, Cosimano and Griffiths67, Reference Johnson, Garcia-Romeu and Griffiths68 obsessive-compulsive disorder (1 study, n = 9),Reference Moreno, Wiegand, Taitano and Delgado69 anorexia nervosa (1 study, n = 10),Reference Peck, Shao and Gruen70 headaches (2 studies, n = 24)Reference Schindler, Sewell and Gottschalk71, Reference Schindler, Sewell and Gottschalk72 and body dysmorphic disorder (1 study, n = 12).Reference Schneier, Feusner and Wheaton73 Much research is still needed before affirming the efficacy of PT in these clinical contexts. Many registered clinical trials are currently evaluating PT’s therapeutic potential in other conditions: substance use disorders (opioid, stimulant, or cannabis), post-traumatic stress disorder, chronic Lyme disease, functional neurological disorder, post-concussive symptoms, and chronic pain.
Limitations in clinical studies on psilocybin therapy
Designing and conducting clinical trials on PT is remarkably challenging, and limitations can be identified in the current literature.
Functional unblinding has rightly been identified as a serious limitation while interpreting results from recent PT trials. Because psilocybin produces powerful and easily recognizable alterations of perceptions, mood, and thoughts, most participants in RCTs can easily deduce if they have received psilocybin or placebo. Similarly, most therapists in RCTs can also recognize the allocation of each participant, which might consciously or unconsciously affect the psychotherapeutic intervention they offer. Unfortunately, studies with inadequate blinding of participants or clinicians are likely to overestimate the efficacy of treatments.Reference Butler, Jelen and Rucker74, Reference Jüni, Altman and Egger75 Limitations stemming from the unblinding of study participants are further amplified by the highly positive expectations of many patients enrolling in psilocybin clinical studies. Indeed, abundant media coverage has led to premature expectations about the efficacy of psychedelic interventions, and many enroll in trials with high hopes. If participants with hopeful expectations receive psilocybin and recognize its effects, psychosomatic factors will likely heighten their treatment response.Reference Aday, Heifets, Pratscher, Bradley, Rosen and Woolley76 On the contrary, participants with high expectations are randomized to receive a placebo, their disappointment may lead to clinical worsening or a nocebo effect.Reference Furukawa, Noma and Caldwell77
In short, high treatment expectancies and functional unblinding of participants likely synergize to inflate the results from PT studies.Reference Aday, Heifets, Pratscher, Bradley, Rosen and Woolley76, Reference Muthukumaraswamy, Forsyth and Lumley78 Some possible solutions have been proposed to mitigate these concerns,79 although each has its limitations. It has been suggested to systematically monitor participants’ expectations before therapy and to use questionnaires measuring the extent of patients’ and therapists’ unblinding. Using consciousness-altering placebos has also been suggested to confound the study participants, although the effectiveness of this measure is still being explored. Some have even proposed to administer psilocybin to patients under general anesthesia,Reference Olson80 which would potentially solve the problem of blinding and expectancy bias. If this unusual measure was applied, it would cancel the effects of the subjective psychedelic experience, psychotherapy intervention, set, and setting. The results of such an experiment could also be confounded by the neuro-pharmacological effects of the anesthetics.
The necessity of psychotherapy in conjunction with psilocybin is still debated, and research trying to optimize these therapeutic interventions is still in its infancy. In studies involving psilocybin, psychotherapy interventions are heterogeneous and often poorly described. Although some research groups have published their psychotherapy protocols with reasonable detail,Reference Ross, Bossis and Guss61, Reference Guss, Krause and Sloshower81–Reference Watts83 the largest industry-sponsored trials have failed to disclose the exact nature of their psychotherapeutic interventions.Reference Goodwin, Aaronson and Alvarez50, Reference Raison, Sanacora and Woolley53 Extensive studies are needed to assess the role of psychotherapy in PT, and specific protocols may be required for different conditions such as mood disorders, anxiety disorders, and substance abuse. Trials with a factorial design could help differentiate the drug efficacy from the effect of psychotherapy.79 Head-to-head comparisons of different psychotherapy models and component studies are also needed to define the essential elements of these interventions. Before such trials are conducted, we can only speculate on important questions. To what extent (if any) does psychotherapy contribute to the efficacy of PT? What are the most effective and most efficient ways of conducting psychotherapy in synergy with psilocybin?
Concerns have been raised regarding the generalizability of the results so far published. Recent PT trials were generally conducted on small samples of demographically homogenous participants. White and educated individuals from high-income countries have formed most of these samples.Reference Haikazian, Chen-Li and Johnson84–Reference Thrul and Garcia-Romeu86 It is therefore unknown if these results could be replicated for patients with more diverse demographic characteristics or those in developing countries. Also, patients with substance use disorders, personality disorders, psychotic illness, or a history of suicide attempts were largely excluded from studies, even though patients with MDD or TRD often suffer from these comorbidities in real-world settings.Reference Haikazian, Chen-Li and Johnson84 Also, PT studies generally have a long list of medical exclusion criteria (e.g. uncontrolled cardio-vascular condition, seizure disorder, BMI > 35 or < 17, etc.). All these exclusion criteria might reduce the generalizability of available results. Since psychiatric or medical comorbidities are associated with treatment resistance, the efficacy of PT in naturalistic settings could be less favorable.
How is psilocybin therapy conducted?
Although significant variations exist in the way PT is conducted among clinicians and researchers, some general principles seem common in most settings.Reference Cavarra, Falzone, Ramaekers, Kuypers and Mento25 As illustrated in Figure 1, PT protocols typically involve a sequence of preparation sessions, single or multiple medication administration session(s), and integration sessions. Rather than being a comprehensive guide for the delivery of PT, the following section offers a brief overview of typical interventions at its core. The summarized material presented here was extracted from published treatment manualsReference Ross, Bossis and Guss61, Reference Guss, Krause and Sloshower81–Reference Watts83 and reviews on the subject.Reference Cavarra, Falzone, Ramaekers, Kuypers and Mento25, Reference Bathje, Majeski and Kudowor87–Reference Zarbo, Tasca, Cattafi and Compare90

Figure 1. Structure of a typical PT protocol.
General considerations
It has long been recognized that psychedelics can produce iatrogenic and unpleasant effects if they are administered in a stressful environment or without proper support.Reference Eisner91, Reference Zinberg92 Therefore, much consideration is given to the set and the setting in which PT is offered.Reference Carhart-Harris, Roseman and Haijen93 The set refers to the states of mind carried by the patient and the therapists, which notably includes their respective expectations, motivations, and beliefs about PT. The setting refers to the actual physical and interpersonal environment in which treatment is delivered. It includes for example the design and atmosphere of the room, the music, and the behavior of the therapists. Components of set and setting are important to promote the safety and efficacy of psychedelic therapy.Reference Hartogsohn94, Reference Leary, Litwin and Metzner95
Therapists should have received specialized training in PT, and they should be in good standing with their regulatory or licensing agency. They should meet the highest standards of ethical integrity and professionalism. Furthermore, therapists must be capable of building and maintaining a sound therapeutic alliance. They should be welcoming, and they should treat patients with compassion, dignity, and respect. Therapists must be mindful of the increased suggestibility elicited by psychedelic substances, and they should be watchful of their countertransference.
In almost all studies published to date, PT was conducted by a dyad of therapists. They are usually encouraged to work in teams to ensure that the patient is always accompanied by at least one person, even if someone must briefly leave the room during the long medication administration session. Dyads could also provide greater safety for participants, diminish the risk of boundary violation, help manage transference and countertransference, and offer learning opportunities from the co-therapist.79, Reference Johnson, Richards and Griffiths82 Other models are being explored, including a hub-and-spoke system where one therapist is with the patient, while another supervises multiple simultaneous dosing sessions.
Preparation sessions
A few hours of preparation are first offered, often spanning over two to three weeks. As discussed below, informed consent should be obtained, although patients have the right to withdraw consent at any step. The preparatory period allows for medical clearance, and screening for possible contraindications to PT. At this time, it is standard to taper medications that could interact with psilocybin or that might interfere with the effectiveness of PT (e.g. antipsychotics, antidepressants, benzodiazepines, etc.).
During the preparation sessions, therapists get a better understanding of the patient’s history and current situation, which helps build trust and alliance. A collaborative process is initiated to define meaningful and realistic treatment goals. Therapists should encourage patients to adopt an engaged and proactive stance since PT is neither miraculous nor effortless.
Preparation also requires thorough explanations about the possible effects of psilocybin, and prior experiences with altered states of consciousness can be explored. Patients should be warned about possible reactions of anxiety, panic, or paranoia, and stress inoculation techniques can be practiced. Breathwork, body scan, and other grounding techniques are commonly taught or reviewed at this stage. Patients are encouraged to welcome rather than resist challenging emotions, imagery, or thoughts. Importantly, there should be a discussion about the potential application of supportive touch (e.g. holding hands or laying a hand on the shoulder) if challenging experiences arise during therapy. If the patient consents to receive supportive touch, clear boundaries must be set and recognized, as to avoid any inappropriate, sexual, or intimate physical contact.
Patients should also be informed about the logistics and structure of the psilocybin session. It is common practice to discuss music and to visit the room where the psychedelic experience will be lived. Patients can be advised to bring comfortable clothes and meaningful items. At the end of the experiential session, patients will be escorted by a companion that should be designated beforehand. If needed, the companion can be met by the therapy team before the dosing day. Finally, the patients’ questions must be addressed during the preparatory period.
Experiential session with psilocybin
On the day of the psilocybin administration session, the patient is welcomed in a comfortable and confidential setting. The room should ideally be calm and demedicalized (see example from Figure 2). The setting typically contains a bed or futon to recline, music, flowers, artwork, snacks, and soft drinks. Some participants may choose to bring significant or symbolic objects. The environment should be safe, and potentially dangerous objects (e.g. sharp edges, high balconies or unsafe windows, glass objects, etc.) should be avoided. Distracting items such as telephones should be removed. Throughout the session, the therapists will demonstrate their benevolent presence and ensure safety.

Figure 2. Design of the therapy room used for psychedelic studies at the Exploratory Therapeutics Laboratory, Stanford University.
A dose of around 25 mg of psilocybin is typically offered, after which the patient is invited to relax. The substance typically starts producing its psychedelic effects 20 to 30 minutes after being ingested, at which point the patient is invited to bring his attention to the unfolding present-moment experience. The experiential session should mainly be an unrushed and inner-directed process. Wearing a blindfold can help focus on internal phenomena such as thoughts and affects, while the music can intensify the experience by offering a sense of guidance and evocating emotions or mental imagery.Reference Kaelen, Giribaldi and Raine96
All internal states, whether pleasant or challenging, should be welcomed and observed with openness and curiosity. Rather than avoiding difficult internal phenomena, patients are encouraged to adopt a non-resisting stance. If the patient expresses the feeling of being stuck, the therapists can foster curiosity about perceived resistance, allow and encourage the patient to express himself or herself, or offer reassurance and encourage the deeper exploration of threatening mental states. If the patient shows signs of intense suffering or dissociation, therapists must stay present and should find a balance between alleviating versus tolerating and validating the patient’s suffering. If the patient has consented to it, a supportive touch can also be offered. Many challenging experiences can be appeased by changing position, distracting, changing the music, doing breathwork, or applying other grounding techniques. In extreme situations, rescue medication such as benzodiazepine or antipsychotic can be proposed.
At the height of the psychedelic experience, the patient should not be urged to understand or interpret everything happening on the spot. The integration sessions are typically the preferred moment to analyze the experience. Therapists can discretely take notes to record meaningful observations, which may later be shared with the patient.
At the end of the session, the effects of psilocybin gradually subside, and patients often express the urge to talk about their psychedelic experience. Curiosity about the experience should be fostered, and it can be reiterated that “understanding” everything is not the current objective. If manifested, early insights or feelings of emotional breakthroughs should be acknowledged. Some patients may require support intervention if they experience distress linked to their psychedelic journey or the return of everyday life concerns.
If the patient has returned to his baseline psychological and physiological state, he can be safely discharged in the presence of a companion. Without attempting to perform therapy, the companion should find the right balance between respecting the patient’s privacy and offering support. Some therapists encourage their patients to write about their experiences. Rest is typically encouraged on the night after the experiential session.
Integration sessions
The integration process is prompted by a reunion with therapists on the day after each session with psilocybin. Other integration sessions are often offered over the following weeks. During these encounters, therapists can foster curiosity for internal phenomena, listen to the patient’s narrative, and raise questions about its interpretation. Self-reflective practices such as journaling, dream analysis, and active imaginationReference Johnson97 can be suggested to revitalize insight and meta-cognition. Increased cognitive flexibilityReference Doss, Považan and Rosenberg98 and new insights may translate into a modified perception of oneself and the environment.Reference Studerus, Kometer, Hasler and Vollenweider33, Reference MacLean, Johnson and Griffiths99 These new ways of thinking and perceiving can help surpass prior obstacles or facilitate grieving and acceptance of difficult situations. Cultivating mindfulness and attending to the present moment is encouraged during the integration process. Therefore, formal and informal meditative practices can be suggested. Some patients may also benefit from expressing themselves through arts such as writing, dancing, or painting.Reference Earleywine, Low, Lau and De Leo88, Reference Zarbo, Tasca, Cattafi and Compare90
It is important to note that the integration process should not be restricted to a fruitless intellectual process. Rather, concrete and tangible change in behavior is hoped for. Changes outside the therapy setting such as behavioral activation, increased socialization, or use of more efficient coping strategies should be applauded. Patients should cultivate a sense of agency, and PT should not be seen as effortless. Statements like “psilocybin may have opened a door, but you have to walk through it” may encourage the patient’s efforts and commitment.
Integration can continue long after the last meeting with therapists. Some have also pointed out that this process could be non-linear, meaning that patients may sometimes experience a short period of stagnation or regression before reaching an improved state. Patience is therefore required to determine if a patient might have benefited from PT. Finally, it must be remembered that we are currently unable to describe the most effective way of conducting the integration process since many different theoretical frameworks have inspired a wide range of interventions. Heterogenous practices stemming from psychodynamics, existential therapy, or ACT have, for example, been tried.Reference Ross, Bossis and Guss61, Reference Guss, Krause and Sloshower81, Reference Watts83, Reference Goodwin, Malievskaia, Fonzo and Nemeroff100
Pharmacology and neurobiology
From hallucinogenic mushrooms to purified psilocybin
Many species of mushrooms naturally synthesize tryptamine alkaloids that can produce psychedelic effects. These psychotropic mushrooms are distributed worldwide and belong to many genera, notably Psilocybe, Panaeolus, Pluteus, Gymnopilus, Pholiotina, Galerina, and Inocybe. Reference Gotvaldová, Borovička, Hájková, Cihlářová, Rockefeller and Kuchař101 Although the exact adaptive function of these psychoactive compounds remains elusive, it has been hypothesized that their presence may offer an evolutionary advantage to these mushrooms by altering the behavior of mycophagous species.Reference Awan, Winter and Turner102 Two opposing theories have been proposed: some believe that tryptamine alkaloids benefit the mushrooms by reducing mycophagy,Reference Reynolds, Vijayakumar, Gluck-Thaler, Korotkin, Matheny and Slot103 while others have suggested these molecules could increase mycophagy and insect-vectored spore dispersal.Reference Gasque, Conway, Huang, Rao and Vosshall104
The mechanisms by which these mushrooms produce their psychedelic effects are complex. Psilocybin and psilocin are likely responsible for most of their psychedelic properties in humans, but these fungi also contain smaller amounts of chemically related compounds such as norbaeocystin, baeocystin, and aeruginascin.Reference Gotvaldová, Borovička, Hájková, Cihlářová, Rockefeller and Kuchař101 These molecules have a chemical structure analogous to psilocin or psilocybin, and their effects on humans have not been fully characterized. Interestingly, a recent study identified that Psilocybe mushrooms can synthesize β-carbolines, which could potentially inhibit the degradation of psilocin by monoamine oxidase (MAO).Reference Blei, Dörner and Fricke105 The significance of this pharmacokinetic interaction remains uncertain, considering the small concentrations of β-carbolines. Moreover, some psychedelic mushrooms contain phenylethylamine, which has amphetamine-like properties that could produce psychostimulant effects and a more pronounced cardiovascular activation.Reference Barnett, Koons, Eynde, Gillman and Bodkin106, Reference Hernandez-Leon, Escamilla-Orozco and Tabal-Robles107
The concentrations of tryptamine alkaloids in hallucinogenic mushrooms can vary tremendously between species and between individual mushrooms, which has an impact on their psychotropic potency. Some species such as P. cyanescens, P. cubensis, and P. mexicana tend to contain relatively high amounts of psilocin and psilocybin, which means they are regarded as medium or high-potency species. Specific strains of these species are widely sold under names such as “Golden teacher” (a cultivar of P. cubensis) or “Blue meanie” (a cultivar of P. cyanescens). On the contrary, species from other genera such as Inocybe or Panaeolus are seldom used recreationally, because they tend to contain low amounts of psychoactive compounds.Reference Gotvaldová, Borovička, Hájková, Cihlářová, Rockefeller and Kuchař101
The fruiting bodies of the mushrooms are composed of a cap and a stipe. The concentration of tryptamines in caps is generally about two-fold higher, although the variability between each mushroom is considerable.Reference Gotvaldová, Hájková, Borovička, Jurok, Cihlářová and Kuchař108 For example, a high-performance liquid chromatography-mass spectrometry analysis revealed that psilocybin concentration can vary from 2.3 to 13.8 mg/g in dry mass between samples of P. cyanescens. Reference Gotvaldová, Borovička, Hájková, Cihlářová, Rockefeller and Kuchař101 The development stage, climatic conditions, and substrate composition can also contribute to this inconsistency. Furthermore, these concentrations can also be influenced by the processing and storage of the mushrooms after their harvest. Degradation of psilocybin happens quickly in lyophilized or frozen fresh fungi, while it can remain unaltered after many years in samples of dried whole mushrooms.Reference Gotvaldová, Borovička, Hájková, Cihlářová, Rockefeller and Kuchař101, Reference Gotvaldová, Hájková, Borovička, Jurok, Cihlářová and Kuchař108
Because fungi contain unpredictable concentrations of psychoactive tryptamines, clinical studies conducted in recent years have used pure psilocybin, either synthesized or extracted from natural sources.109, Reference Yao, Guo and Lu110 These products have the advantage of containing a known and stable dose of psychoactive tryptamines, and no other compounds that may cause pharmacokinetic or pharmacodynamic interactions. If psilocybin is approved for clinical use in the future, good manufacturing practices will ensure the quality and reliability of the prescribed product.
Pharmacokinetics
Psilocybin is an alkaloid zwitterion containing a positively charged amine group and a negatively charged phosphateReference Ballesteros, Ramón, Iturralde and Martínez-Arrieta111, which makes it more soluble in water but reduces its ability to penetrate cell membranes and the blood–brain barrierReference Ballesteros, Ramón, Iturralde and Martínez-Arrieta111. Psilocybin is rapidly dephosphorylated into psilocin by alkaline phosphatases and non-specific esterasesReference Thomann, Kolaczynska and Stoeckmann112. Due to its increased lipophilicity, psilocin is readily absorbed from the upper gutReference Eivindvik, Rasmussen and Sund113 and then undergoes first-pass metabolism. After ingestion, its average absolute bioavailability is around 53%.Reference Hasler, Bourquin, Brenneisen, Bär and Vollenweider114 It first appears in the plasma within 20–30 minutes,Reference Brown, Nicholas and Cozzi115, Reference Kolaczynska, Liechti and Duthaler116 and the time to peak drug concentration is approximately 120 minutes.Reference Holze, Singh, Liechti and D’Souza117 In PK studies and clinical trials, patients typically fast between 2–4 hours to allow for more predictable kinetics,Reference Hasler, Bourquin, Brenneisen, Bär and Vollenweider114 since food in the stomach may delay absorption, reduce peak concentration, and reduce its bioavailability.
Psilocybin demonstrates linear pharmacokinetics in standard dose ranges,Reference Brown, Nicholas and Cozzi115, Reference Holze, Becker, Kolaczynska, Duthaler and Liechti118 which means that doubling its dose would lead to a two-fold increase in the plasma concentration of psilocin. Because of its high lipophilicity and extensive distribution into body tissues, it can be calculated that psilocin has a large volume of distribution.Reference Holze, Becker, Kolaczynska, Duthaler and Liechti118 Contemporary findings indicate that body weight does not significantly influence the subjective effects of psilocybin, nor does it influence plasma concentrations of psilocin.Reference Holze, Becker, Kolaczynska, Duthaler and Liechti118–Reference Spriggs, Giribaldi and Lyons120 Therefore, fixed-dose regimens were given in recent clinical studies, in contrast to the weight-based dosing protocols of earlier studies.
The metabolism of psilocin is summarized in Figure 3. Briefly, nearly 80% of psilocin is metabolized into psilocin-O-glucuronide by UGT enzymes, while the residual fraction is degraded into 4-HTP and 4-HIAA through a minor pathway involving MAO-A.Reference Dinis-Oliveira121–Reference Horita and Weber123 The cytochrome P450 system has a minor role in psilocin’s metabolism, and individuals with variations of CYP activity do not differ in their response to psilocin.Reference Thomann, Kolaczynska and Stoeckmann112 Psilocin has a dose-independent half-life of about 2–4 hours.Reference Hasler, Bourquin, Brenneisen, Bär and Vollenweider114–Reference Kolaczynska, Liechti and Duthaler116, Reference Hasler, Bourquin, Brenneisen and Vollenweider124, Reference Lindenblatt, Krämer, Holzmann-Erens, Gouzoulis-Mayfrank and Kovar125 There is currently no evidence suggesting that psilocin’s metabolites have any significant psychoactive effects.Reference Thomann, Kolaczynska and Stoeckmann112 About 80–85% of metabolites are excreted in the urine (mostly psilocin-O-glucuronide), while the remainder 15–20% is excreted through the biliary system.Reference Dinis-Oliveira121, Reference Kalberer, Kreis and Rutschmann126 Renal function has a minimal impact on the drug’s elimination process. Therefore, dose adjustments may be unnecessary for patients with renal impairment, although limited data is available in this population.Reference Holze, Becker, Kolaczynska, Duthaler and Liechti118, Reference Dodd, Norman and Eyre127 Psilocybin has no significant effect on liver function or creatinine clearance in healthy individuals.Reference Hasler, Grimberg, Benz, Huber and Vollenweider128
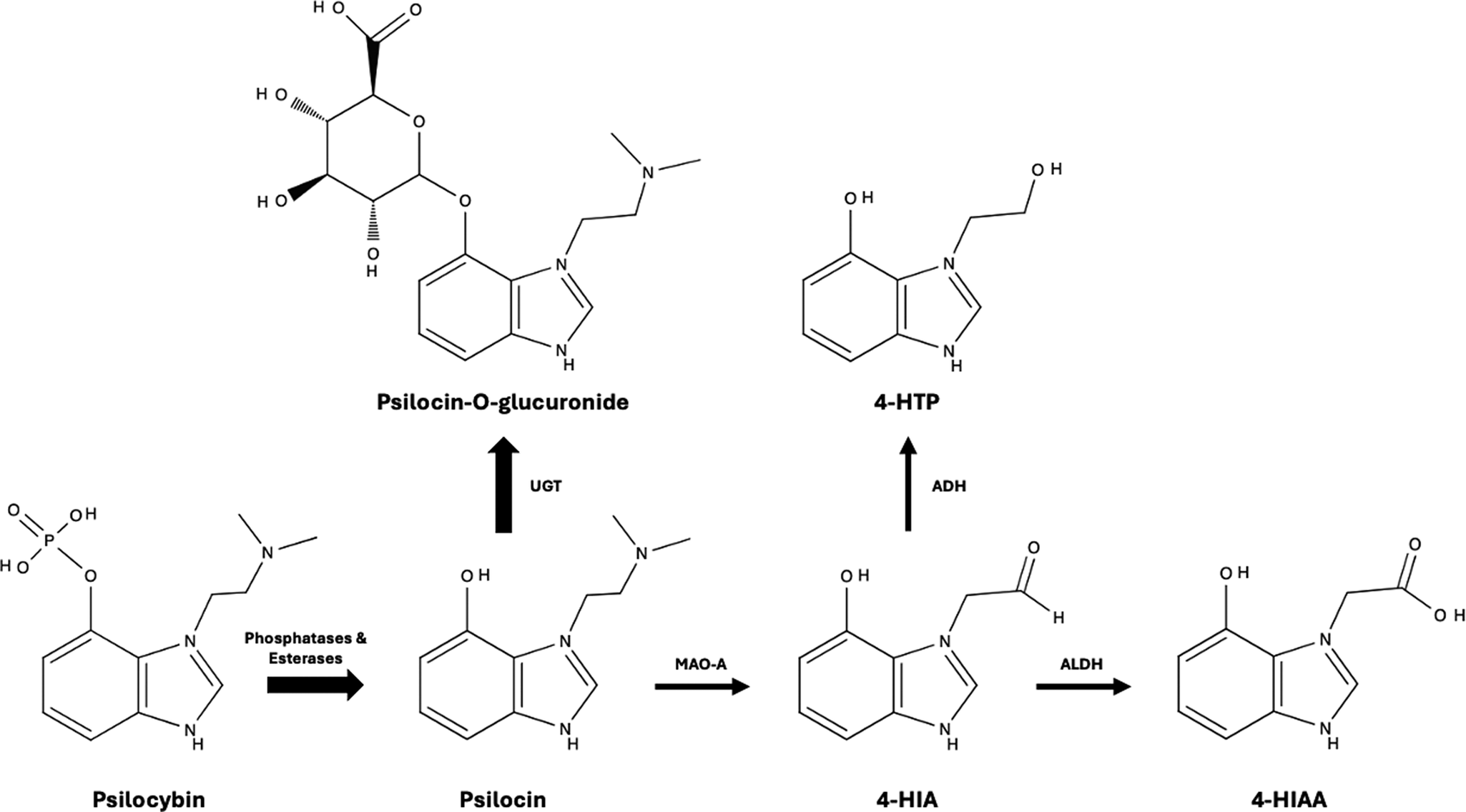
Figure 3. Metabolism of psilocybin and psilocin in humans.
Receptology and downstream signaling
Serotonin and psilocin are tryptamine analogues and share an indole ring structure, which is crucial for binding to 5-HT2A receptors. Like other classic psychedelics such as LSD and mescaline, psilocin is an agonist of the 5-HT2A receptor. Good evidence supports the crucial role of 5-HT2A receptor activation in mediating its psychotropic effects: 5-HT2A receptor occupancy correlates with the subjective effects of classic psychedelics,Reference Holze, Ley and Müller129, Reference Madsen, Fisher and Burmester130 and pre-treatment with 5-HT2A receptor antagonist ketanserin blocks most of psilocybin’s effects.Reference Vollenweider, Vollenweider-Scherpenhuyzen, Bäbler, Vogel and Hell29, Reference Kometer, Schmidt, Bachmann, Studerus, Seifritz and Vollenweider131, Reference Quednow, Kometer, Geyer and Vollenweider132 Upon their activation by psilocin, 5-HT2A receptors initiate complex cascades of downstream signaling. The activation of both canonical Gq/11 and β-arrestin-2 seems necessary to produce psychedelic effects,Reference Wallach, Cao and Calkins133 and so is the coactivation of Gi/o and Src tyrosine kinase.Reference González-Maeso, Weisstaub and Zhou134 These specific pathways are thought to differentiate 5-HT2A receptor agonists with psychedelic properties from other agonists of the same receptor such as ergoline and lisuride that do not have hallucinogenic effects. Many mysteries are yet to be unraveled, as exemplified by the recent discovery that psilocin could activate intracellular 5-HT2A receptors, which results in sustained neuronal growth and structural neuroplasticity.
Although strong evidence supports that 5-HT2A activity mediates most of psilocin’s psychedelic properties, this substituted tryptamine also binds to many other receptorsReference Cameron, Benetatos and Lewis135–Reference Ray137. In fact, psilocin’s binding affinity is even higher for some other serotonin receptors such as 5-HT2C, 5-HT1A, and 5-HT2BReference Ray137. It is currently difficult to determine the clinical significance of psilocin’s interaction with these receptors. Although they do not seem to contribute to the hallucinogenic properties of psilocin, these other serotonin receptors could potentially play a role in mediating its therapeutic effect.Reference Halberstadt and Geyer136, Reference Gresch, Barrett, Sanders-Bush and Smith138–Reference Winter, Rice, Amorosi and Rabin140
Psilocin has a very low affinity for the serotonin transporter (SERT), and it does not interact directly with the norepinephrine transporter (NET) or the dopamine transporter (DAT).Reference Ray137 Although it has the potential to bind with D1 and D3 receptors, it has no direct activity on the widespread D2 receptors.Reference Ray137 It does not interact with adrenergic, opioid, muscarinic, histamine, or cannabinoid receptors.Reference Ray137 Even if psilocin does not directly interact with these receptors, dopamine, noradrenaline, and opioid neurons might be secondarily activated because of the initial disruption in serotonin signaling. The nature and significance of these potential interactions have yet to be understood.Reference Cameron, Benetatos and Lewis135
Interesting data has recently shown that psilocin directly interacts with brain-derived neurotrophic factor (BDNF) signaling,Reference Moliner, Girych and Brunello141 which is crucially involved in neuronal plasticity, cognition, and mood regulation.Reference Autry and Monteggia142–Reference Phillips144 The BDNF/TrkB pathway involves the binding of BDNF to tropomyosin receptor kinase B (TrkB) on the surface of neurons. Psilocin acts as an allosteric modulator within the transmembrane domain and stabilizes TrkB dimers, thus enhancing the action of BDNF.Reference Moliner, Girych and Brunello141, Reference Banushi and Polito145 These results suggest that binding to TrkB could mediate the neuroplastic and antidepressant-like effects of psilocybin in rodents.Reference Moliner, Girych and Brunello141
Neuroendocrine effects
Oxytocin is a neuropeptide that plays an important role in prosociality.Reference Marsh, Marsh, Lee and Hurlemann146 Most serotoninergic psychedelics induce a 5-HT2A-mediated increase in circulating oxytocin,Reference Holze, Avedisian, Varghese, Eckert and Liechti147–Reference Schindler, Wallace, Sloshower and D’Souza149 although psilocybin may not exhibit the same effect.Reference Ley, Holze and Arikci148 More importantly, psilocybin and other psychedelics uniquely have the potential to induce metaplastic restoration of oxytocin-mediated long-term depression in the nucleus accumbens and to reopen the critical period for social reward learning in mice.Reference Nardou, Sawyer and Song150 These neuroendocrine mechanisms could lead to renewed social reward learning, which might explain some of the context-dependent therapeutic effects of psilocybin.
Psilocybin can cause negligible increases in prolactin, TSH, ACTH, and cortisol. These increases are dose-related, and levels return to normal within 5 hours.Reference Hasler, Grimberg, Benz, Huber and Vollenweider128
Effects on cerebral metabolism and brain connectivity
Psilocybin can heighten cerebral glucose metabolism, with the most significant increases being measured in regions with high density of 5-HT2A receptors such as the frontal cortex, and anterior cingulate. Concurrently, relative cerebral blood flow is also increased in the frontal and temporal regions but decreased in the parietal region.Reference Stoliker, Egan, Friston and Razi151
Psilocybin has been shown to decrease the functional connectivity within the default mode network (DMN), a cerebral system engaged during resting states. During acute intoxication, psilocin plasma levels negatively correlate with DMN integrity.Reference Madsen, Stenbæk and Arvidsson152 One possible therapeutic mechanism is that psilocybin modulates DMN activity, which is often increased in MDD.Reference Hamilton, Furman, Chang, Thomason, Dennis and Gotlib153 Although psilocybin leads to decreased within-DMN connectivity, it is also associated with increased connectivity between the DMN and higher-order cognitive networks such as the executive and salience networks.Reference Daws, Timmermann and Giribaldi154 Interestingly, in a small imaging study, psilocybin led to a persistent decrease in connectivity between the DMN and anterior hippocampus (lasting at least 3 weeks), even as whole-brain connectivity returned to baseline.Reference Siegel, Subramanian and Perry155
Potential drug interactions
The published literature on drug–drug interactions involving psilocybin is currently limited.Reference Halman, Kong, Sarris and Perkins156, Reference Sarparast, Thomas, Malcolm and Stauffer157 Therefore, much of the knowledge about potential interactions must be extrapolated from our understanding of psilocybin’s pharmacology, case reports, and surveys of users in unregulated contexts. Data can also be found on drug–drug interactions involving other classic psychedelics that share similar pharmacodynamics (e.g. LSD, DMT, and mescaline). Clinicians can rely on shared decision-making with their patients to decide if and when a concomitant medication should be tapered before PT. To ensure safety and maximize the chances of therapeutic response, case-by-case decision-making should take into consideration the patient’s preferences, our current knowledge of drug interactions, potential problems associated with drug tapering, and comorbid medical conditions.
Clinicians and researchers must also consider that even though some drug interactions can reduce the intensity of psilocybin’s psychedelic effects, it is currently unknown if these interactions would hinder the efficacy of PT. While some data suggests that the intensity of the psychedelic experience predicts treatment response,Reference Griffiths, Johnson and Carducci60, Reference Ross, Bossis and Guss61, Reference Johnson, Hendricks, Barrett and Griffiths158, Reference Roseman, Nutt and Carhart-Harris159 other studies have not replicated this finding.Reference Gukasyan, Davis and Barrett49, Reference Ellis, Bostian and Feng56 Some data suggest that PT might be effective even if patients do not experience subjective psychotropic effects.Reference Rosenblat, Leon-Carlyle, Ali, Husain and McIntyre160 Exciting research is currently underway to understand PT’s therapeutic mechanisms and to explore the possible consequences of drug–drug interactions during PT.Reference Olson80, Reference Cameron, Tombari and Lu161–Reference Lewis, Bonniwell and Lanham163
Antipsychotics
5-HT2A antagonism is a major mechanism of action of second-generation antipsychotics (e.g. olanzapine, risperidone, aripiprazole, etc.), and some first-generation antipsychotics such as chlorpromazine also have significant antagonist effect on this receptor. When taken concomitantly with psilocybin, 5-HT2A antagonists will greatly reduce the intensity of the psychedelic experience.Reference Vollenweider, Vollenweider-Scherpenhuyzen, Bäbler, Vogel and Hell29, Reference Halman, Kong, Sarris and Perkins156, Reference Sarparast, Thomas, Malcolm and Stauffer157 Therefore, therapeutic response might be blunted if PT is performed with concomitant use of 5-HT2A antagonists.
Antidepressants
The subacute or chronic use of selective serotonin reuptake inhibitors (SSRI) or serotonin-norepinephrine reuptake inhibitors (SNRI) leads to a down-regulation of serotonin receptors. This might explain why approximately half the people taking hallucinogenic mushrooms concomitantly with SSRI or SNRI report attenuated psychedelic effects.Reference Gukasyan, Griffiths, Yaden, Antoine and Nayak164 Interestingly, results from an online survey suggest that prior exposure to SSRI or SNRI was associated with attenuated psychedelic experience up to three months after being stopped, which is far longer than the usual discontinuation period before dosing.Reference Gukasyan, Griffiths, Yaden, Antoine and Nayak164
In a small (n = 19) open-label study on patients who did not taper their SSRI before PT,Reference Goodwin, Croal and Feifel54 patients experienced significant psychedelic effects, and no serious adverse event was observed (although two participants presented hypertension and had to receive clonidine). Three weeks after PT, depression had remitted in 42% of patients, which is similar to the effect size observed in other PT studies.Reference Goodwin, Aaronson and Alvarez50 In another exploratory study in healthy controls, a short pretreatment with 20 mg escitalopram did not appear to attenuate the psychedelic effects of psilocybin, but it significantly reduced the occurrence of challenging experiencesReference Becker, Holze and Grandinetti165 and blunted the increase in blood pressure. These results further support that acute or chronic exposure to SERT inhibitors may have different effects when combined with psilocybin, and highlight the need for further studies.
Little is known about the interaction of psilocybin with bupropion or mirtazapine. Online surveys suggest that the acute subjective psilocybin experience is attenuated in approximately half of the cases combining it with mirtazapine, and in approximately a fifth of the cases combining it with bupropion. In the same survey, low rates of negative experiences are reported when combining these molecules with psilocybin.Reference Gukasyan, Griffiths, Yaden, Antoine and Nayak164, Reference Aulet-Leon, Lerche and Woolley166 Trazodone is a 5-HT2A antagonist, and case reports have shown it can reduce the intensity of the experience with psilocybin, although this might not hinder therapeutic response in PT.Reference Rosenblat, Leon-Carlyle, Ali, Husain and McIntyre160, Reference Bonson, Buckholtz and Murphy167
Chronic use of MAO inhibitors causes a downregulation of serotonin receptors, which can suppress the effect of psychedelics.Reference Barnett, Koons, Eynde, Gillman and Bodkin106 On the contrary, short pretreatment with MAO inhibitors can reduce the metabolism of psilocin, thus potentiating its perceptual effects and marginally increasing blood pressure and mydriasis.Reference Barnett, Koons, Eynde, Gillman and Bodkin106, Reference Vojtĕchovský, Hort and Safratová168 Some users have tried combining hallucinogenic mushrooms with herbal concoctions containing natural MAO inhibitors (e.g. Banisteriopsis caapi, Peganum harmala, etc.), which can potentiate the psychoactive effects of psilocybin.Reference Barnett, Koons, Eynde, Gillman and Bodkin106 These results suggest that psilocybin could safely be combined with MAO inhibitors, although such a mix could have unknown consequences on the efficacy of PT.
We could find no information about the consequences of combining psilocybin with tricyclic antidepressants (TCA). Interestingly, combining LSD with TCA could lead to a heightened LSD experience with a faster onset.Reference Bonson and Murphy169
Because psilocybin competitively modulates the activity of 5-HT receptors without increasing levels of synaptic monoamines, the theoretical risk of triggering a serotonin syndrome is low. Empirical data from small clinical trialsReference Goodwin, Croal and Feifel54, Reference Becker, Holze and Grandinetti165 and users in unregulated settingsReference Gukasyan, Griffiths, Yaden, Antoine and Nayak164, Reference Sakai, Bradley and Zamaria170 seems to confirm the very low risk of worrisome serotonin toxicity.
Psychostimulants and MDMA
Methylphenidate acts as an allosteric blocker of the dopamine transporter (DAT), while amphetamines are competitive substrates that interfere with DAT. Their common mechanism of action therefore involves an increase in synaptic dopamine. Since psilocybin has no clear affinity for DAT, NET, or D2 receptors, no worrisome CNS interactions can be predicted with psychostimulants. Nevertheless, psilocybin can increase blood pressure and heart rate, and these cardiovascular effects could be inflated if psychostimulants are added. Caution should therefore be exerted for individuals with uncontrolled hypertension or other cardiovascular conditions.
One study exploring the effects of combining MDMA with psilocybin or LSD found that low-dose MDMA decreased the risk of challenging psychological experience, with no difference in subjective level of physical discomfort.Reference Zeifman, Kettner and Pagni171 In the same study, scores for physical distress on the CEQ scale were slightly higher among people combining classical psychedelics with a medium-high dose of MDMA. In another study, combining MDMA with LSD had additive effects on blood pressure, heart rate, and pupil size, but it did not appear to alter the mind-altering consequences of LSD.Reference Straumann, Ley and Holze172
Anxiolytics
Buspirone has a high affinity for 5-HT1A and 5-HT2A/C receptors, thus it could potentially interact with psilocybin. Indeed, a small trial found that 20 mg buspirone attenuates psilocybin-induced visual perceptual changes.Reference Pokorny, Preller, Kraehenmann and Vollenweider173 Benzodiazepines are allosteric agonists of GABAA receptors. They have been safely used as rescue medications to alleviate panic reactions during PT.Reference Johnson, Richards and Griffiths82 No worrisome interaction can be predicted between anxiolytics and psilocybin, but these molecules could potentially interfere with the efficacy of PT.
Mood stabilizers and anticonvulsants
Many first-person accounts suggest that combining lithium with classic psychedelics could induce seizures (47% of online accounts, n = 62).Reference Nayak, Gukasyan, Barrett, Erowid, Erowid and Griffiths174 Although the mechanism leading to these reactions remains putative, other authors identified that this risk was further increased in patients with a personal or family history of epilepsy.Reference Simonsson, Goldberg, Chambers, Osika, Long and Hendricks175 Lamotrigine seems well tolerated and does not modulate the subjective effect of psychedelics, according to online first-person accounts.Reference Nayak, Gukasyan, Barrett, Erowid, Erowid and Griffiths174 No information could be found on the consequences of combining psilocybin with valproic acid, gabapentin, or pregabalin.
Risks and potential adverse events
Good evidence has been collected on the innocuity of psilocybin and hallucinogenic mushrooms through pre-clinical studies,109, Reference Jerome176 recent RCTs,Reference Hinkle, Graziosi, Nayak and Yaden177, Reference Yerubandi, Thomas, Bhuiya, Harrington, Villa Zapata and Caballero178 and epidemiologic data from large community samples.Reference Krebs and Johansen179–Reference Gable182 The risks associated with psilocybin or hallucinogenic mushrooms can be modulated by the set and setting, so the hazards can be very different in unregulated environments rather than in controlled therapeutic settings.
Direct toxicity
Multiple preclinical studies have shown that psilocybin and its metabolites are not neurotoxic,Reference Hernandez-Leon, Escamilla-Orozco and Tabal-Robles107, Reference Iorgu, Vasilescu and Pfeiffer183, Reference Tombari, Mundy, Morales, Dunlap, Olson and Lein184 and animal studies demonstrate that enormous doses can be delivered without causing physiological toxicity. Indeed, studies with rodents have estimated its LD50 to be around 280 mg/kg, which would only be reached if a 60 kg person ingested an astronomical 17 kg of fresh psilocybin mushrooms.Reference Jerome176, Reference Henríquez-Hernández, Rojas-Hernández, Quintana-Hernández and Borkel185, Reference Usdin and Efron186 When compared to other controlled substances, psilocybin has systematically been classified among the least harmful.Reference Gable181, Reference Gable182 To our knowledge, no deaths have convincingly been recorded because of its physiological toxicity in healthy human subjects.Reference Henríquez-Hernández, Rojas-Hernández, Quintana-Hernández and Borkel185, Reference Gartz, Samorini and Festi187–Reference Lim, Wasywich and Ruygrok189
Psychological distress during acute intoxication
The psychological effects of psychedelics can be highly variable from one episode of use to the other (even among experienced users), and the Challenging Experience questionnaire has been validated to record potential aversive reactions.Reference Barrett, Bradstreet, Leoutsakos, Johnson and Griffiths37 According to a meta-analysis of PT studies, anxiety occurred in approximately 12% of participants (with a relative risk of 3.81 compared to placebo), while paranoia or suspiciousness was recorded in only 3% (RR = 3.04).Reference Yerubandi, Thomas, Bhuiya, Harrington, Villa Zapata and Caballero178 Under the influence of psychedelics, some may experience feelings of isolation, grief, panic, or depression, and some fear losing their mind or recalling the impression of dying.Reference Barrett, Bradstreet, Leoutsakos, Johnson and Griffiths37 Although it is difficult to define the incidence of these reactions, a study suggests that 5% of individuals who have participated in experimental studies rated their experience as “very frightening” and 10% considered it “very unpleasant”.Reference Studerus, Kometer, Hasler and Vollenweider33 During PT, the occurrence of adverse psychological reactions may be limited by careful patient selection, psychological support, and a safe set and setting.Reference Ross, Bossis and Guss61, Reference Guss, Krause and Sloshower81–Reference Watts83, Reference Zinberg92
The use of hallucinogenic mushrooms in uncontrolled environments carries additional risks, particularly if high quantities are combined with other drugs, or if they are taken in an unfavorable set and setting. Distressing “bad trips,” sometimes accompanied by agitation or reckless behavior can also occur, and fatalities have unfortunately been recorded because of polysubstance use or gross disorganization. Cases of accidental falls or defenestration have been recorded,Reference Amsterdam, Opperhuizen and Brink190 and psilocybin intoxication can seriously impair the driving or handling of machinery. When poison centers are called, most cases are managed with supportive measures at homeReference Leonard, Anderson and Klein-Schwartz191 and urgent medical care is rarely required. According to a large anonymous online survey, only 0.2% of past year magic mushroom users sought emergency medical treatment,Reference Kopra, Ferris, Winstock, Young and Rucker192 while the Drug Abuse Warning Network indicates that psilocybin-related emergency visits have an incidence of only 1.9 per 100 000 in the United States.Reference Crane193 Even when consumed in the community, hallucinogens carry a very low risk of causing harm to their users or others around them.Reference Gable181, Reference Gable182
Psychosis is a medical emergency that can rarely result from psilocybin exposure. Many factors make it difficult to estimate its incidence. Clinical expertise is required to differentiate psilocybin’s expected psychedelic effects from substance-induced psychosis, which should only be diagnosed when hallucinations or delusions last longer than the intoxication, and if they are sufficiently severe to require clinical attention.194, 195 In these rare cases, reality testing is impaired, and patients typically fail to recognize that their symptoms are substance-induced.195 Fortunately, no case of psilocybin-induced psychosis has occurred in contemporary PT studies, although patients presenting risk factors for psychosis (e.g. personal or family history of psychotic disorder) were systematically excluded from these trials.Reference Hinkle, Graziosi, Nayak and Yaden177, Reference Nelson, Yuen and Wood196, Reference Yung and Nelson197 The risk of psychotic reactions might be higher in uncontrolled environments where no medical screening is offered before psilocybin exposure. Online surveys and large patient registriesReference Rognli, Heiberg, Jacobsen, Høye and Bramness198 suggest that the incidence of hallucinogen-induced psychosis is very low, even in uncontrolled settings. Most cases of hallucinogen-induced psychosis leading to hospitalization result from polyintoxication with multiple substances.Reference Barbee, Berry-Cabán, Barry, Borys, Ward and Salyer199 In a survey of investigators who had administered LSD or mescaline to 1200 non-patient research participants, a single case of a lasting psychosis was identified, and this unfortunate patient had a monozygotic twin suffering from schizophrenia.Reference Cohen200 The risk of prolonged psychotic reaction was slightly higher among patients undergoing psychotherapy with psychedelics, although the incidence according to this study was only 1.8 per 1000 patients.Reference Cohen200 Although somewhat reassuring, these statistics from old scientific literature must be regarded with caution.Reference Cohen200, Reference Novak201 Even if small, the risk of psilocybin-induced psychosis should not be viewed as trivial.
Physical adverse events
Somatic complaints are not uncommon after the intake of psilocybin or psychedelic mushrooms. According to a meta-analysis of clinical trials, headaches occur in approximately a quarter of participants (RR of 1.99 compared to placebo), while nausea is experienced by about a fifth (RR around 8.85). Dizziness occurs in approximately 5% of PT subjects.Reference Goodwin, Aaronson and Alvarez50, Reference Yerubandi, Thomas, Bhuiya, Harrington, Villa Zapata and Caballero178 The occurrence of other somatic complaints seems negligible and varies between studies. Mild transitory residual symptoms are commonly reported on the day after psilocybin intake: fatigue (experienced by 40 to 60% of subjects), difficulty concentrating (7.5 to 17.5%), lack of appetite (7.5 to 17.5%), headaches (12.5 to 37.5%) or tired legs (0 to 12.5%) are frequent dose-related side effects.Reference Studerus, Kometer, Hasler and Vollenweider33
The cardiovascular effects of psilocybin are usually mild and asymptomatic. In PT trials, peak systolic blood pressure is on average 10–15 mmHg higher with psilocybin than with placebo, while peak diastolic blood pressure is on average 5–10 mmHg higher.Reference Griffiths, Johnson and Carducci60, Reference Ross, Bossis and Guss61 Some patients may nevertheless experience more pronounced increases in blood pressure.Reference Goodwin, Croal and Feifel54, Reference Griffiths, Johnson and Carducci60 Compared to placebo exposure, peak heart rate is on average increased by only 5 bpm.Reference Griffiths, Johnson and Carducci60, Reference Ross, Bossis and Guss61 The elevation in blood pressure and heart rate usually peaks around 120 minutes, which parallels maximal psilocin plasma concentration and subjective effects. It is unclear if these cardiovascular manifestations result from psilocin’s direct physiological effects, or if they are an indirect consequence of its psychological effects. Psilocybin does not significantly increase QTc at doses around 25 mg, but dose-dependent QTc elongation can be expected at supra-therapeutic doses.Reference Dahmane, Hutson and Gobburu202 Some data suggests that chronic exposure to 5-HT2B agonists may be a risk factor for valvular heart disease, but such problems are judged to be unlikely with single-dose psilocybin treatments.79, Reference McIntyre203
Persistent effects on mood, personality, perception, and cognition
Some individuals who have used psychedelics go on to suffer from persistent negative psychological outcomes. This uncommon phenomenon has been well described among people having experimented with psychedelics in uncontrolled settings, but we could not find any case of long-term adverse events in recent PT studies.Reference Hinkle, Graziosi, Nayak and Yaden177, Reference Bremler, Katati, Shergill, Erritzoe and Carhart-Harris204 In a study conducted in the UK, a sample of individuals having suffered from psychedelic “bad trips” and persistent distress completed an online survey and were interviewed.Reference Bremler, Katati, Shergill, Erritzoe and Carhart-Harris204 Almost all responders explained having suffered from anxiety or panic attacks. Some recalled flashbacks of their acute psychedelic experience or intrusive obsessive thoughts. Feelings of isolation, derealization, sleep disturbance, distractibility, or depressive symptoms were also described. Suicidal thoughts or behaviors were even reported by some responders. As mentioned previously, risk factors for prolonged negative psychological experiences may be minimized in a therapeutic setting with adequate support through preparation, dosing, and integration. Avoiding polysubstance use and ingestion of unusually high doses also mitigates the risk of suffering from persistent negative psychological outcomes.
Some evidence suggests that psilocybin may have long-lasting effects on some personality traits, personal values, and attitudes.Reference Goldberg, Shechet and Nicholas205. Although most subjects describe these changes as positive,Reference Studerus, Kometer, Hasler and Vollenweider33 patients should be informed of these possible effects before consenting to PT. In one study, “mystical experience” during PT predicted a durable increase in the openness personality trait of healthy volunteers (n = 52), while other domains from the Big Five personality model were unchanged.Reference MacLean, Johnson and Griffiths99 Also, in an analysis of long-term follow-up questionnaires from healthy volunteers having received psilocybin, about a third reported long-lasting change in aesthetic appreciation, in their relationship with the environment and nature, in the appreciation of their own body, or relationships.Reference Studerus, Kometer, Hasler and Vollenweider33 The overwhelming majority of these responders regarded such changes as positive. In the same study, about a fifth of participants also reported a “positive change in values or world view”.Reference Studerus, Kometer, Hasler and Vollenweider33 Older studies on volunteers having tried psychedelics have also reported long-lasting subjective changes such as better self-understanding, increased tolerance to others, or enhanced appreciation of music, art, and nature.Reference McGlothlin, Cohen and McGlothlin206 A decrease in “materialistic or aggressive orientations” has also been described.Reference McGlothlin, Cohen and McGlothlin206 Results from these old studies must be regarded with caution and contemporary re-evaluation of these outcomes might be warranted. In studies where PT was offered for MDD, it is difficult to distinguish true personality changes from improvement in depressive symptoms.Reference Erritzoe, Roseman and Nour207, Reference Weiss, Ginige and Shannon208
Some authors have suggested that psychedelics could rarely trigger enduring states of “spiritual emergency”,Reference Grof and Grof209 which has been defined as “a process of deep psychological change, often involving non-ordinary states of consciousness, intense emotions, visions, and unusual thoughts […] occurring when a spiritual experience is so overwhelming as to be temporarily non-assimilable”.Reference St. Arnaud and Cormier210 A spiritual emergency is characterized by an abrupt surge of interest in spirituality or transcendental questions, and it can be experienced as overwhelming, chaotic, or destabilizing.Reference St. Arnaud and Cormier210 It has been suggested that patients may even report psychotic-like symptoms such as visual hallucinations and bizarre or unusual thoughts. Unlike most patients suffering from psychosis, those living a so-called spiritual emergency would typically have a partly preserved insight, their adaptative functioning is preserved, and they exhibit no prior history of prodrome or psychosis.Reference Grof and Grof209–Reference Johnson and Friedman211 Although the literature on this phenomenon is very limited, “spiritual emergencies” should cautiously be viewed as rare adverse events that can result from psilocybin exposure.
Sustained perceptual aberrations have been reported after exposure to psychedelic substances. For example, some individuals can reexperience perceptual symptoms that were felt during intoxication such as geometric hallucinations, palinopsia, or flashes of color. Some experience these manifestations episodically, while others have reported them continuously for years without re-exposure to hallucinogens.Reference Espiard, Lecardeur, Abadie, Halbecq and Dollfus212, Reference Halpern213 Although some find them pleasant, others experience these symptoms with vexation and discomfort. When causing significant distress or impairment, a diagnosis of hallucinogen persisting perception disorder (HPPD) can be made according to the DSM-5-TR or ICD-11 nomenclatures.194, 195 The prevalence of HPPD seems very low,Reference Halpern213 and no case could be found after exposure to psilocybin in recent trials.Reference Hinkle, Graziosi, Nayak and Yaden177
Studies have explored the long-term effects of psilocybin on cognition. Notably, a phase I study evaluated the cognitive performances of 89 healthy volunteers up to one month after receiving psilocybin 25 mg, 10 mg, or placebo.Reference Rucker, Marwood and Ajantaival214 This study found no significant change in cognitive capacities on any of the subscales used. According to a scoping review of the subject, assessments of long-term cognition or creativity after psilocybin exposure have mostly shown no change (72% of findings), while studies reporting improvements were more common than studies reporting negative outcomes (22% versus 6% of findings).Reference Bonnieux, VanderZwaag, Premji, Garcia-Romeu and Garcia-Barrera215 All these results suggest that psilocybin has no overall negative impact on cognition.
Low abuse potential
Animal studies, controlled studies in humans, and epidemiologic data accumulated since the 1950s demonstrate that psilocybin and Psilocybe mushrooms carry a very low risk of abuse. Self-administration studies on animals have shown that psilocybin only has weak reinforcing effects or mixed reinforcing and aversive effects.Reference Fantegrossi, Woods and Winger216 As stated earlier, humans taking psilocybin tend to experience an amalgam of pleasant and unpleasant effects, followed by a feeling of contentment. The sum of these subjective effects translates into a low abuse potential.Reference Bogenschutz, Forcehimes, Pommy, Wilcox, Barbosa and Strassman65, Reference Johnson, Griffiths, Hendricks and Henningfield217, Reference Carter and Griffiths218
Psilocybin does not cause physical dependence or withdrawal syndrome.Reference Passie and Halpern32, 195, Reference Johnson, Griffiths, Hendricks and Henningfield217, Reference Isbell, Wolbach, Wikler and Miner219, Reference Martin, Goldberg and Hoffmeister220 When compared to other substances of abuse, psychedelics show the smallest risk of transition from drug use to dependence.Reference Anthony, Warner and Kessler221 The Treatment Episode Datasets (TEDS) show that hallucinogens are responsible for less than 0.1% of drug abuse treatment admissions in the United States.222 No pattern of compulsive or problematic use was observed to date after subjects participated in contemporary psilocybin studies.Reference Studerus, Kometer, Hasler and Vollenweider33, Reference Goodwin, Aaronson and Alvarez50
Reproductive toxicity
Very little is known about the risks of taking psilocybin or hallucinogenic mushrooms during pregnancy or breastfeeding.223 No chromosome breakage or mutagenic activity was identified during the micronucleus test in mice at very high doses,Reference Van Went224 and a single study on mice showed no increase in birth defects after exposure to psilocin.Reference Rolsten225 Considering the paucity of data on the reproductive toxicity of psilocybin, studies have reasonably excluded pregnant or breastfeeding women.
Epistemic harm and risks associated with psychotherapeutic interventions
Psychedelic substances induce a state of vulnerability and increased suggestibility. Therefore, psychotherapeutic interpretations are at increased risk of being misleading and psychologically harmful. Furthermore, boundary violations in the therapeutic setting are possible, and cases of sexual misconduct have been reported in clinical trials with MDMA. These risks surrounding PT must be seriously considered and mitigated.
Other psychedelic substances with therapeutic potential
Other psychedelic drugs with a similar mechanism of action exhibit promising potential for the treatment of mental disorders. MDMA, ayahuasca, and DMT are discussed in other chapters, even though they share some similarities with psilocybin. Ibogaine will not be discussed here, since it has a unique mechanism of action.
Lysergic acid diethylamide (LSD)
The Swiss chemist Albert Hofmann first synthesized LSD in 1938 and serendipitously discovered its psychoactive properties in 1943.Reference Hofmann19 LSD is well known for potently inducing altered states of consciousness, and it was extensively used recreationally in counterculture movements during the 1960s and 1970s. During these decades, abundant scientific research was also conducted to explore its pharmacologic properties and its potential therapeutic use.
LSD is an ergoline derivative that binds with high affinity and activates 5HT2A receptors. It also has significant agonist activity on dopamine D2 receptors, which can produce an activating effect.Reference Passie and Halpern32, Reference Ray137, Reference Nichols226 The mind-altering effect of LSD is phenomenologically similar to that of psilocybin, although LSD has a longer duration of action lasting approximately 8–10 hours.Reference Ley, Holze and Arikci148
LSD therapy has also regained attention in the last two decades. Many small clinical trials have been conducted with LSD to alleviate anxiety and depression in patients suffering from life-threatening medical conditions.Reference Holze, Singh, Liechti and D’Souza117, Reference Gasser, Holstein and Michel227, Reference Gasser, Kirchner and Passie228 These small trials suggest that LSD therapy has the potential to rapidly and sustainably reduce anxiety and depressive symptoms in some patients. Recently, the FDA has given breakthrough therapy status to lysergide d-tartrate, an LSD salt that showed encouraging results in a phase 2B trial for generalized anxiety disorder,Reference Brooks229 although these results are currently unpublished. Low doses of the same molecule are currently being tested for ADHD in adults.230 Through a review of clinical trial registries, a recent Swiss study was found for patients with MDD,231 although its results are yet unpublished. Interestingly, many studies were conducted in the 1960s and 1970s to evaluate the efficacy of LSD for alcohol use disorder, although these studies produced mixed results and presented scientific limitations.Reference Hollister, Shelton and Krieger232–Reference Tomsovic and Edwards235
Mescaline
Mescaline is another psychedelic substance with similarities to psilocybin and LSD. This molecule is naturally present in several species of cacti, most notably the North American peyote (Lophophora williamsii).Reference El-Seedi, De Smet, Beck, Possnert and Bruhn236, Reference Vamvakopoulou, Narine, Campbell, Dyck and Nutt237 It has a long and rich history of being used as an entheogen by indigenous communities in America.Reference El-Seedi, De Smet, Beck, Possnert and Bruhn236, Reference Vamvakopoulou, Narine, Campbell, Dyck and Nutt237 After the Second World War, many psychonauts and intellectuals were introduced to the effects of mescaline, among whom Aldous Huxley famously recalled his experience in the 1954 essay The Doors of Perception. Mescaline is a phenethylamine with a chemical structure closely related to MDMA. Significant cross-tolerance can be observed between mescaline, psilocybin, and LSD, which initially hinted at their common mechanism of action. Indeed, mescaline also primarily acts as a 5HT2A receptor agonist, although with a much lower affinity than other psychedelics. This molecule also binds to 5-HT1A and central α2A adrenergic receptors.Reference Cassels and Sáez-Briones238
Mescaline produces similar mind-altering effects as LSD and psilocybin, but it has a slower onset of action, and its effects typically last between 10 to 12 hours.Reference Ley, Holze and Arikci148 Mescaline often causes nausea, which could hinder its therapeutic use.Reference Bender239 Although mescaline has also benefited from renewed popularity and interest in the scientific community, no recently published clinical trials could be found for the treatment of mental disorders. Our search of clinical trial databases only resulted in finding one Phase I study announced for the treatment of alcohol-use disorder.Reference Bender239
Author contribution
Supervision: T.S.; Writing – review & editing: T.S., A.J.D., C.M.K., M.F.; Writing – original draft: A.J.D., C.M.K., M.F.; Conceptualization: M.F.
Disclosure of Competing interest and financial support
Dr Suppes reported stock options from Psilotec outside the submitted work. The other authors report no financial relationships with commercial interests. They declare no equity ownership, profit-sharing agreements, royalties, patents, and research or other grants from private industry or closely affiliated nonprofit funds.








