Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [Reference Xu1]. The World Health Organization (WHO) claims that COVID-19 has become a global pandemic on 11 March 2020 [2]. As of 30 October 2020, a total of 44 888 869 confirmed cases were reported globally, of which 1 178 475 cases had resulted in mortality [3].
The previous study showed that comorbidities in patients with COVID-19 are risk factors for adverse outcomes and cerebrovascular disease was associated with severe COVID-19 disease, which needs to be monitored in the intensive care unit (ICU) care [Reference Wang4]. A meta-analysis [Reference Pranata5] suggested that cerebrovascular disease was associated with the increased poor composite outcome (RR = 2.04, 95%CI: 1.43 to 2.91, P < 0.001) and another meta-analysis [Reference Xu6] showed similar results. However, the existing meta-analyses only incorporated a small number of samples and most of the studies synthesised came from China. To date, there is still limited evidence regarding the concomitant association between nervous system diseases and COVID-19. Therefore, to address this gap in the literature, it is necessary to conduct a comprehensive meta-analysis. The purpose of this study was to clarify the association between nervous system diseases and severity or mortality in patients with COVID-19.
Methods
To ensure the high quality of our work, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to conduct our study [Reference Liberati7]. We registered this review protocol in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020180567).
Eligibility criteria
We included case−control studies and cohort studies that met the following criteria: (1) patients were diagnosed with COVID-19 by a laboratory test or according to the World Health Organization interim guidance [8]; (2) reported data of pre-existing nervous system diseases, such as cerebrovascular disease, stroke and epilepsy between patients with severe and non-severe illness or between non-survivors and survivors; (3) published in English and Chinese.
We excluded studies with the following characteristics: (1) studies with a sample size of fewer than 20 patients; (2) studies did not report data related to nervous system diseases (e.g. cerebrovascular disease, stroke); (3) studies focused on only suspected cases or confirmed cases and suspected cases; (4) without comparisons (e.g. non-survivors vs. survivors); (5) review articles, protocols, guidelines, consensus, comments, abstracts, letters and editorials.
Literature search
We comprehensively identified all potentially relevant articles through a systematic literature search of the electronic databases: PubMed, EMBASE.com, Web of Science and the Cochrane Central Register of Controlled Trials (CENTRAL). The searches were first performed on 8 May 2020 and updated on 10 October 2020. According to the indices of various databases, we used search terms as follows: ‘COVID-19’, ‘coronavirus disease-19’, ‘new coronavirus’, ‘2019-nCoV’, ‘novel corona virus’, ‘novel coronavirus’, ‘nCoV-2019’, ‘novel coronavirus pneumonia’, ‘2019 novel coronavirus’, ‘coronavirus disease 2019’, ‘SARS-CoV-2’, ‘severe acute respiratory syndrome coronavirus 2’, ‘clinical characteristic’, ‘clinical feature’, ‘risk factor’, ‘prognosis’, ‘comorbidit*’, ‘cerebrovascular disease*’, ‘nervous system disease*’, ‘brain’, ‘neurologic*’, ‘stroke’, ‘cerebral infarction’, ‘dementia’ and ‘epilepsy’. The search strategy of PubMed is shown in Appendix Word 1. We manually searched the reference lists of each included paper to identify potentially eligible studies.
Study selection process
Records were managed by EndNote X8 (Thomson Reuters (Scientific) LLC Philadelphia, PA, US) software to exclude duplicates. At first, two authors independently (YG and YMC) screened the titles and abstracts of the records to determine if they met the inclusion criteria. Then, the same two authors retrieved the full text of all potentially eligible studies and assessed the eligibility of each study according to the inclusion criteria. Disagreements were resolved by discussion or by a third reviewer (JHT). When identified multiple studies from the same team or studies with samples from the same settings, we decided which study to include based on the study time frame and detailed data. For studies with overlapping data, we included studies with larger sample sizes.
Data extraction and quality assessment
We used Microsoft Excel 2019 to construct a standard form to extract research data. The data abstracted included: (1) study characteristics (first author, year of publication, journal name, publication language, country of the first author, recruitment time frame, study design, study setting); (2) population characteristics (sex, age, sample size); (3) outcomes of interest (number of nervous system diseases patients, severe cases, non-severe cases, non-survivors, and survivors). The severe disease was defined as patients with acute respiratory distress syndrome (ARDS), needing mechanical ventilation, vital life support or intensive care unit admission [Reference Liu9–Reference Gao12]. We defined nervous system diseases according to the international classification of diseases -11 (ICD-11) [Reference Shakir13, 14].
We used the Newcastle−Ottawa quality assessment scale (NOS) to assess the quality of the included studies [Reference Stang15]. Studies with more than 7 stars were regarded as high quality, 5–7 stars were regarded as moderate quality and lower than 5 stars were regarded as low quality. In our study, one reviewer (YG, YMC, ML or ZWS,) evaluated the quality of each study according to the scale and another (MLY and MMN) reviewed it. In the case of incongruity, the third researcher (JHT) was invited to discuss.
Statistical analysis
We used Stata (13.0; Stata Corporation, College Station, Texas, USA Stata) to perform all meta-analyses. We conducted pairwise meta-analyses to compute the odds ratio (OR) with 95% confidence interval (95%CI) to estimate the association between nervous system diseases and COVID-19 severity or mortality. The meta-analyses used the inverse variance method with the random-effects model to estimate the average effect. We used the I 2 statistic and Cochran's Q test to assess statistical heterogeneity. The I 2 statistic results were interpreted as <25%, 26–50% and >50%, representing low, moderate and high heterogeneity, respectively [Reference Higgins16].
Sensitivity analyses were applied by excluding studies published in Chinese to assess the stability of results. We further performed univariate meta-regression analyses to assess if the OR varied with study sample size. The funnel plot and Egger's test were used to detect publication bias for outcomes with studies no fewer than 10. The statistical level of significance was set at P < 0.05.
Results
Screening results
Totally, 16 286 records were identified through the literature search. After removing duplicates, 7360 records were excluded, and after reviewing the titles and abstracts, 8474 records were excluded. Through full-text evaluation of the remaining 452 records, 383 studies were further excluded, we finally included 69 studies [Reference Wang4, Reference Argenziano17–Reference Zou84] in our meta-analyses. The flowchart of the screening process is presented in Fig. 1.
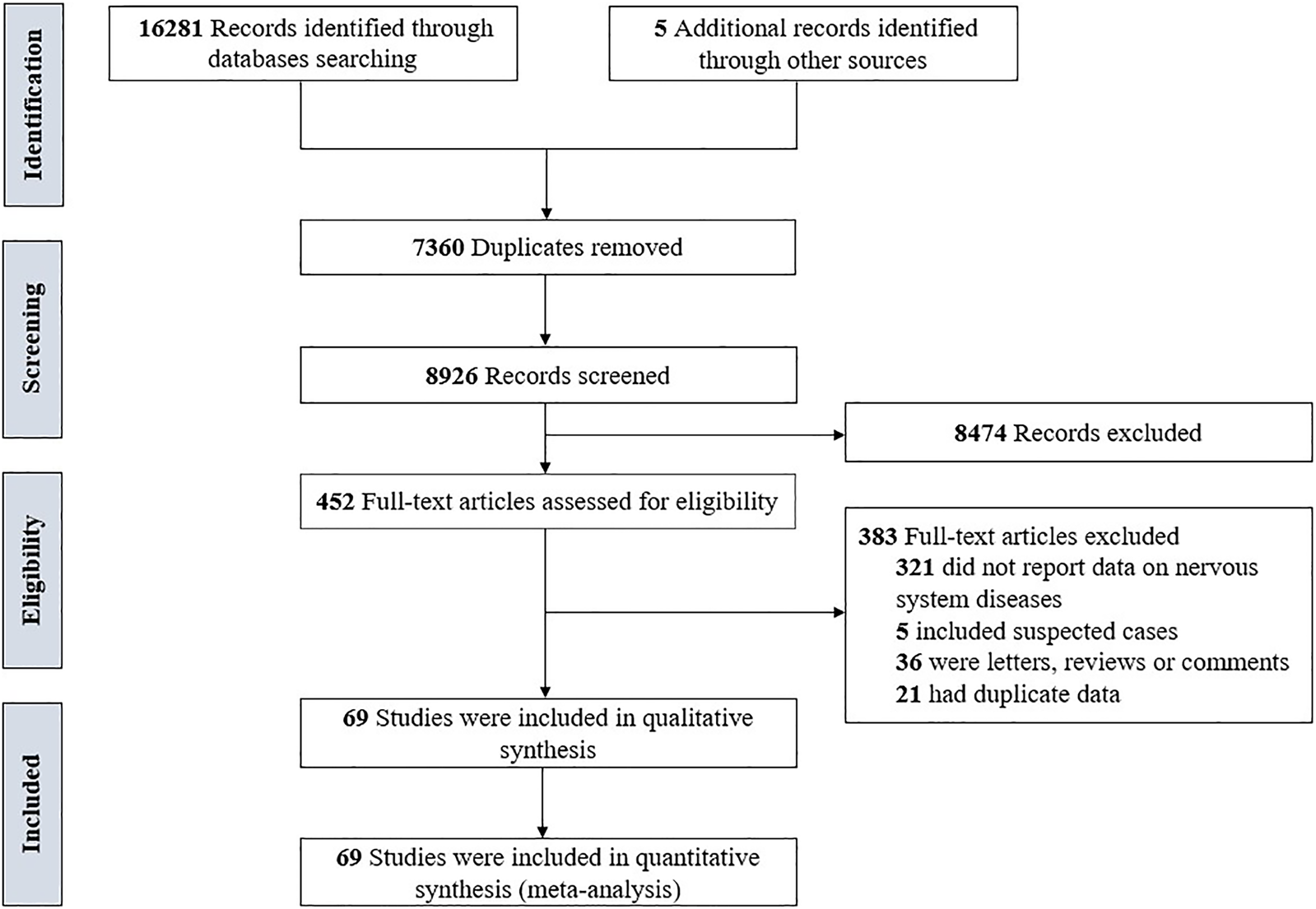
Fig. 1. The flowchart of the screening process.
General characteristics and quality of studies
All included studies were published online in 2020, incorporated patients between 11 December 2019 and 27 June 2020. In all, 68 studies [Reference Wang4, Reference Argenziano17–Reference Zheng83] were published in English and 1 study [Reference Zou84] published in Chinese. Out of which 54 studies [Reference Wang4, Reference Cao18–Reference Cheng24, Reference Feng29–Reference Gao31, Reference Guan34–Reference Huang39, Reference Lei45–Reference Lyu53, Reference Pan55, Reference Qin56, Reference Wang59–Reference Zou84] were from China, 3 studies [Reference Argenziano17, Reference Gayam32, Reference Maeda54] were from the USA, 3 studies [Reference Chon25, Reference Hwang40, Reference Lee44] were from Korea, 2 studies [Reference Colombi26, Reference d'Arminio Monforte27] were from Italy and the remaining 7 studies [Reference Dupley, Oputa and Bourne28, Reference Götzinger33, Reference Itelman41–Reference Kutluhan43, Reference Romero-Sánchez57, Reference Shabrawishi58] were from Austria, Iran, Israel, Saudi Arabia, Spain, Turkey, and UK. The sample size per study ranged from 27 to 1590 (total 17 879; 9686 males). Considering methodological quality in items of NOS scale, 23 studies [Reference Wang4, Reference Argenziano17, Reference Chen19, Reference Chen21, Reference Cheng24, Reference d'Arminio Monforte27–Reference Feng29, Reference Götzinger33, Reference Guan34, Reference Hu37, Reference Li46, Reference Liu49, Reference Pan55, Reference Shabrawishi58, Reference Wang61, Reference Wang65, Reference Wang67–Reference Wu69, Reference Yan73, Reference Yang75, Reference Yang76] were rated as high quality (>7 stars) and 46 studies [Reference Cao18, Reference Chen20, Reference Chen22, Reference Cheng23, Reference Chon25, Reference Colombi26, Reference Fu30–Reference Gayam32, Reference Han35, Reference Hu, Yao and Qiu36, Reference Huang38–Reference Lei45, Reference Li47, Reference Li48, Reference Liu50–Reference Maeda54, Reference Qin56, Reference Romero-Sánchez57, Reference Wang59, Reference Wang60, Reference Wang62–Reference Wang64, Reference Wang66, Reference Wu70–Reference Xie72, Reference Yan74, Reference Yuan77–Reference Zou84] were rated as moderate quality (5−7 stars). The detailed characteristics and quality of the included studies are summarised in Table 1.
Table 1. Characteristics of included studies
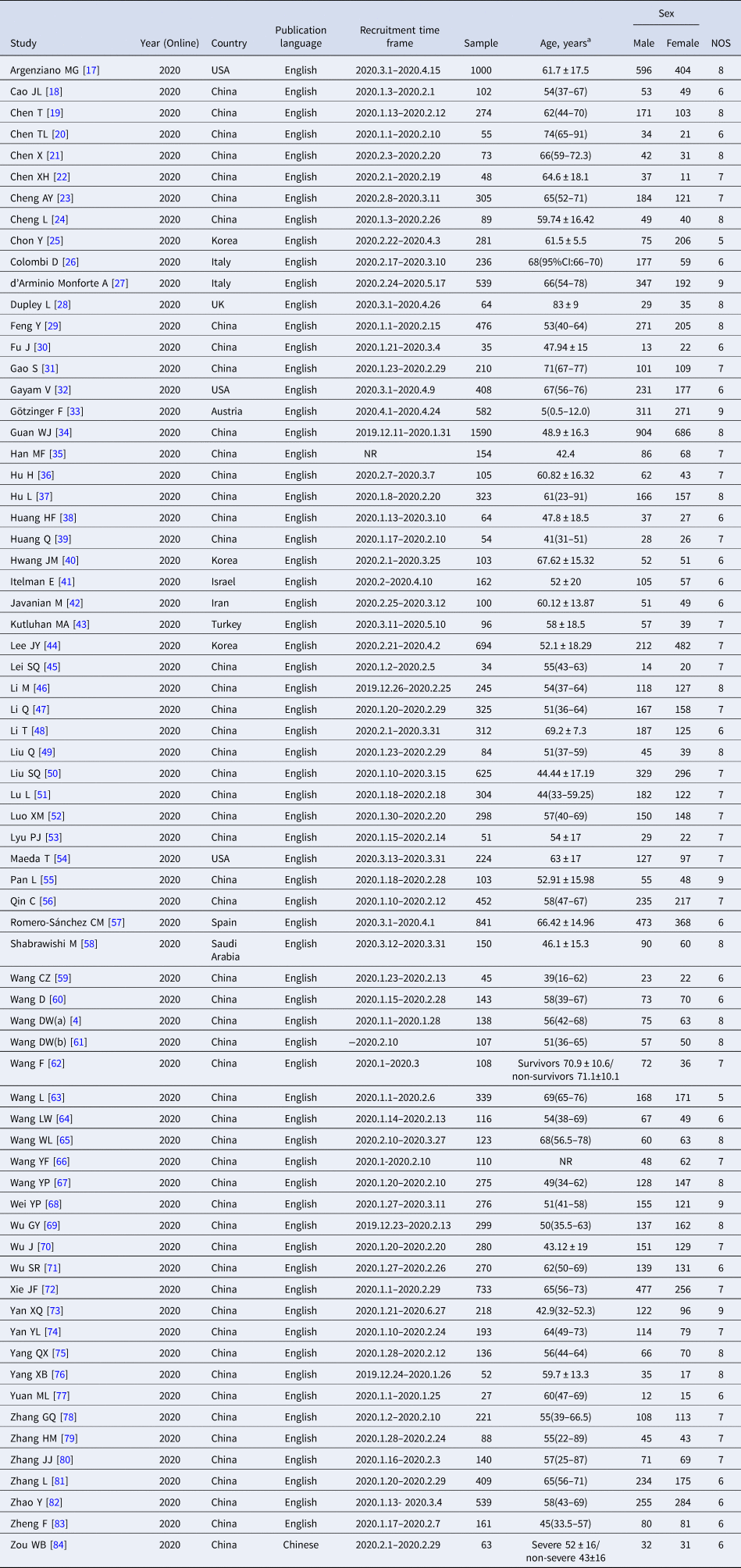
a Age data presented as median (IQR) or mean ± s.d.. NR, not reported.
Association between nervous system diseases and the severity and mortality of COVID-19
In all, 42 studies [Reference Wang4, Reference Argenziano17, Reference Chen22, Reference Chon25, Reference Colombi26, Reference Feng29, Reference Fu30, Reference Götzinger33–Reference Han35, Reference Hu37–Reference Huang39, Reference Itelman41, Reference Kutluhan43–Reference Lei45, Reference Li47–Reference Lu51, Reference Lyu53, Reference Pan55, Reference Qin56, Reference Shabrawishi58–Reference Wang60, Reference Wang64, Reference Wang66–Reference Wu71, Reference Yan73, Reference Yang75, Reference Zhang78–Reference Zhang80, Reference Zheng83, Reference Zou84] totaling 11 213 patients reported prevalence of nervous system diseases among COVID-19 patients with the severe and non-severe disease. The meta-analysis demonstrated that nervous system diseases were associated with COVID-19 severity (OR = 3.19, 95%CI: 2.37 to 4.30, P < 0.001; I 2 = 31.0%) (Fig. 2). We observed a significant association (OR = 3.19, 95%CI: 2.36 to 4.32, P < 0.001) between nervous system diseases and COVID-19 severity after excluding a Chinese study [Reference Zou84] (Appendix Fig. 1).
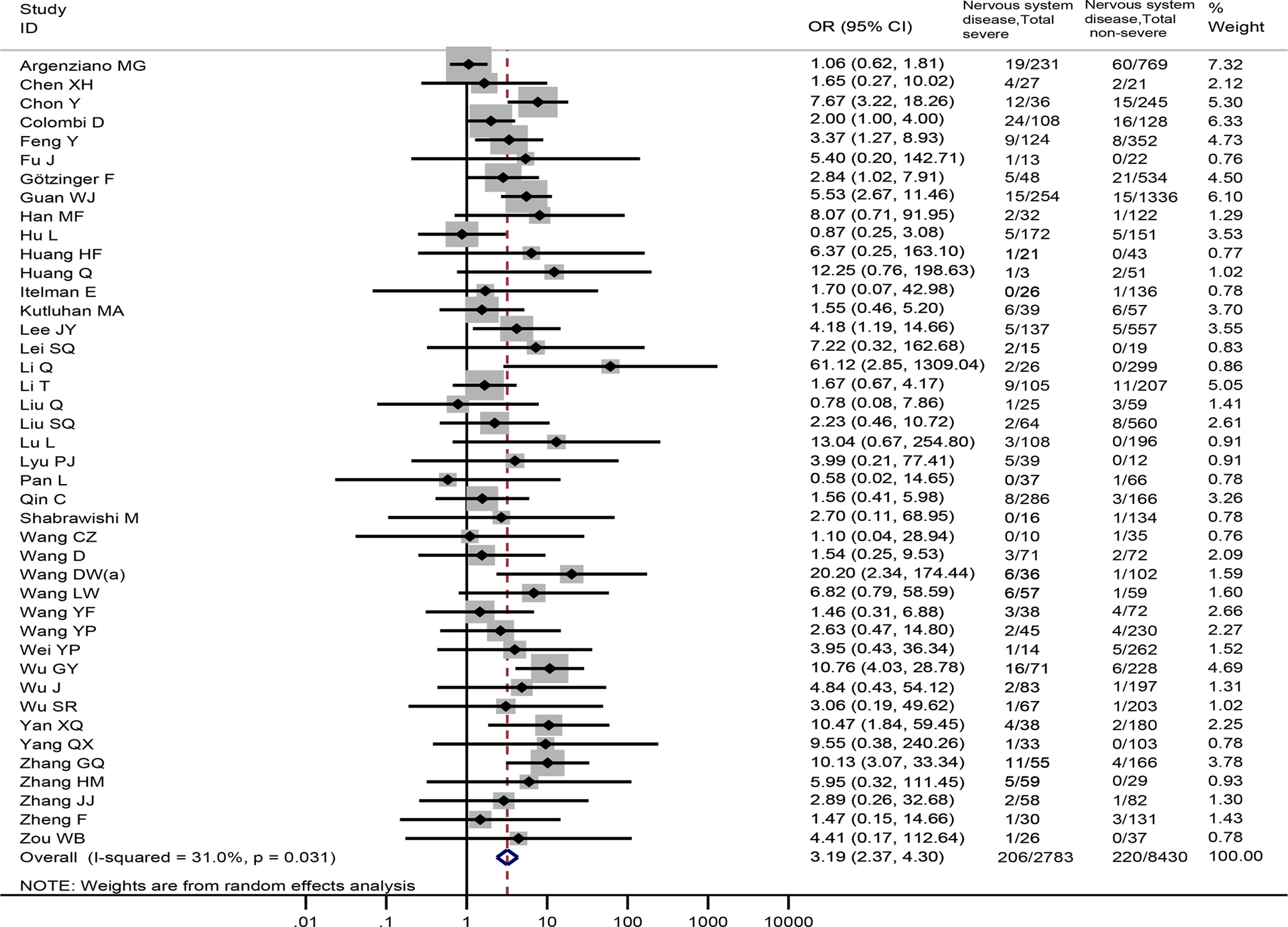
Fig. 2. Association between nervous system diseases and the severity of COVID-19.
Overall, 23 studies [Reference Cao18–Reference Chen21, Reference Cheng23, Reference Cheng24, Reference d'Arminio Monforte27, Reference Gao31, Reference Gayam32, Reference Guan34, Reference Hu, Yao and Qiu36, Reference Javanian42, Reference Li46, Reference Luo52, Reference Wang61–Reference Wang63, Reference Xie72, Reference Yan74, Reference Yang76, Reference Yuan77, Reference Zhang81, Reference Zhao82], involving 6900 patients provided nervous system diseases data between non-survivors and survivors. The result revealed that nervous system diseases were associated with a significantly enhanced risk of death (OR = 3.75, 95%CI: 2.68 to 5.25, P < 0.001; I 2 = 35.6%) (Fig. 3).
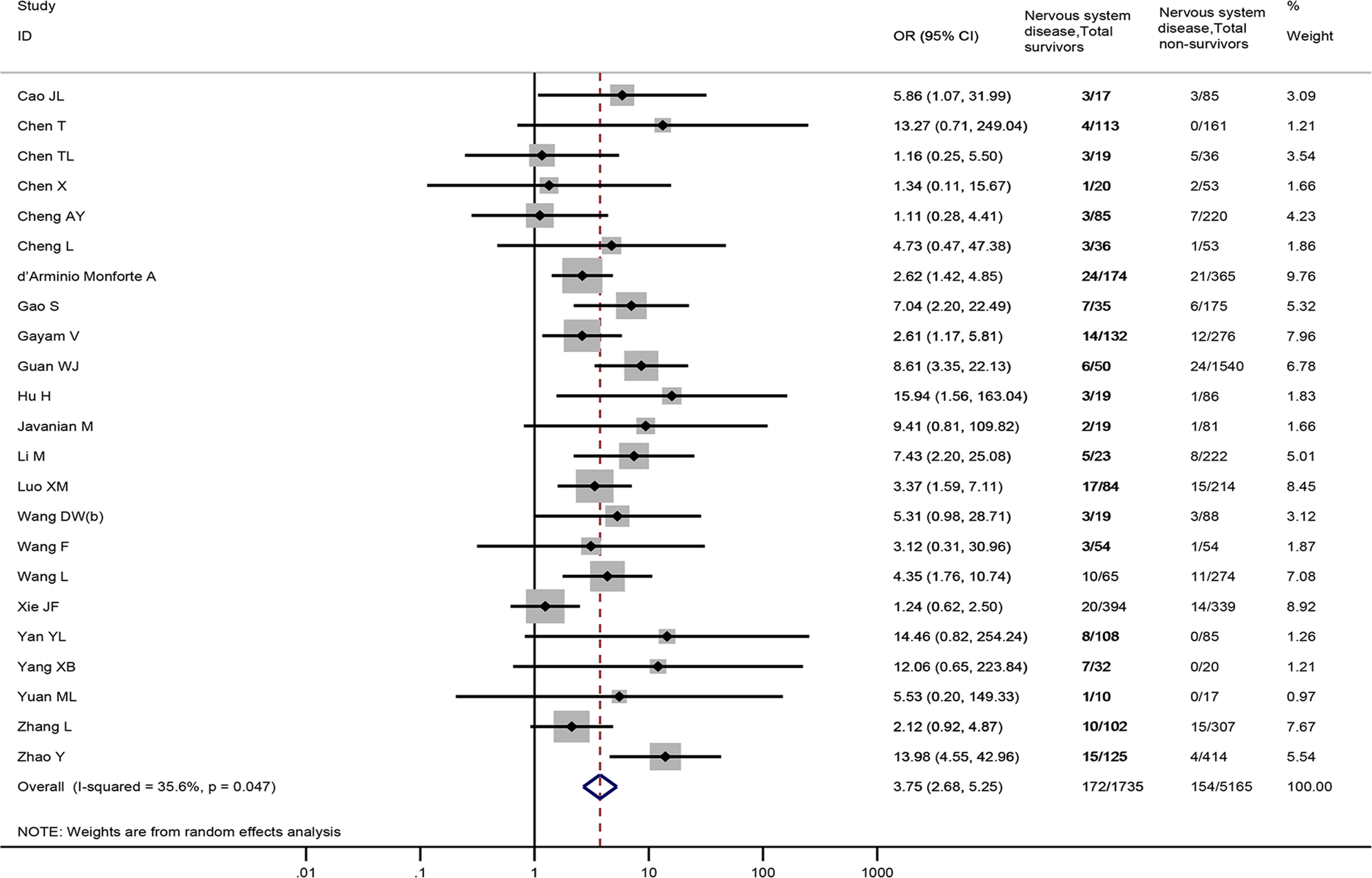
Fig. 3. Association between nervous system diseases and the mortality of COVID-19.
Association between cerebrovascular disease and the severity and mortality of COVID-19
Thirty-seven studies [Reference Wang4, Reference Argenziano17, Reference Feng29, Reference Fu30, Reference Guan34, Reference Han35, Reference Hu37–Reference Huang39, Reference Kutluhan43, Reference Lei45, Reference Li47–Reference Lu51, Reference Lyu53, Reference Maeda54, Reference Qin56–Reference Wang60, Reference Wang64–Reference Wu69, Reference Wu71, Reference Yan73, Reference Yang75, Reference Zhang78–Reference Zhang80, Reference Zheng83, Reference Zou84], totaling 10 015 samples, reported the prevalence of cerebrovascular disease between severe and non-severe COVID-19 patients. Cerebrovascular disease was observed to be associated with a significantly enhanced risk of severe COVID-19 disease (OR = 3.10, 95%CI: 2.21 to 4.36, P < 0.001; I 2 = 38.6%), Fig. 4. Sensitivity analysis by excluding a Chinese study [Reference Zou84] showed similar results (OR = 3.10, 95%CI: 2.19 to 4.39), Appendix Fig. 2.
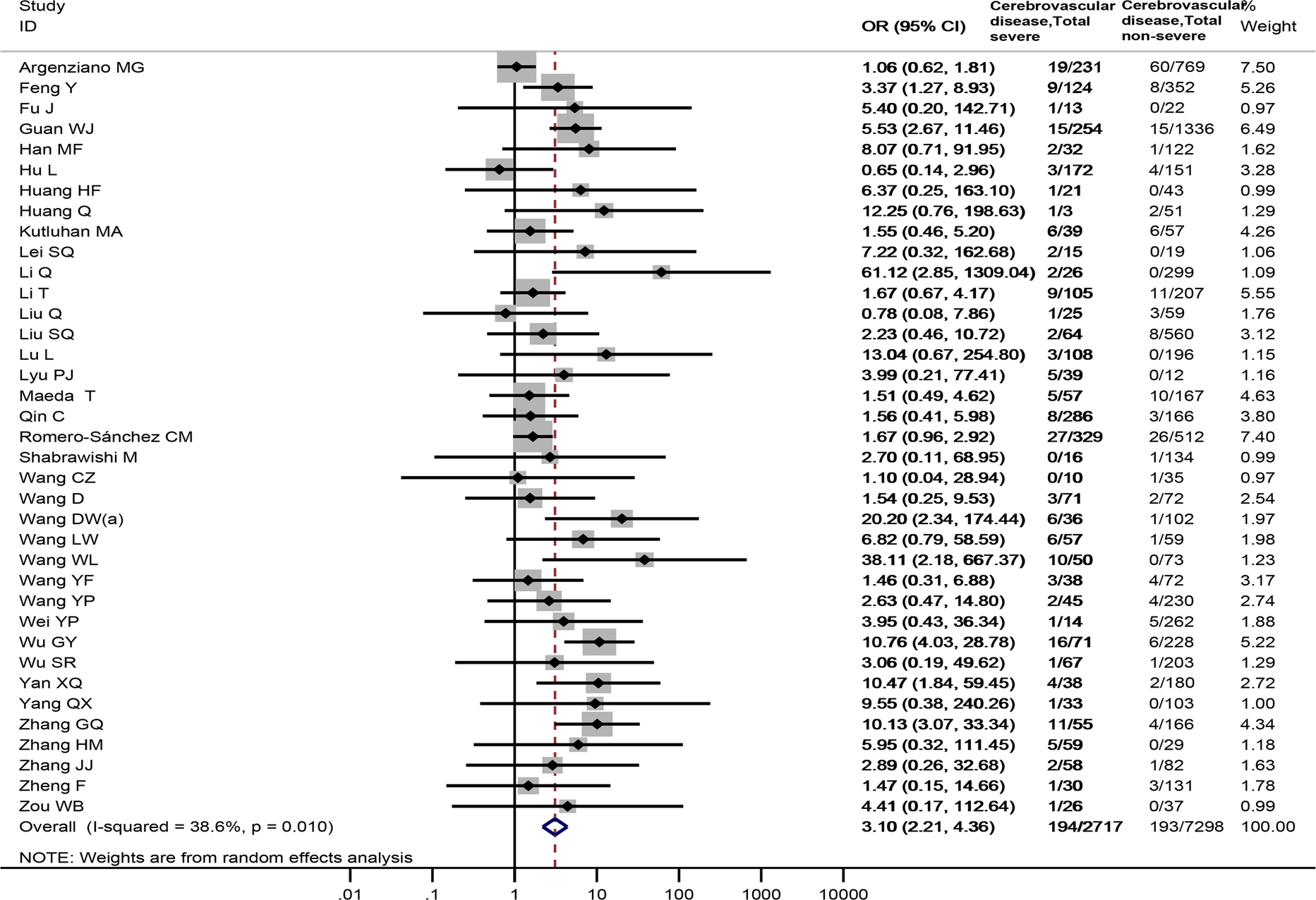
Fig. 4. Association between cerebrovascular disease and the severity of COVID-19.
Twenty-four studies [Reference Cao18–Reference Chen21, Reference Cheng23, Reference Cheng24, Reference d'Arminio Monforte27, Reference Dupley, Oputa and Bourne28, Reference Gao31, Reference Gayam32, Reference Guan34, Reference Hu, Yao and Qiu36, Reference Hwang40, Reference Javanian42, Reference Luo52, Reference Wang61–Reference Wang63, Reference Xie72, Reference Yan74, Reference Yang76, Reference Yuan77, Reference Zhang81, Reference Zhao82], including 6822 patients, reported cerebrovascular disease data between non-survivors and survivors. The meta-analysis demonstrated that cerebrovascular disease was associated with death in COVID-19 patients (OR = 3.45, 95% CI: 2.46 to 4.84, P < 0.001; I 2 = 35.2%) (Fig. 5).
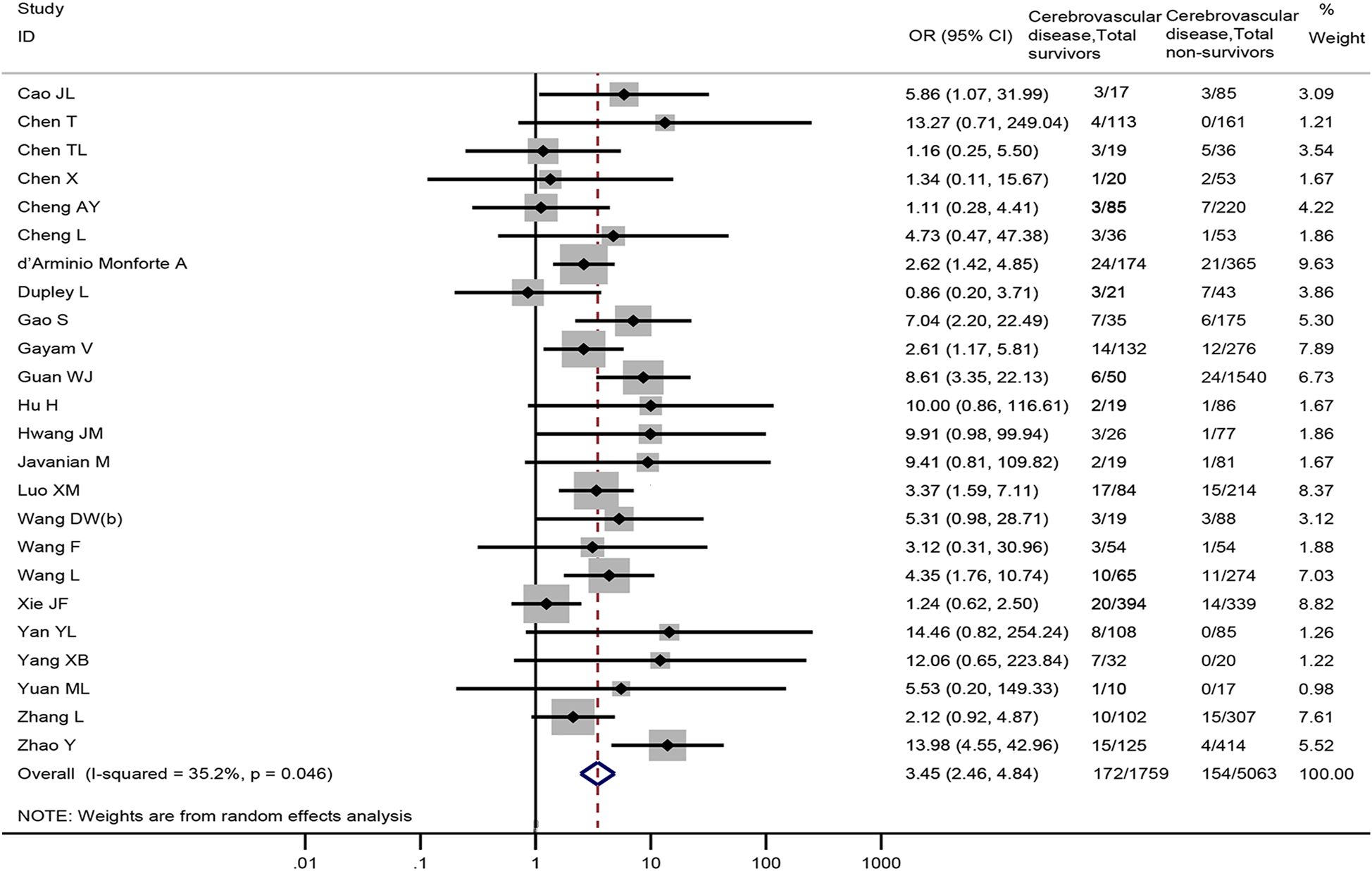
Fig. 5. Association between cerebrovascular disease and the mortality of COVID-19.
Association between stroke, epilepsy, dementia and the severity and mortality of COVID-19
As for specific nervous system diseases, our meta-analysis showed that stroke was associated with severe COVID-19 disease (8 studies [Reference Argenziano17, Reference Huang38, Reference Liu50, Reference Romero-Sánchez57, Reference Wang64, Reference Wang65, Reference Wu71, Reference Zhang80], 3178 patients; OR = 1.95, 95%CI: 1.11 to 3.42, P = 0.020; I 2 = 30.2%) (Fig. 6A). There were no significant differences in the prevalence of epilepsy (2 studies [Reference Itelman41, Reference Romero-Sánchez57], 1003 patients; OR = 1.00, 95%CI: 0.42 to 2.35, P = 0.994; I 2 = 0.0%) and dementia (3 studies [Reference Lee44, Reference Maeda54, Reference Wang65], 1041 patients; OR = 2.39, 95%CI: 0.55 to 10.48, P = 0.247; I 2 = 61.9%) between severe and non-severe patients (Fig. 6B and C).
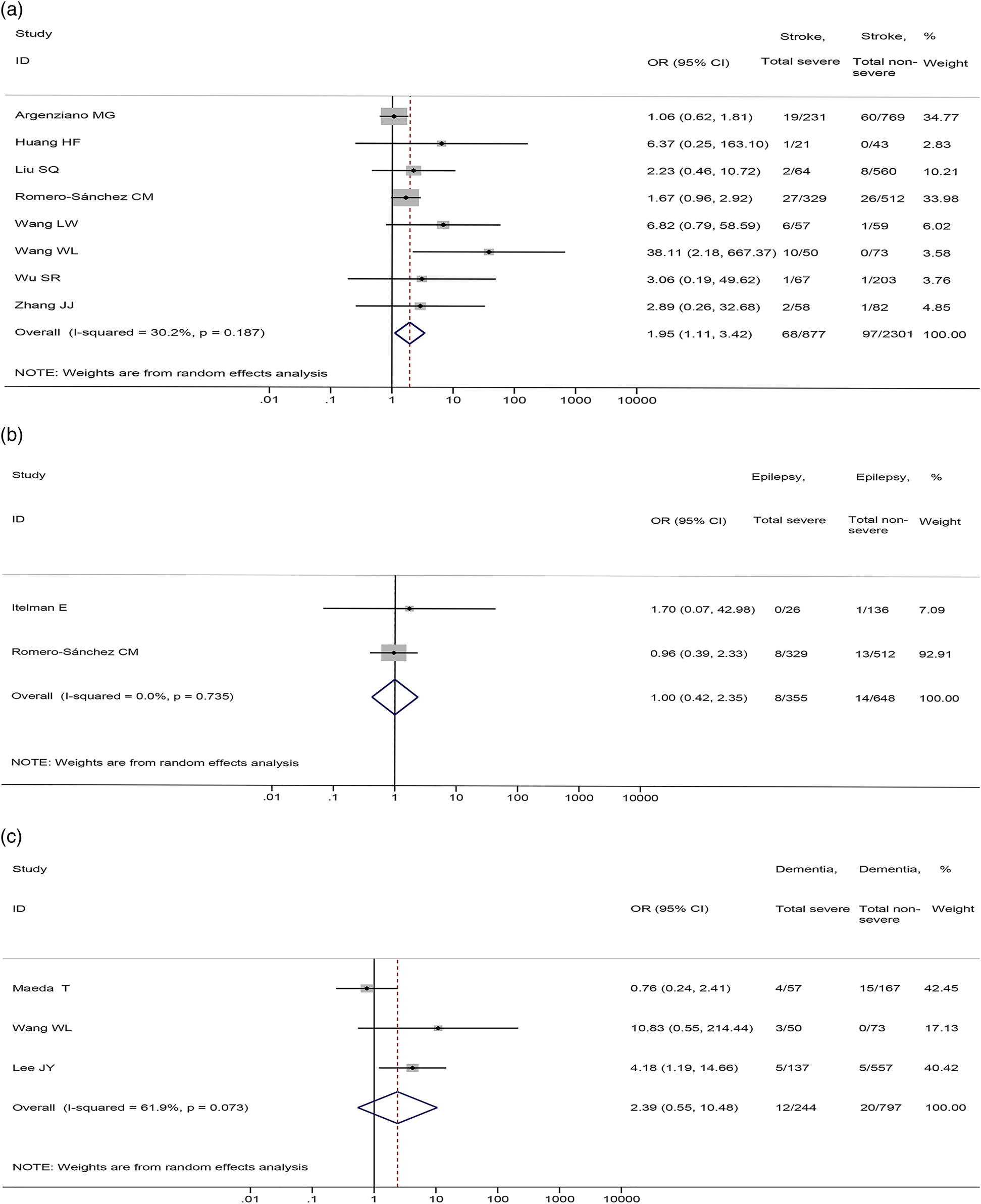
Fig. 6. Association between (a) stroke, (b) epilepsy, (c) dementia and the severity of COVID-19.
No significant differences were found in the prevalence of stroke (4 studies [Reference Chen21, Reference Hwang40, Reference Xie72, Reference Yuan77], 936 patients; OR = 1.79, 95%CI: 0.76 to 4.23, P = 0.185; I 2 = 13.0%) and epilepsy (2 studies [Reference Dupley, Oputa and Bourne28, Reference Hwang40], 167 patients; OR = 2.08, 95%CI: 0.08 to 50.91, P = 0.654; I 2 = 92.0%) between non-survival and survival patients (Fig. 7).
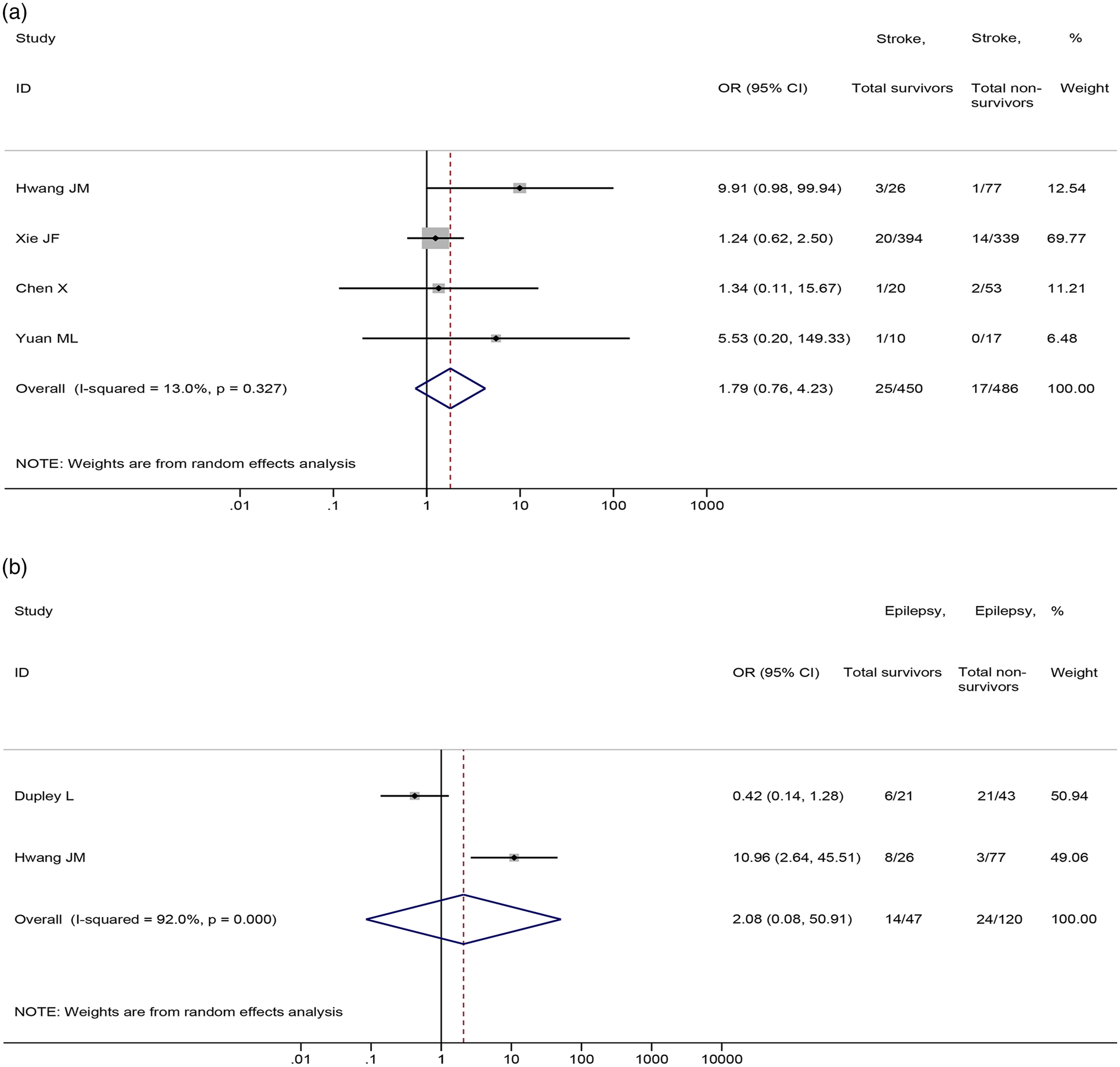
Fig. 7. Association between (a) stroke and (b) epilepsy and the mortality of COVID-19.
Meta-regression analyses
Univariate meta-regression analyses revealed that the sample size of individual study was not the source of heterogeneity or the factor affecting the association between nervous system diseases and COVID-19 severity or mortality (Appendix Figs 3 and 4) and the association between cerebrovascular disease and COVID-19 severity or mortality (Appendix Figs. 5 and 6).
Publication bias
The funnel plot and Egger's test revealed that there was no statistically significant publication bias of nervous system diseases associated with severity (P = 0.090) (Appendix Fig. 7) and mortality of COVID-19 (P = 0.061) (Appendix Fig. 8). We found that there was a possibility of publication bias for the association between cerebrovascular disease and COVID-19 severity (P = 0.011) (Appendix Fig. 9). There was no statistically significant publication bias for the association between cerebrovascular disease and COVID-19 mortality (P = 0.100) (Appendix Fig. 10).
Discussion
Principal findings
This study included 69 studies and systematically assessed the association between nervous system diseases and the severity and mortality of patients with COVID-19. Specifically, we also conducted meta-analyses to explore the association between cerebrovascular disease and severity or mortality of patients with COVID-19, as well as the association between stroke, epilepsy, dementia and COVID-19 severity and mortality. Our meta-analyses revealed that nervous system diseases were associated with severity and mortality of patients with COVID-19. Cerebrovascular disease was associated with severity and mortality of patients with COVID-19. Severe COVID-19 patients were more likely to have a stroke compared with non-severe patients. There were no significant associations between epilepsy and dementia and COVID-19 severity or mortality. Sensitivity analyses suggested that the results did not change substantially after excluding studies published in Chinese.
Comparison with other studies
A previous meta-analysis, including three studies with a total sample size of 1299, demonstrated that a significant relationship between patients with severe COVID-19 and cerebrovascular disease (OR = 3.89, 95% CI: 1.64 to 9.22, P = 0.002) [Reference Wang85]. Another meta-analysis, including seven studies involving 2585 patients, showed that cerebrovascular disease was significantly associated with severe COVID-19 disease (RR = 1.88, 95% CI: 1.00 to 3.51, P = 0.05) and five studies involving 936 patients revealed that cerebrovascular disease was associated with COVID-19 mortality (RR = 2.38, 95%CI: 1.92 to 2.96, P < 0.001) [Reference Pranata5]. Compared with these two studies, our study reached similar conclusions. However, it has distinct advantages and our results are more comprehensive. Our study meta-analysed 37 studies involving a total of 10 015 COVID-19 patients between cerebrovascular disease and COVID-19 severity, at the same time, 24 studies with a total sample size of 6822 between cerebrovascular disease and COVID-19 mortality. Therefore, our meta-analysis has the advantage of expanding the sample size and including more research studies. To the best of our knowledge, the two previous meta-analyses that included studies completely came from China. In this study, we included 15 studies from the USA, Korea, Italy, UK, Austria, Iran, Israel, Saudi Arabia, Spain and Turkey, which expanded our research scope. Another difference between our study and previous meta-analyses is that we also analysed the relationship between detailed nervous system diseases including stroke, epilepsy, dementia and the severity and mortality of patients with COVID-19. Furthermore, we also performed sensitivity analyses and meta-regression analyses and investigated the publication bias, and these analyses indicated that results of our study were stable. Therefore, the results of our study are more systematic and comprehensive.
Implications for research and practice
Previous studies have reported that SARS and Middle East respiratory syndrome (MERS) patients with nervous system diseases are at a higher risk of poor outcomes [Reference Chen86, Reference Lee, Khang and Lim87]. Our study revealed that nervous system diseases were associated with severity and mortality of patients with COVID-19. Previous studies have shown that SARS-CoV-1 can invade the nerves and cause direct central nervous system infection [Reference Gu88, Reference Xu89], which may also be one of the pathogenic pathways of SARS-CoV-2. Furthermore, the SARS-CoV-2 virus may enter the cerebral circulation, and the interaction between the viral spike proteins and the ACE2 receptors expressed in the brain capillary endothelium may destroy the blood−brain barrier [Reference Patel90, Reference Baig91]. SARS-CoV-2 can infect cardiomyocytes through ACE2 receptors and cause vascular damage and inflammation, making thrombus easy to form and increasing the risk of stroke [Reference Hamming92, Reference Lee93]. COVID-19 could also cause viral encephalitis and haemorrhagic necrosis in the mesial temporal lobes and thalamus [Reference Lee93]. These may be the potential mechanisms for the poor prognosis of COVID-19 patients with nervous system diseases. However, the exact mechanism of increased severity of COVID-19 in patients with nervous system diseases remains unclear, which requires further research to clarify.
Our meta-analyses found that cerebrovascular disease was associated with severity and mortality of patients with COVID-19. These findings highlight the need for neurologists to be vigilant to the high risk of serious illness and death associated with COVID-19 infection in patients with nervous system diseases. A systematic review showed that an increasing number of reports of COVID-19 patients with neurological disorders have added emergent experimental models with neuro-invasion, which is a reasonable concern because SARS-CoV-2 is a new neuropathogen [Reference Montalvan94]. However, at present, there is a lack of treatment strategies for COVID-19 patients with nervous system diseases. Therefore, protecting patients with nervous system diseases from COVID-19 is a problem worthy of our attention. To the best of our knowledge, there is currently no recommendation regarding the treatment strategies for nervous system diseases patients with COVID-19. The results of our meta-analysis also provide the latest references for the development of new guidelines. There is an urgent need for high-quality evidence-based guidelines to clarify the protective measures for patients with nervous system diseases, as well as care and treatment strategies for nervous system disease patients with COVID-19.
Strengths and limitations
Despite comprehensive analyses, our meta-analysis has many limitations. First, we found that some patients of included studies were still hospitalised at the end of the study and no studies reported the specific time period of nervous system diseases. Second, since we included cohort studies and case−control studies, there might be confounding factors that influence the relationship between nervous system diseases and COVID-19 severity as well as mortality. Third, there was much variation in eligibility for SARS-CoV-2 testing between studies or over time within studies. Fourth, we conducted meta-regression analysis and sensitivity analysis to explore the sources of heterogeneity, but the selected factors were not the sources of heterogeneity and the results of some meta-analyses may be affected by the high heterogeneity. Finally, the total number of patients with nervous system diseases included in analyses is relatively small even in this comprehensive literature review, resulting in some wide confidence intervals. As described above, these limitations showed that caution is required before drawing any firm conclusions in the absence of high-quality, comprehensive evidence.
Conclusions
Nervous system diseases were associated with severity and mortality of patients with COVID-19. Among them, cerebrovascular disease was associated with a high risk of severity and mortality of patients with COVID-19. However, due to the limitations of this study, more high-quality, large sample, multicentre trials are needed to provide robust evidence to support clinical practice.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268821000376.
Acknowledgements
The authors thank all investigators and supporters involved in this study.
Author contributions
YG, YMC and JHT planned and designed the study. YG, YMC, ML, MMN, MLY and ZWS participated in the literature search and data collection. YG, YMC and ML analysed the data. YG and YMC drafted the paper. YG, YMC and JHT revised the paper. All authors read and approved the final paper.
Financial support
This study was funded by the Emergency Research Project of Key Laboratory of Evidence-based Medicine and Knowledge Translation of Gansu Province (grant no. GSEBMKT-2020YJ01).
Role of the Funding Source
The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the paper; and decision to submit the paper for publication.
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
Not applicable.
Consent for publication
Not applicable.
Data
All datasets generated for this study are included in the manuscript.











