The use of aggressive chemotherapeutic and immunosuppressive agents as well as broad-spectrum antimicrobials has created a large population of patients who are at increased risk of acquiring infections from fungal organisms, especially Candida spp. C. albicans accounts for over the half of all cases of invasive candidiasis but C. glabrata has emerged as the second most common cause and several other yeast species are also increasing in frequency [Reference Tortorano1, Reference Pfaller and Diekema2]. The first comprehensive survey of fungal infection in Belgium was carried out in 2002 [Reference Swinne3] and the species of yeast, potential risk factors and susceptibilities to antifungal agents were defined for 211 isolates from 207 patients in 28 hospitals. We repeated the survey over a 15-month period and involved twice the number of patients and hospital centres to assess whether the earlier findings hold true.
Fifty-five centres contributed isolates to the study between June 2005 and September 2006. Isolates were subcultured on Sabouraud's agar for 24 h at 35°C and identified by standard methods [Reference Barnett, Payne and Yarrow4, Reference Hennequin5].
Susceptibility testing was performed and interpreted as described in the Clinical Laboratory Standards Institute (CLSI) breakpoints guidelines [6]. Antifungal drugs tested were amphotericin B, itraconazole, fluconazole and voriconazole. As official CLSI interpretative breakpoints are not available for amphotericin B, the breakpoints recommended by Pfaller & Diekema were used [Reference Pfaller and Diekema2]. The amount of glucose in the RPMI medium was doubled to 2% to support optimal growth of isolates [Reference Odds, Vranckx and Woestenborghs7].
A total of 412 yeast isolates was collected from 402 patients; 10 patients had a double yeast infection. As found in the 2002 survey [Reference Swinne3], the majority of patients (54·8%) were male and aged >65 years of age (60%), a finding consistent with the literature [Reference Pfaller8, Reference Charlier9] and the same five most common risk factors in 2002 were also associated, i.e. antimicrobial therapy (45%), stay in intensive care units (34%), the presence of in-dwelling catheters (32%), underlying cancer (23%), and major surgery (11%). Most patients had more than one predisposing factor.
Data for 292 patients showed that fluconazole alone or in combination with another antifungal agent was the treatment of choice for 253 patients (86·6%) Fluconazole monotherapy was prescribed for 231 patients (79·1%); echinocandins (caspofungin or micafungin) were used singly for 21 patients (7·2%); voriconazole, amphotericin B and itraconazole singly for six, four and three patients, respectively. One patient received amphotericin B and 5-fluorocytosine and 26 (8·9%) received different sequential monotherapies. An increase in the use of echinocandins reflects practice in other Belgian centres [Reference Lagrou10].
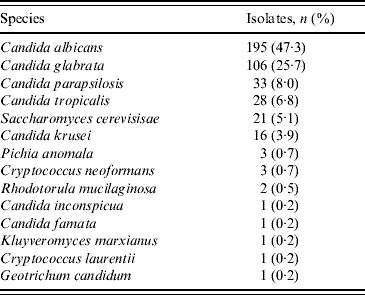
Table 1 shows that of the 412 isolates, 195 (47·3%) were C. albicans; the other most common species were C. glabrata (25·7%), C. parapsilosis (8·0%) and C. tropicalis (6·8%). The incidence of C. albicans and C. parapsilosis was lower than in 2002 (55% and 13% in 2002, respectively). C. glabrata and C. tropicalis increased (22% and 2·8% in 2002, respectively). It is noteworthy that C. glabrata infections are more commonly associated with the elderly and this may explain the increase observed in the present study which involved more elderly patients than the 2002 survey. By contrast C. parapsilosis is generally more frequent in children and the low number of isolates recovered in this group probably reflects the small number of patients (4·4%) aged <15 years [Reference Pfaller8]. There was about a threefold increase in the frequency of Saccharomyces cerevisiae and 19/21 isolates gave a profile of the variety boulardii which is widely used in probiotics, although biotherapy was only cited three times as a risk factor. In the 10 patients with double yeast infections, C. albicans was associated with C. glabrata in five, with C. parapsilosis in two, and with S. cerevisiae in a single patient. C. glabrata was isolated with C. parapsilosis and C. tropicalis in separate patients.
Table 1. Yeast species isolated from blood cultures
The antimicrobial susceptibility of the 217 non-C. albicans isolates is shown in Table 2. Compared with the results obtained using the Sensititre YeastOne commercial system (Trek Diagnostic Systems, East Grinstead, UK) in 2002, resistance increased to amphotericin B (6·4% vs. 1% in 2002), itraconazole (30·4% vs. 7%) and fluconazole (20·7% vs. 3%). Dose-dependent susceptibility was exhibited by 29·9% and 36·4% of isolates to itraconazole and fluconazole, respectively, and this represented an increase from the 2002 survey. Indeed, only 39·6% of isolates were fully susceptible to itraconazole and 42·8% to fluconazole (69% and 82% in 2002, respectively). C. glabrata isolates were mostly responsible for the decrease in susceptibility to these latter agents which was also observed by Lagrou et al. [Reference Lagrou10] using the Sensititre YeastOne commercial system. Resistance to fluconazole was observed in all but three of the C. krusei isolates, a frequently intrinsically resistant species [Reference Charlier9]. Voriconazole showed excellent activity with 87·1% of isolates inhibited by ⩽1 μl/ml. Eleven out of the 15 resistant isolates were C. glabrata and 13 (11 C. glabrata and two Rhodotorula mucilaginosa) showed cross-resistance to the other azoles tested. Susceptibility to voriconazole was not tested for in the 2002 survey but an earlier study from this laboratory showed excellent potency and broad spectrum activity against non-C. albicans isolates [Reference Swinne, Watelle and Nolard11]; this finding is confirmed here.
Table 2. Susceptibilities of 217 non-C. albicans yeast isolates from blood cultures to antifungals
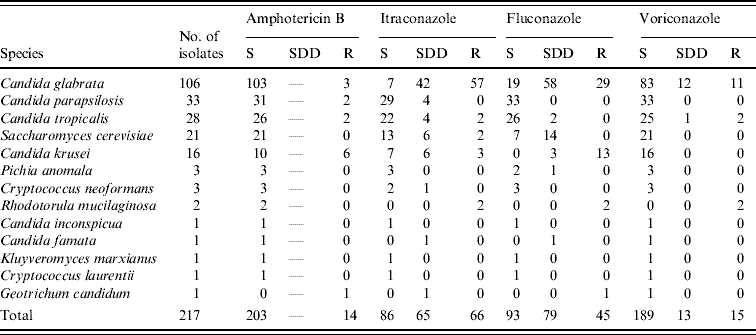
S, Susceptible; SDD, susceptible dose-dependent; R, resistant.
Amphotericin B (S: MIC ⩽1 μg/ml; SDD: not relevant; R: MIC ⩾2 μg/ml).
Itraconazole (S: MIC ⩽0·125 μg/ml; SDD: MIC between 0·25 and 0·5 μg/ml; R: MIC ⩾1 μg/ml).
Fluconazole (S: MIC ⩽8 μg/ml; SDD: MIC between 16 and 32 μg/ml; R: MIC ⩾64 μg/ml).
Voriconazole (S: MIC ⩽1 μg/ml; SDD: MIC=2 μg/ml, R: MIC ⩾4 μg/ml).
In conclusion, the percentage of bloodstream infections due to C. albicans isolates is decreasing in Belgium, probably as a consequence of the use of azoles selecting for more resistant non-C. albicans species, of which C. glabrata remains the most difficult to treat effectively.
ACKNOWLEDGEMENTS
This study was supported by a grant from Pfizer Pharmaceuticals. We thank the participants of the Belgian Institutes for the contribution of isolates. Thanks are also due to Frédéric Fauche, Isabelle Seel, Marc Van der Flaes, Anne-Marie Verhaegen for excellent technical help as well as Christel Binard for administrative support.
APPENDIX
Participants from Belgian institutes contributing isolates
Academisch Ziekenhuis Vlaamse Universiteit, Brussels (S. Lauwers, D. Piérart); Algemeen Ziekenhuis Damiaan, Oostende (G. Alliet); Algemeen Ziekenhuis Groeninge, Kortrijk (J. Colaert); Algemeen Ziekenhuis Middelheim, Antwerpen (A. Mertens); Algemeen Ziekenhuis Jan Palfijn, Merksem (M. Mollemans); Algemeen Ziekenhuis Klina, Braaschaat (G. Eykens); Algemeen Ziekenhuis Sint Augustinus, Wilrijk (J. Van Schaeren); Algemeen Ziekenhuis Sint Dimpna, Geel (G. de MÛelenaere); Algemeen Ziekenhuis St Elisabeth, Herentals (L. De Wolf); Algemeen Ziekenhuis St Jan, Brugge (E. Nulens); Algemeen Ziekenhuis Sint Lucas, Gent (A. M. Van Den Abeele); Algemeen Ziekenhuis Sint Maarten, Mechelen (A. Lemmens, K. Vermaelen); Algemeen Ziekenhuis M. Middelares-St Jozef, Gent (J. Dierick); Algemeen Ziekenhuis Stuivenberg, Antwerpen (K. Camps); Clinique Edith Cavell, Brussels (Y. De Gheldre); Clinique de l‘Europe-site: Sainte-Elisabeth, Brussels (D. Allemeersch); Clinique Générale Saint-Jean, Brussels (B. Mulongo, M. Wegge); Clinique Joseph Bracops, Brussels (A. I. de Moreau); Clinique Notre-Dame de Grâce, Gosselies (N. Fonteyn, B. Gualtieri); Clinique Notre-Dame, Tournai (P. Struyven); Clinique du Parc Léopold, Brussels (D. Bosson, C. Tilmant); Clinique Sainte-Elisabeth, Namur (P. Gavage); Clinique Saint-Luc, Bouge (K. Laffineur); Clinique Sud Luxembourg, Arlon (P. Goffinet); Cliniques Universitaires St Pierre, Ottignies (J. Wautelet); Cliniques Universitaires Saint-Luc, Brussels (J. Gigi); Centre Hospitalier Notre-Dame et Reine Fabiola, Charleroi (N. Thys); Centre Hospitalier Régional, Huy (C. Sion); Centre Hospitalier Régional, Namur (N. Thys); Centre Hospitalier Universitaire Brugmann, Brussels (G. Mascart); Centre Hospitalier Universitaire Mont-Godinne, Yvoir (Y. Glupszynski); Centre Hospitalier Universitaire Tivoli, La Louvière (C. Potvliege); Centre Hospitalier Universitaire André Vésale, Montigny-le-Tilleul (D. Govaerts); Dodoenziekenhuis, Mechelen (A. Lemmens); Heilig Hart Ziekenhuis, Lier (W. Laffut); Heilig Hart Ziekenhuis, Mol (P. Verbeeck); Heilig Hart Ziekenhuis, Roeselaere (I. Surmont, A. Verlinde); Henri Serruys Ziekenhuis, Oostende (M. Van Kerkhove); Hôpital Ambroise Paré, Mons (M. Vatlet); Hôpital Saint-Joseph, Charleroi (M. Fossion); Hôpital Erasme, Université libre, Brussels (M. J. Struelens, H. Villalobos); Hôpital de Jolimont, Haine-Saint-Paul (F. Meunier); Hôpital Militaire Reine Astrid, Brussels (M. Pauwels); Onze Lieve Vrouwziekenhuis Aalst (A. Boel); Onze Lieve Vrouw van Lourdes, Waregem (A. Vuye); Sint Andriesziekenhuis, Tielt (C. Verdonckt); Sint Augustinuskliniek, Veurne (A. Verhaeghe); Sint Elisabethziekenhuis, Geel (T. Van Thienen); Stedelijk Ziekenhuis, Roeselaere (R. De Smedt); Universitair Ziekenhuis, Antwerpen (G. Ieven); Universitair Ziekenhuis, Gent (G. Claeys; G. Verschraegen); Virga Jesse Ziekenhuis, Hasselt (R. Cartuyvels, K. Magerman); J. Yperman Ziekenhuis, Ieper (P. Vandecandelaere); ZOL Campus Sint Jan, Genk (G. Coppens).
DECLARATION OF INTEREST
None.




