INTRODUCTION
Hantaviruses belong to the genus Hantavirus, family Bunyaviridae. Infection with hantaviruses may result in several illnesses with different clinical course and prognosis. Depending on the hantavirus serotype the infection can range from asymptomatic infection to serious and fatal diseases: haemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) [Reference Schmaljohn and Hjelle1]. HFRS is endemic in Europe and HPS in North and South America. Two hantaviruses, Puumala virus (PUUV) and Dobrava virus (DOBV) have been shown to cause HFRS in Europe [Reference Brummer-Kurvenkontio2]. Bosnia and Herzegovina (B&H) has been recognized as a highly endemic region for hantavirus infections for over 50 years [Reference Hukić3]. Several HFRS outbreaks in B&H have been reported up to the present time. The first documented HFRS outbreak was reported in 1967 [Reference Gaon4], followed by two major outbreaks in the former Yugoslavia in 1986 and 1987 with more than 1000 cases recorded, the majority of which were located in B&H [Reference Gligić5]. The most important HFRS epidemic occurred in 1989, with 144 patients and a mortality rate of 4·86%. From 1989 to 1991, 55 additional cases were detected in B&H with a mortality rate of 7·3% [Reference Gligić5, Reference Markotić6]. During the war in B&H (1995), more than 300 patients, most of them soldiers exposed in the field from northeast Bosnia, were hospitalized with acute hantavirus disease due either to PUUV or DOBV as first documented by IgG and IgM ELISA [Reference Hukić7] and later confirmed by focus reduction neutralization test [Reference Lundkvist8]. To our knowledge, this was the first report of an epidemic caused by two different hantaviruses each carried by a different rodent species, which were apparently synchronistic and sympatric in a same limited but highly endemic area. The latest HFRS outbreak occurred again in 2002. The following causative agents of HFRS were confirmed: PUUV, DOBV and unidentified hantavirus serotypes responsible for 49·84%, 23·15% and 27·00% of hospitalized cases, respectively [Reference Hukić3]. These findings suggest that at least three distinct serotypes are endemic throughout the Balkans and that easily accessible tests are needed to permit a different diagnosis, given the totally different prognosis for each infection. Hantaviral infections are predominant in rural areas [Reference Lednicky9]. Asymptomatic or non-specific mild infections result in an underestimation of the number of hantavirus infections. The ratio of subclinical to clinical infection is from 5:1 to 10:1 in Europe, and the ratio could be as high as 14:1 to 20:1 for some hantaviruses [Reference Hart and Bennett10]. Occupation is a dominant risk factor, with animal trappers, forestry workers, farmers and military personnel at highest risk [Reference Zeitz11, Reference LeDuc12]. Epidemiological investigations have linked viral exposure to activities such as heavy farm work, threshing, sleeping on the ground, military exercises, and lower socioeconomic status [Reference Hart and Bennett10, Reference LeDuc12].
Presently, data about the seroprevalence of Hantaan virus (HTV) in the general population or in different risk groups of people at high risk of exposure to hantaviruses are still required. Several outbreaks have opened up the necessity of determining to what extent public health is affected by hantavirus presence in order to achieve reasonable coverage and monitoring of hantavirus infections.
The aim of the study was to document the prevalence of hantavirus antibodies in human population-based samples from previously documented endemic and non-endemic regions in B&H and to assess the seroprevalence of hantavirus in different risk groups from the general population.
METHODS
The study included a total of 1331 volunteers without previous history or symptoms related to HFRS. The study population was selected from various geographical regions in B&H including seven cantons in the Federation of B&H (Tuzla, Herzegovina-Neretva, West Bosnia, Zenica-Doboj, Una-Sana, Posavina and Sarajevo cantons) and the Republic of Srpska. The endemic region was defined as the area with previously documented HFRS outbreaks, mainly located in central and north-eastern Bosnia and including mainly rural areas from Tuzla, Zenica-Doboj and Central Bosnian Canton (Fig. 1). The study included 741 subjects from the endemic region, 499 females (67·3%) and 242 (32·7%) males. Mean age (±s.d.) of the population from the endemic region was 42·84±16·53 (range 6–83) years. The non-endemic region population included 590 subjects, 378 (64·1%) males and 212 (35·9%) females. Mean age of subjects from the non-endemic region was 47·49±13·58 (range 18–83) years. Subjects were selected randomly both from rural and urban areas and different geographical regions of B&H including central, western and north-eastern parts of mostly mountainous, forest region with a modified Pannonia or Alpine climate, and the southern region characterized by valleys and hilly terrain with a modified Mediterranean climate (Fig. 1).

Fig. 1. Geographic map of Bosnia and Herzegovina showing the distribution of the study population. 1, Tuzla Canton; 2, Zenica-Doboj Canton; 3, Sarajevo Canton; 4, Herzegovina-Neretva Canton; 5, West Bosnia Canton; 6, Una-Sana Canton; 7, Posavina Canton; 8, Republic of Srpska; ![]() , endemic region.
, endemic region.
With particular interest in assessing hantavirus seroprevalence in occupational risk groups all participants were asked to complete a questionnaire and identify their occupation. Farmers, forestry workers and former soldiers who actively served the military during the war in B&H (1992–1996) were classified as being in the ‘occupational risk group’.
Our serosurvey included 93 former soldiers free from symptoms or history related to hantaviral infections. Former soldiers were subjects who served in the military in the endemic region during the war in B&H in 1992–1996 and were therefore classified as a separate subgroup with two hantavirus risk factors: occupation and high-risk region (endemic). In the non-endemic region group 198 subjects reported their occupation as farmers, forestry workers and soldiers and were therefore classified as ‘occupational risk group from non-endemic region’. We classified all participants in two groups, i.e. endemic and non-endemic regions:
Endemic region
(1) General population included 648 subjects; 160 (24·7%) males and 488 (75·3%) females with mean age 43·8±17·2 (range 6–83) years.
(2) Former soldiers included 93 subjects with mean age 36·3±8·7 (range 21–63) years. There were 82 (88·2%) males and 11 (11·8%) females.
Non-endemic region
(1) General population included 394 subjects; 248 (62·9%) males and 146 (37·1%) females with mean age 48·6±14·0 (range 18–83) years.
(2) Occupational risk group included 196 subjects; 130 males (66·3%) and 66 females (33·7%) with mean age 45·3±12·5 (range 19–82) years.
Sera samples were collected prospectively from subjects attending healthcare centres for various reasons during the average period of 3 months in the years 2005–2006, with the exception of sera from the former soldiers group which were collected in 2000. Blood was drawn into tubes without additives and aliquots of serum samples were frozen and held at −70°C until analysis. Sera were taken according to the ethical regulations in B&H and written informed consent was obtained from all participants included in the study.
All sera samples were examined for the presence of antibodies reactive with the DOBV, PUUV, HTV and Seoul (SEO) antigens by using IgG ELISA tests and Western blot test.
IgG enzyme-linked immunoassay
Anti-hantavirus IgG antibodies were detected using the commercially available enzyme immunoassay kit (Focus Technologies, USA). The Focus kit uses a pool of baculovirus-recombinant N-truncated protein from several hantaviruses as the antigen. The protocol described in the kit manual was followed. Briefly, serum samples from patients were diluted 1:100 and incubated for 1 h at room temperature in antigen-coated, 96-well plates. Peroxidase-coupled anti-human IgG was used as the secondary antibody, and was incubated with the plates for 30 min at room temperature. The substrate was reacted and colour development was assessed by measuring absorbance at 450 nm.
Bunyavirus immunoblot
All serum samples were additionally analysed by recomLine Bunyavirus immunoblot IgG (Microgen, Germany). The Bunyavirus immunoblot contains recombinant antigens, i.e. combined PUU/Sin Nombre+HTV/DOB/SEO virus antigens, separate antigens of PUU, HTV, DOB and SEO virus antigens and has previously been found to be sensitive (90%) and specific (100%) in hantavirus diagnostic external quality assurance and in the diagnosis of nephropathia epidemica [Reference Biel13].
Statistical analysis
Data are presented as percentages or as mean±standard deviation. Differences in proportions were tested using χ2 test or Fisher's exact test where appropriate in SPSS for Windows (version 16.0; SPSS Inc., USA).
RESULTS
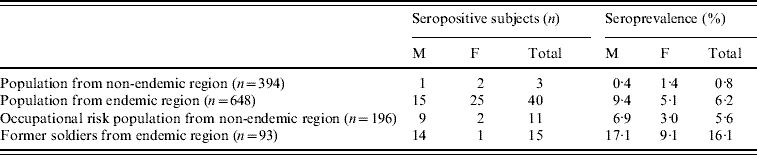
There were 55/741 hantavirus-seropositive subjects from the endemic region and 14/590 from the non-endemic region. Hantavirus seroprevalence in the overall population from the endemic region was 7·4% which was significantly higher than in the population from the non-endemic region (2·4%) (χ2=17·03, P<0·001) (data not shown).
In the general population from the endemic region, 40 sera were found to be hantavirus positive, with a seroprevalence of 6·2%. The mean age of seropositive subjects from the general endemic population was 49·9±16·7 (range 11–79) years.
Former soldiers from the endemic region had the highest hantavirus seroprevalence; 15/93 tested soldiers were seropositive and the seroprevalence was 16·1%. The mean age of seropositive former soldiers was 36·27±7·7 (range 24–49) years.
In the overall population from the non-endemic region regardless of occupational risk factors, the seroprevalence was highest in the Herzegovina-Neretva Canton (4·4%), while hantavirus seroprevalence was lower in the Republic of Srpska (1·9%) and West Bosnia Canton (2·1%).
Hantavirus seroprevalence in the occupational risk group from the non-endemic region (5·6%) and in the general population from the endemic region (6·2%) was significantly higher compared to seroprevalence in the general population from the non-endemic region (0·8%) (χ2=13·29, χ2=18·14, respectively, P<0·001). The mean age of seropositive subjects in the general population from the non-endemic region was 44·3±21·5 (range 22–65) years.
In the former soldiers group from the highly endemic region the seroprevalence was highest (16·1%), and significantly higher compared to the general population from both endemic and non-endemic regions and the occupational risk group from the non-endemic region (P<0·01) (Table 1).
Table 1. Hantavirus seroprevalence in general and risk groups from endemic and non-endemic regions
M, Males; F, females.
Hantavirus seropositivity was more frequent in males from the endemic region group (χ2=3·76, P=0·052) (Table 1) while no significant difference in hantavirus seroprevalence between gender in other study groups was observed. In occupational risk group from the non-endemic region hantavirus-seropositive farmers and forestry workers were equally present (Table 2). The mean age of seropositive subjects was 50·55±12·64 (range 32–72) years.
Table 2. Hantavirus seropositive subjects and occupation in population from the non-endemic region

There was no significant difference in hantavirus seroprevalence in age groups. Hantavirus seroprevalence was 10% in the 55–74 years and 8·3% in the ⩾75 years age groups in the general population from the endemic region. In children from the endemic region three females (9·1%) tested seropositive with a mean age of 13·0 (range 11–14) years (Fig. 2).

Fig. 2. Frequency of hantavirus-seropositive subjects in age groups in the population from the highly endemic region, non-endemic region, former soldiers group and occupational risk group.
In the overall population from the endemic region PUUV seroreactivity was observed in 80% of seropositive subjects, while PUUV and DOBV seroreactivity was equally present (50% for each) in the overall population from the non-endemic region. In the overall population from the endemic region the seroprevalence of PUUV was 5·97% and DOBV 1·5%, while in the population from the non-endemic region the seroprevalence of PUUV and DOBV was 1·2% (Table 2).
Table 3. Seroprevalence of Puumala (PUUV) and Dobrava (DOBV) in general and risk population from endemic and non-endemic region

Out of 40 hantavirus-seropositive subjects from the endemic region 33 (82·4%) subjects were PUUV seropositive and seven (19·6%) were DOBV seropositive with a significant predominance of PUUV compared to DOBV serum reactivity (χ2=6·2, P<0·05) (Fig. 3). In the former soldiers group PUUV seroreactivity accounted for 73·33% of seropositive former soldiers (Fig. 4). In the occupational risk group from the non-endemic region PUUV seroreactivity accounted for 63·6% and DOBV for 36·4% seropositivity (Fig. 5), while in the general population from the same region all three subjects were DOBV seropositive.

Fig. 3. Puumala (□) and Dobrava (![]() ) seropositive subjects from the endemic region by age group.
) seropositive subjects from the endemic region by age group.

Fig. 4. Puumala (□) and Dobrava (![]() ) seropositive former soldiers from the endemic region by age group.
) seropositive former soldiers from the endemic region by age group.

Fig. 5. Puumala (□) and Dobrava (![]() ) seropositive subjects with occupational risk from the non-endemic region by age group.
) seropositive subjects with occupational risk from the non-endemic region by age group.
Although no significant difference in PUUV compared to DOBV seroreactivity in age groups was observed, PUUV-seropositive subjects were more frequent in age groups >15 years in the general population and in the former soldiers group from the endemic region. DOBV-seropositive subjects were more frequent in the 0–14 years age group (Figs 3, 4). In the occupational risk group PUUV-seropositive subjects were more frequent in the <55 years age group, whereas DOBV-seropositive subjects were more frequent in the 55–74 years age group (Fig. 5).
DISCUSSION
This study demonstrated, for the first time, hantavirus seroprevalence in B&H and confirmed that B&H is Europe's hotspot for hantavirus infections. Hantavirus seroprevalence in B&H was found to be 2·4% in the overall population from the non-endemic region and 7·4% in the overall population from the endemic region.
Our results on seroreactivity in the population from B&H are considerably higher compared to other European countries. In Austria, a serosurvey on internal medicine patients revealed an overall prevalence of 1·2% (n=1215), ranging from 0·2% to 1·8% in different areas of the country [Reference Aberle14]. A serosurvey in Slovakia demonstrated PUUV and/or HTV-IgG in 0·84% sera of the average population (n=2133), ranging from 0·54% (western/central) to 1·91% (eastern) [Reference Sibold15]. In residents of Germany the overall hantavirus-specific seroprevalence was about 1·63% [Reference Zollër16], 0·5% in Switzerland [Reference Schultze17], 0·9% for The Netherlands [Reference Groen18], 1·6% for Belgium [Reference Van der Groen19], <1% for France [Reference Mailles20] and 4·0% for Greece [Reference Papadimitriou and Antoniadis21].
However, higher prevalence was recorded in Estonia and Finland. In Estonia 2·1–8·1% of blood donor sera were positive for IgG antibodies against three hantavirus serotypes [Reference Golovljova22] and in Finland in 0–12% of subjects were positive; depending on the region [Reference Brummer-Kurvenkontio2].
Unexpectedly, our study indicated that the southern part of B&H, alongside Neretva river valley, previously considered as a non-endemic region, appeared to be the region with a high hantavirus seroprevalence, confirmed in 4·4% of residents. A serosurvey conducted in 1989 in the Sarajevo region revealed the presence of specific IgG antibodies to hantaviruses in 2·51% of tested persons (A. Markotić unpublished observations).
In the endemic region, which is located in north-eastern part of the country, several HFRS outbreaks have been reported to date. The most recent outbreaks occurred during the war in B&H in 1995 affecting 300 subjects, mostly soldiers [Reference Hukić3]. The outbreak escalated again in 2002, when more than 300 cases with acute hantavirus disease were hospitalized [Reference Hukić7].
The seroprevalence of hantavirus in healthy persons without previous symptoms of hantaviral disease in the endemic region ranged from 6·2% in the general population, mostly from suburban areas, up to 16·1% in former soldiers from the endemic region.
The high seropositivity in former soldiers who actively served in the military during the war in B&H is no surprise. They were exposed to several different risk factors: military action in the endemic region during the HFRS outbreak with an increased density of the rodent population. Transmission of hantavirus occurs mainly through contact with infected animal excreta as well as through the aerosol [Reference Zhenqiang23]. Exposure to rodents has been confirmed as the most important risk factor for developing hantavirus disease and seems an unavoidable aspect in the life of the soldier at war.
Even exercises imitating war conditions can put the soldier at risk: the most important cluster of hantavirus disease in Americans abroad was reported in U.S. soldiers exercising in January 1990 in southern Germany and camping under tent in a mice-infested area. Within 2 weeks, 24 acute PUUV infections were documented, and 14 soldiers had to be hospitalized with varying degrees of acute renal failure (no deaths), whereas no outbreak occurred in the civilian population of the surrounding area [Reference Clement24].
In the non-endemic region the seroprevalence was significantly lower in the general population, mostly from urban areas compared to professionally exposed subjects (0·8% vs. 5·6%). Our study revealed no difference in seropositivity between farmers, forestry workers and soldiers from the non-endemic region. The probable reasons for relatively low seroprevalence of hantavirus in soldiers from non-endemic regions could be due to their young age at the time of recruitment (18–24 years) and no previous exposure to occupational risk for a long period of time, and lack of usual field training because of the danger from mines. Similar results were obtained in Slovakia with significantly more positive sera documented from forestry workers (5·88%) than those from the general population of eastern Slovakia [Reference Sibold15] and in Germany between professionally exposed forest workers and the general population [Reference Zollër16]. By contrast, two independently conducted studies in Germany revealed only minor differences between forestry workers and the general population [Reference Ulrich25, Reference Martens26].
The pattern of age-related seroprevalence rates in our study is similar to those from other European studies [Reference Ahlm27], with the highest prevalence for the 55–74 years age group. The general population from the endemic region and the occupational risk group from the non-endemic region had similarly high seroprevalences of 10·0% and 10·3%, respectively. In Finland a seroprevalence up to 30% has been found in elderly subjects [Reference Brummer-Kurvenkontio2]. Antibodies against hantavirus remain detectable for decades after infection [Reference Settergren28] and, therefore, a progressive increase in seroprevalence can be expected.
Although seroprevalence rates in children aged <15 years are low in Europe [Reference Piechotowski29], in our study 9·1% of children from the endemic region in B&H were seropositive which might reflect the highest rate reported in Europe. Seropositive rates similar to ours were observed only in children from South America [Reference Armien30]. It is difficult to offer reasons which might have influenced such a result. The seropositive children are mostly from rural regions; however, we must consider the lower hygienic conditions during the war, as well as in the post-war period when some of the children entered or re-entered endemic regions devastated by war.
The current study found no significant difference in seroprevalence by sex, inaccord with other reports [Reference Ahlm27, Reference Niklasson31], although there was a trend towards significantly higher seroprevalence in males from the endemic region (P=0·052).
PUUV is the most common cause of HFRS in Europe. According to previous studies, PUUV seroprevalence is ~5% in Finland, 5–9% in Northern Sweden, and 2% in Estonia [Reference Brummer-Kurvenkontio2, Reference Ahlm27, Reference Lundkvist32]. PUUV infections have also been diagnosed and reported as the causative agent of HFRS in the endemic region in B&H, responsible for 49·8% of HFRS cases [Reference Hukić3]. Our study showed that PUUV seroprevalence in the overall healthy population from the endemic region is almost 6%, while DOBV seroprevalence in the endemic and non-endemic regions was 1·5% and 1·2%, respectively. Reports from many regions of Eastern Europe and the Balkans showed that DOBV is a hantavirus serotype present in these regions and in some cases responsible for a more severe form of HFRS [Reference Lundkvist8, Reference Antoniadis33–Reference Golovljova35].
Hantavirus infections are widely distributed in Europe with the exception of the far north and the Mediterranean regions. Over the past few decades, the understanding and recognition of hantaviral infections throughout the world has greatly improved. Given the unclear clinical picture and the benign clinical symptoms in a number of patients, some cases escape the surveillance systems. Environmental changes may affect the geographic distribution, abundance, and dynamics of the rodent carrier, and hence the epidemiology of hantavirus infections [Reference Clement24].
Well-known natural hosts of hantaviruses (Apodemus flavicolis and Myodes glareolus) are the most widely spread species of small rodents, and the increase in their population is closely related to outbreaks of epidemics of HFRS. During the epidemic years in B&H, the average monthly temperatures in February were 4·3 times higher than the average temperatures during the non-epidemic years, which may have influenced the early reproduction of rodents and development of ‘mouse years’. In B&H several different rodent species have been detected so far: A. flavicollis, A. sylvaticus, A. agrarius, M. glareolus and Pytimus subterraneus. M. glareolus was found to be predominant in regions with an altitude >1160 m and Apodemus spp. in regions with an altitude <670 m [Reference Hukić3]. The rodent population density changes seasonally and cyclically. During the epidemic years the rodent population density was marked as very high, whereas during the non-epidemic years it was designated as low to moderate. The proportion of humans infected with PUUV and DOBV correlated with the number of natural hosts of hantaviruses in the areas of HFRS outbreaks [Reference Hukić3].
High hantavirus seroprevalence in the population from B&H especially in high-risk groups implies the need for better and more efficient ways to control hantaviral diseases, preferably by reducing human exposure to infected rodents and their excreta. Monitoring the hantavirus prevalence in the rodent population and rodent population density can prevent more HFRS cases.
ACKNOWLEDGEMENTS
The study was supported by the Federal Ministry of Science and Education in Bosnia and Herzegovina.
DECLARATION OF INTEREST
None.










