Sub-Saharan Africa (SSA) has long been battling with communicable diseases; namely HIV, malaria and tuberculosis. However, the region's disease burden continues to grow given the onset of the nutrition transition, which has led to an increase in prevalence of non-communicable diseases (NCD) such as type-2 diabetes(Reference Steyn and McHiza1). Out of the 371 million people with diabetes worldwide, Africa is home to 27·5 million (7·4 %) of them. Table 1 presents a summary of data on prevalence of type-2 diabetes in SSA in people aged 20–79 years and Table 2 presents these data in relation to highest prevalence. However, the full extent of the diabetes epidemic in Africa may be underestimated given poor disease surveillance and a lack of local diagnostic data(Reference Mensah2–Reference Mbanya, Motala and Sobngwi4). Table 3 presents the regional perspective on proportion of undiagnosed type-2 diabetes in 20–79 year olds. By 2030 the total burden of NCD in Africa is expected to surpass that of communicable diseases.
Table 1. Sub-Saharan countries with the highest number of people aged 20–79 years with type-2 diabetes(Reference Peer, Kengne and Motala5)

Table 2. Sub-Saharan countries with the highest percentage of people aged 20–79 years with type-2 diabetes(Reference Peer, Kengne and Motala5)

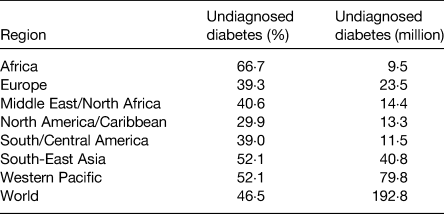
Table 3. Regional estimates of the proportion and number of undiagnosed type-2 diabetes among adults ages 20–79 years(Reference Ogurtsova, da Rocha Fernandes and Huang6)
High rates of type-2 diabetes and other NCD are mainly due the nutrition transition in African countries that are advancing economically. Nine out of the ten fastest growing economies are in Africa; bringing with it changes in the environmental and nutritional landscape of a country that has embraced globalisation(Reference Taylor and Jacobson7). Some of these changes include rapid urbanisation and shifting of traditional diets towards more globalised foods including sugar-sweetened beverages (SSB)(Reference Mbanya, Assah and Saji8). This is reflected in predictions that Africa will experience the largest increase in type-2 diabetes cases (estimated 41·4 million by 2035); and that nine out of ten countries with the highest rates of type-2 diabetes will be in Africa(Reference Mbanya, Assah and Saji8, Reference Zhang, Zhang and Brown9). This is supported by data from the International Diabetes Federation, which estimates that between 2010 and 2030, Africa will see a 98·1 % increase in diabetic patients; the highest in the world(Reference Bosu10).
Also, countries experiencing high levels of globalisation are more likely to import and consume sugar-based processed products including SSB. Country-specific fixed-effects models exploring the relationship between sugar and processed foods imports, globalisation and average BMI revealed an association between sugar and processed food imports and increasing BMI(Reference Lin, Teymourian and Tursini11).
Sugar-sweetened beverage, obesity and type-2 diabetes
Obesity is a risk factor for diabetes and other NCD, and excess bodyweight is the sixth most important risk factor contributing to the overall global burden of disease(Reference Hu and Malik12). The link between SSB and obesity is well established(Reference Hu and Malik12, Reference Buhler, Raine and Arango13). Its growing intake plays an important role in the onset of type-2 diabetes(Reference Kengne, Echouffo-Tcheugui and Sobngwi3, Reference Manyema, Veerman and Chola14).
Studies conducted in both high-income and low- to middle-income countries (LMIC) have provided clear evidence of this(Reference Hu and Malik12, Reference Imamura, Micha and Khatibzadeh15–Reference Malik, Popkin and Bray17). Data from the Euromonitor Global Market Information Database, the WHO and the International Diabetes Federation were analysed to estimate the association between SSB consumption and overweight, obesity and diabetes prevalence in seventy-five countries. It was shown that there was a global increase in SSB consumption from 9·5 gallons per person per year in 1997 to 11·4 gallons in 2010. There was also a 1 % rise in SSB consumption that was linked to an additional 4·8 overweight, 2·3 obese and 0·3 diabetic adults per 100 people. Such results were found to be particularly robust in LMIC(Reference Basu, Vellakkal and Agrawal18).
The average 330 ml serving of sugar-sweetened soda or fruit juice contains anywhere between 40 and 45 g sugar(Reference Manyema, Veerman and Chola14). Owing to their popularity and often lack of nutritional knowledge, SSB contribute to a considerable proportion of total per capita sugar and energy consumption(Reference Manyema, Veerman and Chola14). Using data from 165 countries, it was shown that diabetes prevalence was strongly correlated to per capita sugar consumption(Reference Weeratunga, Jayasinghe and Perera19). Therefore, regular SSB consumption can raise both glucose and insulin levels in an individual; leading to a high dietary glycaemic load, and eventual weight gain(Reference Hu and Malik12). Estimates indicate that one 330 ml SSB serving containing between 586J (140 calories) and 626J (150 calories) can result in a weight increase of up to 6·8 kg in one year in a person consuming a standard American diet over a year(Reference Hu and Malik12). This is plausible given the considerably large quantities of rapidly absorbed carbohydrates such as sucrose and high-fructose maize syrup in most SSB. The flavour-enhancing effect of these added carbohydrates comes with adverse effects for the consumer. When consumed in large quantities, the likelihood of developing glucose intolerance and insulin resistance increases, as well as inflammation and cell dysfunction(Reference Manyema, Veerman and Chola14). This indicates that SSB can lead to diabetes independently of obesity(Reference Malik, Popkin and Bray17).
A meta-analysis conducted by Malik et al. provided a clear association between SSB consumption and type-2 diabetes. It was shown that participants with the highest SSB intake had a 26 % higher risk of developing type-2 diabetes compared with those with the lowest intake(Reference Malik, Popkin and Bray17).
Furthermore, it has even been identified that a high sugar intake can inevitably reduce micronutrient intake by lowering the density of key micronutrients in the diet such as zinc, magnesium, calcium, iron and vitamins A, C and B12(Reference Steyn and Temple16, Reference Brown, Stamler and Van Horn20).
Implications for Africa
The type-2 diabetes epidemic is having a devastating impact on SSA, resulting in higher mortality and morbidity than any other region. This is largely due to factors such as inadequate public healthcare system and medication supply, along with inadequate funding for diabetes research, and disparities between urban and rural diabetic patients(Reference Pastakia, Pekny and Manyara21).
Despite recent advances, African healthcare systems still have challenges for mapping the epidemiological transition from communicable diseases to NCD, and/or effectively dealing with the shift in disease patterns(Reference Byass, de Savigny and Lopez22). These inadequacies range from a lack of key diagnostic tools, essential medications, as well as standardised protocols for treatment monitoring and specialist referral(Reference Kane, Landes and Carroll23). In addition, the poor integration of diabetic care into African healthcare systems is widespread(Reference Nuche-Berenguer and Kupfer24).
SSA spends the least on healthcare despite having some of the highest disease burdens. Healthcare costs related to type-2 diabetes alone are expected to rise by 50 % between 2010 and 2030; to reach an estimated UD$2 billion by 2030(Reference Zhang, Zhang and Brown9). Yet countries such as Ivory Coast, Ethiopia, Madagascar and Niger spend <US$20 per diabetic person; which is not sufficient to cover the cost of the most basic pharmaceutical treatment(Reference Zhang, Zhang and Brown9). In total, the eighteen African countries belonging to International Diabetes Federation cumulatively spend only 0·3 % of the global diabetic bill, compared with 52·7 % paid by the USA(Reference Zhang, Zhang and Brown9). Less than 10 % of the world's expenditure on diabetes will come from the least developed countries, despite having close to 70 % of the world's diabetic patients(Reference Zhang, Zhang and Brown9).
In a conceptual review, Renzaho(Reference Renzaho25) highlighted several challenges facing diabetes prevention, treatment and management programmes in Africa. These include (1) inadequate risk factor documentation, (2) demographic shifts (urbanisation and ageing populations), (3) co-morbidities, namely tuberculosis and HIV, (4) nutrition emergencies, (5) lack of political prioritisation and (6) an insufficiently regulated food and beverage industry(Reference Renzaho25).
Meanwhile, population-based surveys conducted across Africa between 1980 and 2014 for BMI and diabetes revealed an increase in (age-standardised) mean BMI from 21·0 to 23·0 kg/m2 in men and 21·9 to 24·9 kg/m2 in women; while (age-standardised) prevalence of diabetes grew from 3·4 to 8·5 % in men and from 4·1 to 8·9 % in women. These estimates were significantly higher in Northern and Southern Africa, more so than the global average(Reference Kengne, Sobngwi and Echouffo-Tcheugui26).
Prevalence data from twelve SSA countries showed a median diabetes prevalence of 5 % (2–14 % range) and a median overweight/obesity prevalence of 27 % (16–68 % range)(Reference Manne-Goehler, Atun and Stokes27). Such trends are also observed in the lower income countries. Data collected between 2013 and 2016 from adults in Malawi showed overweight and obesity prevalence of 18 and 44 % in urban men and women, respectively; and 9 and 27 % in rural men and women, respectively. Meanwhile, diabetes prevalence was 3 % in urban residents and 2 % in rural residents, irrespective of sex(Reference Price, Crampin and Amberbir28). This presents an urgent need for action particualrly on prevention of disease.
Consumption in early years (childhood)
Childhood years, particularly infancy and adolescence, are critical to the physical growth and cognitive development of an individual(Reference Tzioumis and Adair29). Optimum nutrition during this time is essential to achieving positive health outcomes in adulthood. Hence many chronic diseases experienced during adulthood can be traced to dietary factors during these early development years.
The current trend observed in LMIC is a shift from childhood diseases associated with undernutrition to a double burden of under- and overnutrition-related conditions(Reference Tzioumis and Adair29).
Although increase in age remains a risk factor for type-2 diabetes, it is predicted that a shift towards earlier onset will be witnessed as the epidemic matures(Reference Mbanya, Motala and Sobngwi4). In 2013, most diabetic cases in Africa were among individuals under age 60 years, with peak onset found between 40 and 59 years(Reference Mbanya, Motala and Sobngwi4, Reference Werfalli, Musekiwa and Engel30). This is likely to fall to an even lower age, given the trend for early age consumption of SSB in SSA. In some instances, infants are given SSB as a means of weaning them off breast milk. A Nigerian cross-sectional survey revealed that close to 90 % of 6–18 months old babies were given sugar-sweetened chocolate drinks, while 79·9 % were given fruit juices. Just over 70 % of infants were given carbonated drinks, of which approximately 16 % received it daily(Reference Bankole, Aderinokun and Odenloye31). In a similar study in rural South Africa, 54 % of infants between 4 and 24 months in one area and 37 % in another area were given carbonated drinks up to three times per week(Reference MacKeown and Faber32). It is thus unsurprising that carbonated drinks are the third most commonly consumed beverage among infants aged 12–24 months in urban South Africa(Reference Igumbor, Sanders and Puoane33).
A cross-sectional survey conducted in eleven South African primary school shops revealed that children had a daily sugar intake of 34 g from carbonated drinks; in addition to 13 g from flavoured milk and 24 g from mixed fruit blends, all consumed at school(Reference Wiles NL and Veldman34). Two of the shops also sold 500 ml and 1 litre servings of carbonated drinks; raising flags of concern over the lack of nutritional knowledge regarding the dangers of regularly consuming SSB in such large quantities(Reference Oldewage-Theron, Egal and Moroka35).
In a descriptive survey conducted in Cape Town, school children aged 9–13 years were consuming up to 730 g carbonated drinks daily, equalling a sugar intake of 40–80 g each day from carbonated drinks alone(Reference Louwrens and Otty36). Such high intake levels persist into adolescence. In urban Uganda, a 2004 cross-sectional survey involving 614 adolescents aged 10–14 years found that an average of 30 % were consuming carbonated drinks on a daily basis(Reference Kiwanuka and Trovik37).
Available national data on sugar consumption in children from the 1999 South African National Food Consumption Survey showed an average daily consumption in 1–9 years old of 10·4 g sugar from squash, and 6 g sugar from carbonated drinks(Reference Steyn and Temple16).
Sugar consumption in urban South Africa for adolescents and adults (over 10 years) was reported to be 12·3 % of total energy intake, which exceeds WHO recommendations of <10 % of total energy intake; and <5 % for additional health benefits(38, Reference Ronquest-Ross and Sigge39). From 1999 to 2012, there was a 68·9 % increase in carbonated drink consumption in South Africa; with consumption by rural women increasing from 25 % in 2005 to 56 % in 2010, and from 33 to 63 % in men during the same period(Reference Ronquest-Ross and Sigge39). Carbonated drinks were second only to fruit as the most commonly consumed street food in South Africa(Reference Ronquest-Ross and Sigge39).
Much of this frequent and early consumption stems from the affordability of SSB. Goryakin et al.(Reference Goryakin, Monsivais and Suhrcke40)assessed the relationship between sales and prices of carbonated soft drinks and BMI, overweight, obesity and diabetes in seventy-eight countries. Interestingly, the study discovered a significantly positive relationship only in lower to middle-income countries. It was then suggested that restricting sales of SSB could prove beneficial towards minimising the health impact in least developed countries(Reference Goryakin, Monsivais and Suhrcke40).
Nutritional knowledge/education
A lack of nutritional knowledge/education of the risks involved with frequent SSB intake may also play a role in early consumption. Data from high- and low-income high school students in peri-urban South Africa showed an inverse relationship between level of mothers’ education and students’ frequency of carbonated drink consumption(Reference Audain and Veldman41).
Despite the association between SSB and diabetes, there are little to no data on the level of consumer knowledge of the adverse effects of frequent SSB consumption within the African context. In a US study, it was observed that less than a third of survey respondents correctly identified the daily energy recommendations for a typical adult; and these were also found to consume on average nine fewer SSB per month compared with individuals who responded incorrectly(Reference Gase, Robles and Barragan42).
A South African study evaluated the effect of a nutrition education programme on diabetes knowledge and attitudes of adults with type-2 diabetes; and it was discovered that the intervention group that received weekly group education, follow-up meetings and education materials had higher mean diabetes knowledge scores than the control group that received education materials only. However, it was also observed that the scores were below 50 %. The study concluded that education programmes should focus on addressing misconceptions about healthy eating and unhealthy eating practices, increasing self-efficacy regarding purchasing and preparation of healthy food, and representing diverse cultures while paying attention to issues related to availability and affordability(Reference Muchiri, Gericke and Rheeder43).
Policy regulations
Globally, concerted efforts are being made to reduce SSB consumption and curb the obesity epidemic, one of which is implementing a taxation on sugar and sugar-based products. The UK Chancellor of the Exchequer announced a levy on SSB in 2016, following a report by the Scientific Advisory Committee on Nutrition; which inexplicably linked SSB with obesity in children and teenagers, as well as type-2 diabetes independently of obesity. The levy, which commenced in 2018, involves companies paying a higher level tax for drinks with more than 8 g sugar/100 ml(Reference Jones44). The Scientific Advisory Committee on Nutrition also recommended that <5 % of total dietary energy intake for adults and children from the age of 2 years should be from free sugars(Reference Swan, Powell and Knowles45).
In response, the UK government published two childhood obesity plans, which included the announcements on a soft drink industry levy and a wider sugar reformulation programme(46, 47). The soft drink industry levy applied to drinks with sugar content of 8 and 5 %, with a view to incentivise manufacturers to reduce sugar content in their products in advance of the levy being applied. In 2018, the Public Health England published a progress report on these programmes, demonstrating an 11 % reduction in sugar sales in the UK as a result of the soft drink industry levy; and a 2 % reduction in sugar in the nine food categories covered by the reformulation programme(48).
In the USA, SSB are the main source of added sugars in the average diet, which includes sweeteners such as sucrose, high-fructose maize syrup or fruit juice concentrates(Reference Malik, Popkin and Bray17). From 2006, the city of New York began disseminating educational messages via mass media campaigns on the adverse health effects of SSB consumption. In addition, city agencies reduced the availability of SSB; and policies on excise tax and capping drink portion sizes in the food service industry were proposed. From 2007 to 2013, a 35 % decrease in consumption of one or more SSB daily by the average adult was observed; as well as a 27 % decrease among adolescents(Reference Kansagra, Kennelly and Nonas49).
Whereas, researchers highlighted in India, 11·2 million cases of overweight and obesity and 400 000 cases of type-2 diabetes could be prevented, should they implement a 20 % excise duty on SSB from 2014 to 2023(Reference Sachan50).
South Africa was the first and to date remains the only African country to attempt a taxation on sugary foods. Effective from April 2017, the tax came amidst heavy criticism from the sugar industry. Sugar directly employs up to 79 000 people in South Africa, with another one million depending on sugar for their livelihood(51). At the same time, the country faces nearly seventeen million annual health centre visits as a result of diabetes and hypertension alone(52). This has spurred the South African Department of Health to recommend considering the taxation on sugary foods as a strategy for reducing obesity and NCD prevalence(52). A life table-based model to determine the impact of SSB taxation on nutritional status (BMI) was constructed by Manyema et al.(Reference Manyema, Veerman and Chola14) using consumption data from the South African National Health and Nutrition Examination Survey in 2012, along with own- and cross-price elasticities of SSB and energy balance equations. It was noted that an increase in SSB tax of 20 % over a 20-year period could lower the diabetes incidence rate in women and men by 106 000 and 54 000, respectively. This could lead to the avoidance of 21 000 diabetes-related deaths, 374 000 daily-adjusted life years and over US$650 million in healthcare expenditure(Reference Manyema, Veerman and Chola14).
The South African government also made efforts to limit the exposure of its young population to aggressive marketing tactics. SSB are aggressively marketed to younger people through multiple avenues in SSA such as the sponsoring of high profile sporting and entertainment(Reference Jones44). Data from South Africa identified television as the most influential advertising medium that encourages the consumption of SSB and other unhealthy food choices(Reference McHiza, Temple and Steyn53). It was revealed that most advertisements shown during children's viewing time (15·00 hours–17·00 hours) were for unhealthy foods including desserts and sweets (28 %), fast foods (17 %) and SSB (11 %)(Reference McHiza, Temple and Steyn53). In 2007, the government drafted the Foodstuffs, Cosmetics & Disinfectants Act, which included restrictions of the advertising of foods considered ‘non-essential to a healthy lifestyle’ such as SSB to children younger than 16 years(Reference Igumbor, Sanders and Puoane33).
The national policies to address obesity and diabetes in LMIC and particularly SSA are currently insufficient. Some trade-related policies have been proposed, including an increase in duties and import taxes on sugar products. However, such initiatives may have marginal effectiveness, if any at all, owing to the normalisation of frequent SSB consumption among many populations. Hence local and community organisations can develop strategies to target individual practices surrounding SSB consumption(Reference Lin, Teymourian and Tursini11). Nutrition education campaigns may be necessary to sensitise individuals to the health impact of regular SSB consumption. Efforts cannot afford to be one-sided and should involve a multi-sectoral approach to tackling this concern.
Conclusion
This aim of this review is to explore trends of early consumption of SSB in SSA, within the context of growing child and adolescent obesity and escalating type-2 diabetes prevalence. We explored efforts to mitigate this drawing on a review of the literature. We found that there is a paucity of published literature for many regions in Africa to inform the review and this should be an area of future research to inform country-level policy. However, despite this we conclude this review with a number of points.
The contribution of SSB to obesity and diabetes in SSA is real and escalating rapidly; therefore, immediate action to mitigate its impact must be taken. Attention should be paid to the early consumption of SSB as well as its marketing to Africa's youthful population. The adoption of nutrition education programmes at the school level to provide information on the health risks of excess SSB consumption and their contribution to obesity, diabetes and other NCD should be encouraged. Best practices and lessons learned from other countries should be reviewed and adopted within context. These include but are not limited to nutrition education and health behaviour change initiatives. The beverage industry in Africa should be actively monitored and regulated as they are in a number of industrialised countries; given the challenges for healthcare systems to cope with growing NCD epidemics. Governments should also be informed of the medium- to long-term health and financial benefits of investing in obesity prevention measures such as taxation on sugary foods. African nutritionists and dietitians must play a prominent role in providing this information, by collating the evidence needed to inform policy and through the use of advocacy to influence policy change.
Acknowledgements
The authors are grateful to Bilge Seyhan (Research Assistant, University of Chester) for her assistance in the final preparations of this paper.
Financial Support
This research received no other specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.





